Reducing the Risks of Esophagectomies: A Retrospective Comparison of Hybrid versus Full-Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE) Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients
2.3. Surgical Technique
2.4. Postoperative Care
2.5. Endpoints
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Uhlenhopp, D.J.; Then, E.O.; Sunkara, T.; Gaduputi, V. Epidemiology of esophageal cancer: Update in global trends, etiology and risk factors. Clin. J. Gastroenterol. 2020, 13, 1010–1021. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; Ruurda, J.P.; Verhage, R.J.; van der Horst, S.; Haverkamp, L.; Siersema, P.D.; Borel Rinkes, I.H.; Ten Kate, F.J.; van Hillegersberg, R. Oncologic long-term results of robot-assisted minimally invasive thoraco-laparoscopic esophagectomy with two-field lymphadenectomy for esophageal cancer. Ann. Surg. Oncol. 2015, 22, 1350–1356. [Google Scholar] [CrossRef]
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.C.C.M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Kingma, B.F.; Grimminger, P.P.; van der Sluis, P.C.; van Det, M.J.; Kouwenhoven, E.A.; Chao, Y.-K.; Tsai, C.-Y.; Fuchs, H.F.; Bruns, C.J.; Sarkaria, I.S.; et al. Worldwide Techniques and Outcomes in Robot-assisted Minimally Invasive Esophagectomy (RAMIE). Ann. Surg. 2020, 276, e386–e392. [Google Scholar] [CrossRef] [PubMed]
- Mariette, C.; Piessen, G.; Briez, N.; Gronnier, C.; Triboulet, J.P. Oesophagogastric junction adenocarcinoma: Which therapeutic approach? Lancet Oncol. 2011, 12, 296–305. [Google Scholar] [CrossRef]
- Babic, B.; Müller, D.T.; Jung, J.O.; Schiffmann, L.M.; Grisar, P.; Schmidt, T.; Chon, S.H.; Schröder, W.; Bruns, C.J.; Fuchs, H.F. Robot-assisted minimally invasive esophagectomy (RAMIE) vs. hybrid minimally invasive esophagectomy: Propensity score matched short-term outcome analysis of a European high-volume center. Surg. Endosc. 2022, 36, 7747–7755. [Google Scholar] [CrossRef] [PubMed]
- Hoelzen, J.P.; Sander, K.J.; Sesia, M.; Roy, D.; Rijcken, E.; Schnabel, A.; Struecker, B.; Juratli, M.A.; Pascher, A. Robotic-Assisted Esophagectomy Leads to Significant Reduction in Postoperative Acute Pain: A Retrospective Clinical Trial. Ann. Surg. Oncol. 2022, 29, 7498–7509. [Google Scholar] [CrossRef]
- Kingma, B.F.; de Maat, M.F.G.; van der Horst, S.; van der Sluis, P.C.; Ruurda, J.P.; van Hillegersberg, R. Robot-assisted minimally invasive esophagectomy (RAMIE) improves perioperative outcomes: A review. J. Thorac. Dis. 2019, 11 (Suppl. 5), 735–742. [Google Scholar] [CrossRef]
- van der Sluis, P.C.; van der Horst, S.; May, A.M.; Schippers, C.; Brosens, L.A.A.; Joore, H.C.A.; Kroese, C.C.; Mohammad, N.H.; Mook, S.; Vleggaar, F.P.; et al. Robot-assisted minimally invasive thoracolaparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer. Ann. Surg. 2019, 269, 621–630. [Google Scholar] [CrossRef]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of Surgical Complications: A New Proposal With Evaluation in a Cohort of 6336 Patients and Results of a Survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Low, D.E.; Kuppusamy, M.K.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.; Davies, A.; D’journo, X.B.; Gisbertz, S.S.; Griffin, S.M.; et al. Benchmarking complications associated with esophagectomy. Ann. Surg. 2019, 269, 291–298. [Google Scholar] [CrossRef]
- Mariette, C.; Markar, S.R.; Dabakuyo-Yonli, T.S.; Meunier, B.; Pezet, D.; Collet, D.; D’journo, X.B.; Brigand, C.; Perniceni, T.; Carrère, N.; et al. Hybrid Minimally Invasive Esophagectomy for Esophageal Cancer. N. Engl. J. Med. 2019, 380, 152–162. [Google Scholar] [CrossRef]
- Biere, S.S.; Van Berge Henegouwen, M.I.; Maas, K.W.; Bonavina, L.; Rosman, C.; Garcia, J.R.; Gisbertz, S.S.; Klinkenbijl, J.H.G.; Hollmann, M.W.; de Lange, E.S.; et al. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: A multicentre, open-label, randomised controlled trial. Lancet 2012, 379, 1887–1892. [Google Scholar] [CrossRef]
- Straatman, J.; van der Wielen, N.; Cuesta, M.A.; Daams, F.; Garcia, J.R.; Bonavina, L.; Rosman, C.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; van der Peet, D.L. Minimally invasive versus open esophageal resection: Three-year follow-up of the previously reported randomized con-trolled trial: The TIME trial. Ann. Surg. 2017, 266, 232–236. [Google Scholar] [CrossRef]
- van Hillegersberg, R.; Boone, J.; Draaisma, W.A.; Broeders, I.A.; Giezeman, M.J.; Rinkes, I.B. First experience with robot-assisted thoracoscopic esophagolymphadenectomy for esophageal cancer. Surg. Endosc. 2006, 20, 1435–1439. [Google Scholar] [CrossRef] [PubMed]
- Booka, E.; Takeuchi, H.; Suda, K.; Fukuda, K.; Nakamura, R.; Wada, N.; Kawakubo, H.; Kitagawa, Y. Meta-analysis of the impact of postoperative complications on survival after oesophagectomy for cancer. BJS Open 2018, 2, 276–284. [Google Scholar] [CrossRef] [PubMed]
- Kamarajah, S.K.; Griffiths, E.A.; Phillips, A.W.; Ruurda, J.; van Hillegersberg, R.; Hofstetter, W.L.; Markar, S.R. Robotic Techniques in Esophagogastric Cancer Surgery: An Assessment of Short- and Long-Term Clinical Outcomes. Ann. Surg. Oncol. 2022, 29, 2812–2825. [Google Scholar] [CrossRef] [PubMed]
- Tagkalos, E.; Goense, L.; Hoppe-Lotichius, M.; Ruurda, J.P.; Babic, B.; Hadzijusufovic, E.; Kneist, W.; van der Sluis, P.C.; Lang, H.; van Hillegersberg, R.; et al. Robot-assisted minimally invasive esophagectomy (RAMIE) compared to conventional minimally invasive esophagectomy (MIE) for esophageal cancer: A propensity-matched analysis. Dis. Esophagus 2020, 33, doz060. [Google Scholar] [CrossRef]
- Berlth, F.; Mann, C.; Uzun, E.; Tagkalos, E.; Hadzijusufovic, E.; Hillegersberg, R.; Li, H.; Egberts, J.H.; Lang, H.; Grimminger, P.P. Technical details of the abdominal part during full robotic-assisted minimally invasive esophagectomy. Dis. Esophagus 2020, 33 (Suppl. 2), 84. [Google Scholar] [CrossRef]
- Yang, Y.M.; Li, B.M.; Yi, J.M.; Hua, R.M.; Chen, H.M.; Tan, L.M.; Li, H.M.; He, Y.M.; Guo, X.M.; Sun, Y.M.; et al. Robot-assisted Versus Conventional Minimally Invasive Esophagectomy for Resectable Esophageal Squamous Cell Carcinoma: Early Results of a Multicenter Randomized Controlled Trial: The RAMIE Trial. Ann. Surg. 2022, 275, 646–653. [Google Scholar] [CrossRef]
- Angeramo, C.A.; Bras Harriott, C.; Casas, M.A.; Schlottmann, F. Minimally invasive Ivor Lewis esophagectomy: Robot-assisted versus laparoscopic–thoracoscopic technique. Systematic review and meta-analysis. Surgery 2021, 170, 1692–1701. [Google Scholar] [CrossRef]
- Park, S.; Hwang, Y.; Lee, H.J.; Park, I.K.; Kim, Y.T.; Kang, C.H. Comparison of robot-assisted esophagectomy and thoracoscopic esophagectomy in esophageal squamous cell carcinoma. J. Thorac. Dis. 2016, 8, 2853–2861. [Google Scholar] [CrossRef]
- de Groot, E.M.; Goense, L.; Ruurda, J.P.; van Hillegersberg, R. State of the art in esophagectomy: Robotic assistance in the abdominal phase. Updates Surg. 2021, 73, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Grimminger, P.P.; Staubitz, J.I.; Perez, D.; Ghadban, T.; Reeh, M.; Scognamiglio, P.; Izbicki, J.R.; Biebl, M.; Fuchs, H.; Bruns, C.J.; et al. Multicenter Experience in Robot-Assisted Minimally Invasive Esophagectomy—A Comparison of Hybrid and Totally Robot-Assisted Techniques. J. Gastrointest. Surg. 2021, 25, 2463–2469. [Google Scholar] [CrossRef]
- Grimminger, P.P.; Hadzijusufovic, E.; Lang, H. Robotic-assisted Ivor Lewis esophagectomy (RAMIE) with a standardized intrathoracic circular end-to-side stapled anastomosis and a team of two (surgeon and assistant only). J. Thorac. Cardiovasc. Surg. 2017, 66, 404–406. [Google Scholar] [CrossRef]
- Low, D.E.; Alderson, D.; Cecconello, I.; Chang, A.C.; Darling, G.E.; D’Journo, X.B.; Griffin, S.M.; Hölscher, A.H.; Hofstetter, W.L.; Jobe, B.A.; et al. International Consensus on Standardization of Data Collection for Complications Associated With Esophagectomy: Esophagectomy Complications Consensus Group (ECCG). Ann. Surg. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Grimminger, P.P.; Tagkalos, E.; Hadzijusufovic, E.; Corvinus, F.; Babic, B.; Lang, H. Change from Hybrid to Fully Minimally Invasive and Robotic Esophagectomy is Possible without Compromises. J. Thorac. Cardiovasc. Surg. 2019, 67, 589–596. [Google Scholar] [CrossRef]
- Al-Batran, S.-E.; Hofheinz, R.D.; Pauligk, C.; Kopp, H.-G.; Haag, G.M.; Luley, K.B.; Meiler, J.; Homann, N.; Lorenzen, S.; Schmalenberg, H.; et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. Lancet Oncol. 2016, 17, 1697–1708. [Google Scholar] [CrossRef]
- van Hagen, P.; Hulshof, M.C.; van Lanschot, J.J.; Steyerberg, E.W.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.; Richel, D.J.; Nieuwenhuijzen, G.A.; Hospers, G.A.; Bonenkamp, J.J.; et al. CROSS Group. Preoperative chemoradiotherapy for esophageal or junctional cancer. N. Engl. J. Med. 2012, 366, 2074–2084. [Google Scholar] [CrossRef]
- Grimminger, P.P.; Hadzijusufovic, E.; Ruurda, J.P.; Lang, H.; van Hillegersberg, R. The da Vinci Xi Robotic Four-Arm Approach for Robotic-Assisted Minimally Invasive Esophagectomy. J. Thorac. Cardiovasc. Surg. 2018, 66, 407–409. [Google Scholar] [CrossRef]
- Low, D.E.; Allum, W.; De Manzoni, G.; Ferri, L.; Immanuel, A.; Kuppusamy, M.; Law, S.; Lindblad, M.; Maynard, N.; Neal, J.; et al. Guidelines for Perioperative Care in Esophagectomy: Enhanced Recovery after Surgery (ERAS®) Society Recommendations. World J. Surg. 2019, 43, 299–330. [Google Scholar] [CrossRef] [PubMed]
- Kingma, B.F.; Hadzijusufovic, E.; Van der Sluis, P.C.; Bano, E.; Lang, H.; Ruurda, J.P.; van Hillegersberg, R.; Grimminger, P.P. A structured training pathway to implement robot-assisted minimally invasive esophagectomy: The learning curve results from a high-volume center. Dis. Esophagus 2020, 33 (Suppl. 2), 47. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, H.F.; Collins, J.W.; Babic, B.; DuCoin, C.; Meireles, O.R.; Grimminger, P.P.; Read, M.; Abbas, A.; Sallum, R.; Müller-Stich, B.P.; et al. Robotic-assisted minimally invasive esophagectomy (RAMIE) for esophageal cancer training curriculum-a worldwide Delphi consensus study. Dis. Esophagus 2022, 35, 55. [Google Scholar] [CrossRef]
- Chon, S.H.; Brunner, S.; Müller, D.T.; Lorenz, F.; Stier, R.; Streller, L.; Eckhoff, J.; Straatman, J.; Babic, B.; Schiffmann, L.M.; et al. Time to endoscopic vacuum therapy-lessons learned after > 150 robotic-assisted minimally invasive esophagectomies (RAMIE) at a German high-volume center. Surg. Endosc. 2023, 37, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Buunen, M.; Gholghesaei, M.; Veldkamp, R.; Meijer, D.W.; Bonjer, H.J.; Bouvy, N.D. Stress response to laparoscopic surgery: A review. Surg. Endosc. 2004, 18, 1022–1028. [Google Scholar] [CrossRef] [PubMed]
- Visser, E.; Marsman, M.; van Rossum, P.S.N.; Cheong, E.; Al-Naimi, K.; van Klei, W.A.; Ruurda, J.P.; van Hillegersberg, R. Postoperative pain management after esophagectomy: A systematic review and meta-analysis. Dis. Esophagus 2017, 30, 1–11. [Google Scholar] [CrossRef]
- Tagkalos, E.; van der Sluis, P.C.; Berlth, F.; Poplawski, A.; Hadzijusufovic, E.; Lang, H.; van Berge Henegouwen, M.I.; Gisbertz, S.S.; Müller-Stich, B.P.; Ruurda, J.P.; et al. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus minimally invasive esophagectomy for resectable esophageal adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC Cancer 2021, 21, 1060. [Google Scholar] [CrossRef]


| Full-RAMIE (n = 105) n (%) | Hybrid-RAMIE (n = 63) n (%) | p-Value | |
|---|---|---|---|
| Age, years | p = 0.05 | ||
| <65 | 46 (43.8) | 39 (61.9) | |
| 65–75 | 41 (39.1) | 14 (22.2) | |
| >75 | 18 (17.1) | 10 (15.9) | |
| Sex | p = 1 | ||
| F | 17 (16) | 10 (15.9) | |
| M | 88 (84) | 53 (84.1) | |
| Ethnicity | |||
| White | 105 (100) | 63 (100) | |
| BMI, kg/m2 | p = 0.113 | ||
| <20 | 8 (7.6) | 2 (3.2) | |
| 20–30 | 77 (73.3) | 41 (65.1) | |
| >30 | 20 (19) | 20 (31.7) | |
| ASA score | p = 0.314 | ||
| 1 | 3 (2.9) | 2 (3.2) | |
| 2 | 50 (47.6) | 35 (55.6) | |
| 3 | 52 (49.5) | 26 (41.3) | |
| 4 | 0 (0) | 0 (0) | |
| Type of carcinoma | p = 0.513 | ||
| Adenocarcinoma | 87 (82.9) | 55 (87.3) | |
| Squamous cell carcinoma | 18 (17.1) | 8 (12.7) | |
| Locations of tumor | p = 0.555 | ||
| Upper third | 1 (1.0) | 0 (0) | |
| Middle third | 12 (11.4) | 4 (6.3) | |
| Gastroesophageal junction | 92 (87.6) | 59 (93.7) | |
| Neoadjuvant therapy | p = 0.269 | ||
| Chemotherapy | 31 (29.5) | 24 (38.1) | |
| Chemoradiotherapy | 65 (61.9) | 31 (49.2) | |
| None | 9 (8.6) | 8 (12.7) | |
| Charlson Comorbidity Index | p = 0.07 | ||
| 1 | 7 (6.7) | 0 (0) | |
| 2 | 8 (7.6) | 1 (1.6) | |
| 3 | 21 (20.0) | 11(17.5) | |
| 4 | 24 (22.9) | 13 (20.6) | |
| 5 | 14 (13.3) | 19 (30.2) | |
| 6 | 13 (12.4) | 10 (15.9) | |
| 7 | 8 (7.6) | 4 (6.3) | |
| 8 | 5 (4.8) | 3 (4.8) | |
| 9 | 4 (3.8) | 2 (3.2) | |
| 10 | 0 (0) | 0 (0) | |
| 11 | 1 (1.5) | 0 (0) | |
| pretherapeutic T-status | p = 0.645 | ||
| T1 | 10 (9.5) | 7 (11.1) | |
| T2 | 20 (19.0) | 13 (20.6) | |
| T3 | 73 (69.5) | 42 (66.7) | |
| T4 | 2 (1.9) | 1 (1.6) | |
| pretherapeutic N-status | p < 0.01 | ||
| N0 | 57 (54) | 16 (25) | |
| N+ | 48 (46) | 47 (74) |
| Full-RAMIE (n = 105) | Hybrid-RAMIE (n = 63) | p-Values | |
|---|---|---|---|
| Operating time, min * | 410 (367, 470) | 494 (409, 593) | p < 0.001 |
| Blood loss, mL * | 0 (0, 125) | 0 (0, 300) | p = 0.5 |
| Conversions [n (%)] | 0 (0) | 0 (0) | |
| Conversion thorax | 0 (0) | NA | |
| Conversion abdomen | 0 (0) | 0 (0) | |
| Pathological T-status [n (%)] | p = 0.8 | ||
| T0 | 26 (24.8) | 14 (22.2) | |
| T1 | 12 (11.4) | 12 (19.1) | |
| T2 | 20 (19) | 6 (9.5) | |
| T3 | 47 (44.8) | 31 (49.2) | |
| Pathological N-status [n (%)] | p = 0.848 | ||
| N0 | 57 (54.3) | 35 (55.6) | |
| N1 | 23 (21.9) | 9 (14.3) | |
| N2 | 16 (15.2) | 13 (20.6) | |
| N3 | 9 (8.6) | 6 (9.5) | |
| Radicality of surgery [n (%)] | p = 1.00 | ||
| R0 | 102 (97.1) | 61 (96.8) | |
| R1 | 3 (2.9) | 2 (3.2) |
| Full-RAMIE (n = 105) | Hybrid-RAMIE (n = 63) | p-Values | |
|---|---|---|---|
| Morphine-equivalent-dose total, mg * | 194 (105, 360) | 345 (215, 750.5) | p < 0.001 |
| Morphine-equivalent-dose total/kg bw, mg/kg * | 2.28 (1.12, 4.83) | 3.66 (2.54, 7.83) | p < 0.001 |
| Morphine-equivalent-dose total/kg bw/days hospital stay, mg/kg/days * | 0.13 (0.07, 0.26) | 0.24 (0.14, 0.36) | p < 0.001 |
| NRS pain POD 1 * | |||
| rest | 0 (0, 1) | 2 (1, 3) | p < 0.001 |
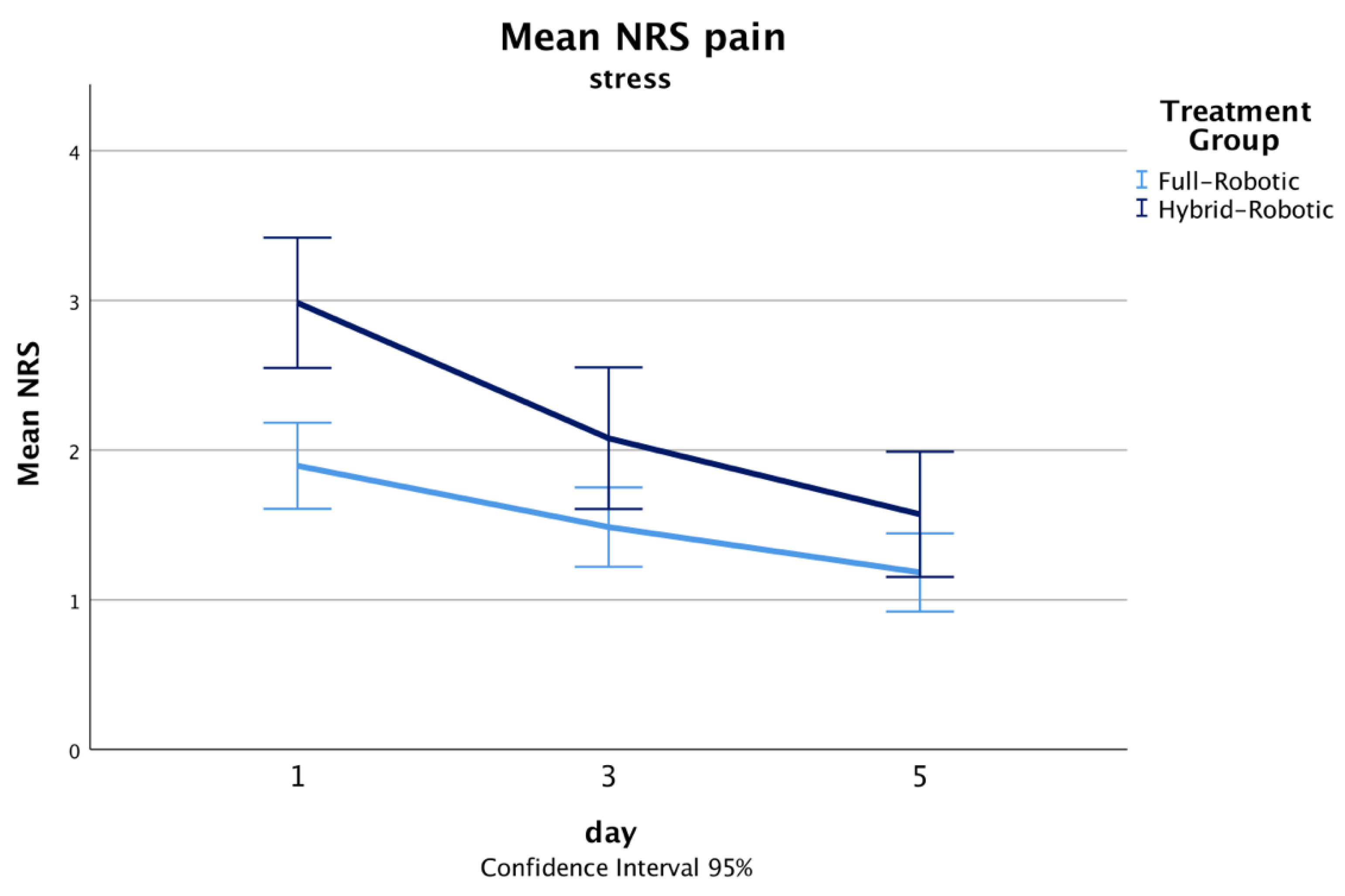
| stress | 2 (1, 3) | 3 (2, 4) | p < 0.001 |
| NRS pain POD 3 * | |||
| rest | 0 (0, 1) | 1 (0, 2) | p = 0.006 |
| stress | 1 (0, 2) | 2 (1, 3) | p = 0.058 |
| NRS pain POD 5 * | |||
| rest | 0 (0, 1) | 0 (0, 1) | p = 0.14 |
| stress | 1 (0, 2) | 1 (0, 3) | p = 0.152 |
| Complications, MCDC * | 0 (0, 3) | 2 (0, 3) | p = 0.004 |
| Severe complication, MCDC ≤ 3b [n (%)] | 9 (8.5) | 9 (14.3) | p = 0.304 |
| Pneumonia [n (%)] | 8 (7.6) | 13 (20.6) | p = 0.017 |
| Anastomotic leakage a [n (%)] | 15 (14.2) | 14 (22.2) | p = 0.21 |
| Type I (conservative) | 0 (0) | 0 (0) | |
| Type II (nonsurgical intervention) | 13 (12.3) | 10 (15.9) | |
| Type III (surgical intervention) | 2 (1.9) | 4 (6.3) | |
| Reoperations [n (%)] | 5 (4.7) | 7 (11.1) | p = 0.134 |
| Hospital stay, days * | 18 (14, 25) | 19 (14, 27) | p = 0.479 |
| ICU stay, days * | 2 (1, 3) | 3 (2, 6) | p < 0.001 |
| Epidural anesthesia, days * | 5 (5, 6) | 5 (5, 6) | p = 0.007 |
| Time until bowel movement, days * | 5 (4, 7) | 4 (3, 6) | p < 0.001 |
| 30-Day mortality [n (%)] | 1 (0.9) | 0 (0) | p = 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoelzen, J.P.; Frankauer, B.E.; Szardenings, C.; Roy, D.; Pollmann, L.; Fortmann, L.; Merten, J.; Rijcken, E.; Juratli, M.A.; Pascher, A. Reducing the Risks of Esophagectomies: A Retrospective Comparison of Hybrid versus Full-Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE) Approaches. J. Clin. Med. 2023, 12, 5823. https://doi.org/10.3390/jcm12185823
Hoelzen JP, Frankauer BE, Szardenings C, Roy D, Pollmann L, Fortmann L, Merten J, Rijcken E, Juratli MA, Pascher A. Reducing the Risks of Esophagectomies: A Retrospective Comparison of Hybrid versus Full-Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE) Approaches. Journal of Clinical Medicine. 2023; 12(18):5823. https://doi.org/10.3390/jcm12185823
Chicago/Turabian StyleHoelzen, Jens Peter, Brooke E. Frankauer, Carsten Szardenings, Dhruvajyoti Roy, Lukas Pollmann, Lukas Fortmann, Jennifer Merten, Emile Rijcken, Mazen A. Juratli, and Andreas Pascher. 2023. "Reducing the Risks of Esophagectomies: A Retrospective Comparison of Hybrid versus Full-Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE) Approaches" Journal of Clinical Medicine 12, no. 18: 5823. https://doi.org/10.3390/jcm12185823
APA StyleHoelzen, J. P., Frankauer, B. E., Szardenings, C., Roy, D., Pollmann, L., Fortmann, L., Merten, J., Rijcken, E., Juratli, M. A., & Pascher, A. (2023). Reducing the Risks of Esophagectomies: A Retrospective Comparison of Hybrid versus Full-Robotic-Assisted Minimally Invasive Esophagectomy (RAMIE) Approaches. Journal of Clinical Medicine, 12(18), 5823. https://doi.org/10.3390/jcm12185823






