Oncological and Reproductive Outcomes after Fertility-Sparing Surgery in Patients with Advanced-Stage Serous Borderline Ovarian Tumor: A Single-Center Retrospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Basic Characteristics
2.2. Statistical Analyses
3. Results
3.1. Patients’ Characteristics
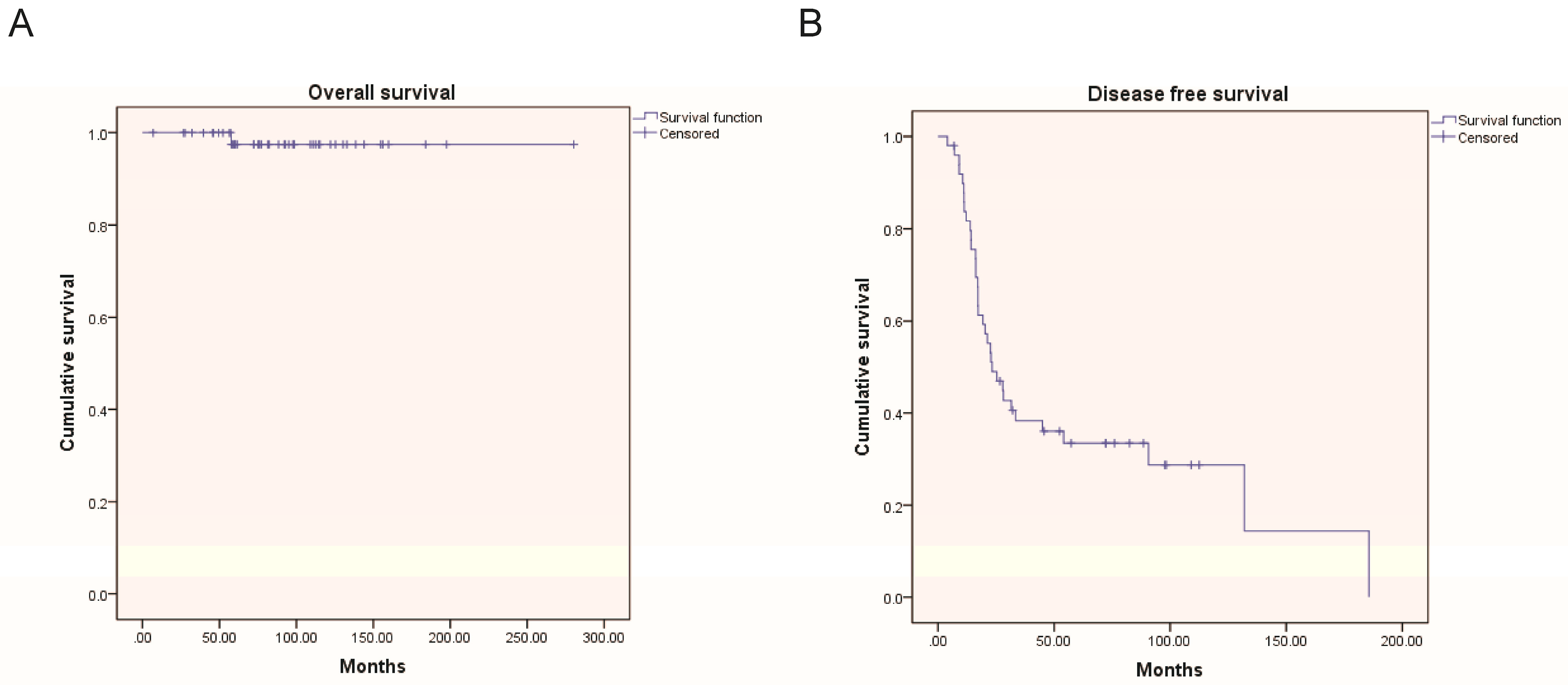
3.2. Oncological Outcomes
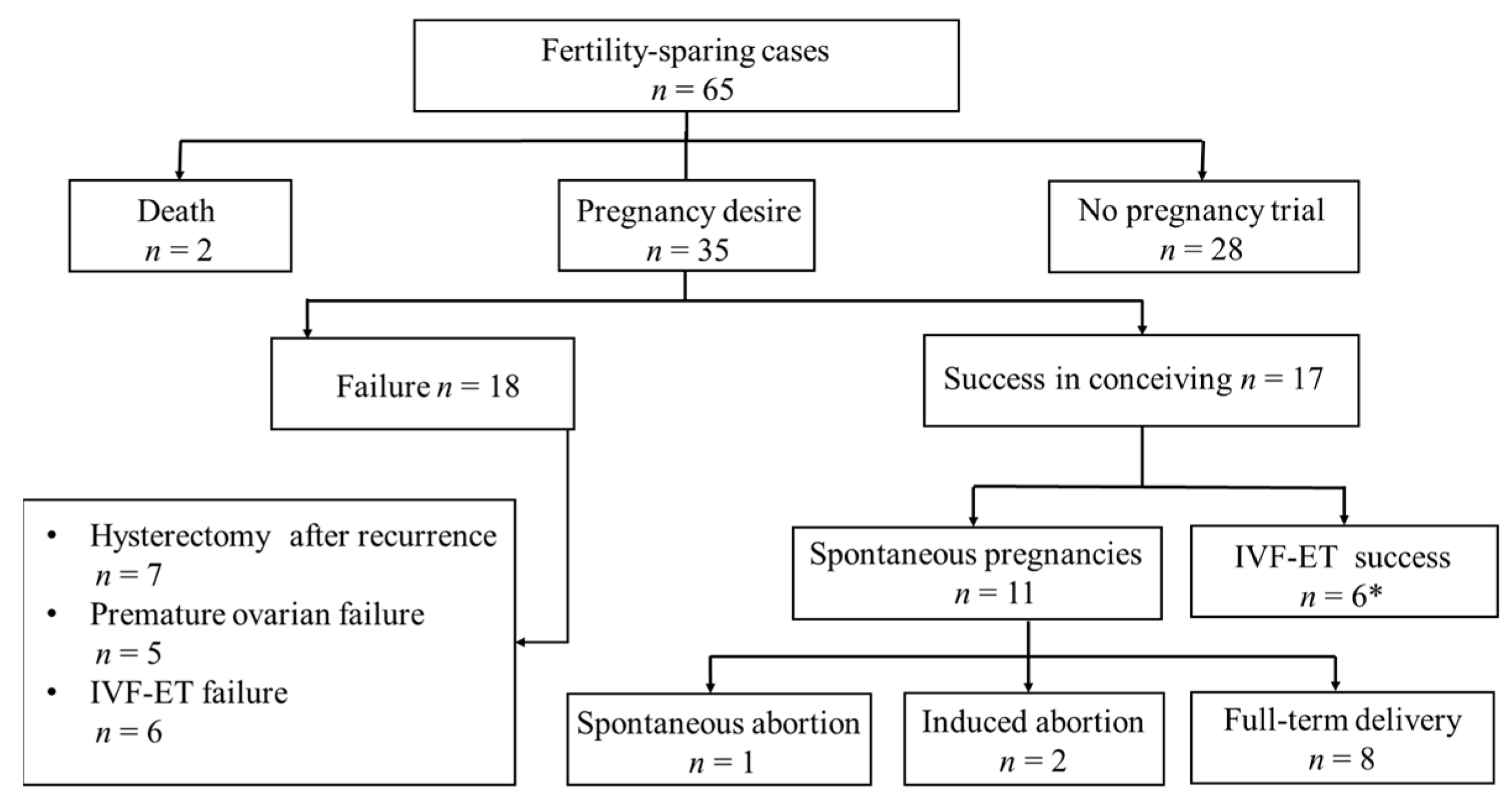
3.3. Reproductive Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Characteristics | |
|---|---|
| Surgical treatment | |
| Unilateral cystectomy | 3 |
| Bilateral cystectomy | 25 |
| Unilateral salpingo-oophorectomy | 6 |
| Unilateral salpingo-oophorectomy and contralateral cystectomy | 31 |
| Peritoneal cytology | 19 |
| Positive | 15 |
| Negative | 4 |
| Appendectomy | 14 |
| Lymphadenectomy | 11 |
| Omentectomy | 31 |
| Residual tumor | |
| No residual tumor | 44 |
| Residual disease | 21 |
References
- Ureyen, I.; Karalok, A.; Tasci, T.; Turkmen, O.; Boran, N.; Tulunay, G.; Turan, T. The Factors Predicting Recurrence in Patients with Serous Borderline Ovarian Tumor. Int. J. Gynecol. Cancer 2016, 26, 66–72. [Google Scholar] [CrossRef]
- Gershenson, D.M. Management of borderline ovarian tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Seong, S.J.; Kim, D.H.; Kim, M.K.; Song, T. Controversies in borderline ovarian tumors. J. Gynecol. Oncol. 2015, 26, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.; Zhao, L.; Chen, X.; Yu, A.; Xia, L.; Zhang, P. The impact of clinicopathologic and surgical factors on relapse and preg-nancy in young patients (≤40 years old) with borderline ovarian tumors. BMC Cancer 2018, 18, 1147. [Google Scholar] [CrossRef]
- Ushijima, K.; Kawano, K.; Tsuda, N.; Nishio, S.; Terada, A.; Kato, H.; Tasaki, K.; Matsukuma, K. Epithelial borderline ovarian tumor: Diagnosis and treatment strategy. Obstet. Gynecol. Sci. 2015, 58, 183–187. [Google Scholar] [CrossRef]
- Hauptmann, S.; Friedrich, K.; Redline, R.; Avril, S. Ovarian borderline tumors in the 2014 WHO classification: Evolving concepts and diagnostic criteria. Virchows Arch. 2016, 470, 125–142. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zhang, W.; Wang, Y. The feasibility of fertility-sparing surgery in treating advanced-stage border-line ovarian tumors: A meta-analysis. Taiwan J. Obstet. Gynecol. 2016, 55, 319–325. [Google Scholar] [CrossRef]
- Falcone, F.; Breda, E.; Ferrandina, G.; Malzoni, M.; Perrone, A.M.; Cormio, G.; Di Donato, V.; Frigerio, L.; Mangili, G.; Raspagliesi, F.; et al. Fertility-sparing treatment in advanced-stage serous borderline ovarian tumors. An analysis from the MITO14 study database. Gynecol. Oncol. 2021, 161, 825–831. [Google Scholar] [CrossRef]
- Bell, D.A.; Weinstock, M.A.; Scully, R.E. Peritoneal implants of ovarian serous borderline tumors: Histologic features and prognosis. Cancer 1988, 62, 2212–2222. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent diseasedagger. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- De Iaco, P.; Ferrero, A.; Rosati, F.; Melpignano, M.; Biglia, N.; Rolla, M.; De Aloysio, D.; Sismondi, P. Behaviour of ovarian tumors of low malignant potential treated with conservative surgery. Eur. J. Surg. Oncol. 2009, 35, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Zanetta, G.; Rota, S.; Chiari, S.; Bonazzi, C.; Bratina, G.; Mangioni, C. Behavior of Borderline Tumors with Particular Interest to Persistence, Recurrence, and Progression to Invasive Carcinoma: A Prospective Study. J. Clin. Oncol. 2001, 19, 2658–2664. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Choi, C.H.; Kim, H.-J.; Lee, W.; Lee, Y.-Y.; Kim, T.-J.; Lee, J.-W.; Bae, D.-S.; Kim, B.-G. Oncologic and reproductive outcomes in patients with advanced-stage borderline ovarian tumors. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 156, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Morice, P.; Uzan, C.; Fauvet, R.; Gouy, S.; Duvillard, P.; Darai, E. Borderline ovarian tumour: Pathological diagnostic dilemma and risk factors for invasive or lethal recurrence. Lancet Oncol. 2012, 13, e103–e115. [Google Scholar] [CrossRef]
- Jung, H.-J.; Park, J.-Y.; Kim, D.-Y.; Suh, D.-S.; Kim, J.-H.; Kim, Y.-M.; Kim, Y.-T.; Nam, J.-H. Comparison of Laparoscopic and Open Surgery for Patients with Borderline Ovarian Tumors. Int. J. Gynecol. Cancer 2018, 28, 1657–1663. [Google Scholar] [CrossRef]
- Hart, W.R. Borderline epithelial tumors of the ovary. Mod Pathol. 2005, 18 (Suppl. 2), S33–S50. [Google Scholar] [CrossRef]
- Du Bois, A.; Ewald-Riegler, N.; De Gregorio, N.; Reuss, A.; Mahner, S.; Fotopoulou, C.; Kommoss, F.; Schmalfeldt, B.; Hilpert, F.; Fehm, T.; et al. Borderline tumours of the ovary: A cohort study of the Arbeitsgmeinschaft Gynakologische Onkologie (AGO) Study Group. Eur. J. Cancer 2013, 49, 1905–1914. [Google Scholar] [CrossRef]
- Kurman, R.J.; Carcangiu, M.L.; Herrington, C.S.; Young, R.H. WHO Classification of Tumours of Female Re-Productive Organs, 4th ed.; IARC: Lyon, France, 2014. [Google Scholar]
- Network, National Comprehensive Cancer. NCCN Clinical Practice Guidelines in Oncology. Ovarian Can-cer Including Fallopian Tube Cancer and Primary Peritoneal Cancer, Version 3. 2021. Available online: https://www.nccn.org/professionals/physiciangls/pdf/ovarian.pdf (accessed on 26 February 2021).
- Trillsch, F.; Mahner, S.; Woelber, L.; Vettorazzi, E.; Reuss, A.; Ewald-Riegler, N.; de Gregorio, N.; Fotopoulou, C.; Schmalfeldt, B.; Burges, A.; et al. Age-dependent differences in borderline ovarian tumours (BOT) regarding clinical characteristics and outcome: Results from a sub-analysis of the Arbeitsgemeinschaft Gynaekologische Onkologie (AGO) ROBOT study. Ann. Oncol. 2014, 25, 1320–1327. [Google Scholar] [CrossRef]
- Kane, A.; Uzan, C.; Rey, A.; Gouy, S.; Camatte, S.; Pautier, P.; Lhommé, C.; Haie-Meder, C.; Duvillard, P.; Morice, P. Prognostic Factors in Patients with Ovarian Serous Low Malignant Potential (Borderline) Tumors with Peritoneal Implants. Oncol. 2009, 14, 591–600. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Sehouli, J.; Ewald-Riegler, N.; De Gregorio, N.; Reuss, A.; Richter, R.; Mahner, S.; Kommoss, F.; Schmalfeldt, B.; Fehm, T.; et al. The Value of Serum CA125 in the Diagnosis of Borderline Tumors of the Ovary: A Subanalysis of the Prospective Multicenter ROBOT Study. Int. J. Gynecol. Cancer 2015, 25, 1248–1252. [Google Scholar] [CrossRef]
- Uzan, C.; Muller, E.; Kane, A.; Rey, A.; Gouy, S.; Bendiffallah, S.; Duvillard, P.; Fauvet, R.; Darai, E.; Morice, P. Prognostic factors for recurrence after conservative treatment in a series of 119 patients with stage I serous borderline tumors of the ovary. Ann. Oncol. 2014, 25, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.; de Sousa Mendes, M. Conservative surgery in ovarian borderline tumours: A meta-analysis with emphasis on recurrence risk. Eur. J. Cancer 2015, 51, 620–631. [Google Scholar] [CrossRef] [PubMed]
- Palomba, S.; Falbo, A.; Del Negro, S.; Rocca, M.; Russo, T.; Cariati, F.; Annunziata, G.; Tolino, A.; Tagliaferri, P.; Zullo, F. Ultra-conservative fertility-sparing strategy for bilateral borderline ovarian tumours: An 11-year follow-up. Hum. Reprod. 2010, 25, 1966–1972. [Google Scholar] [CrossRef] [PubMed]
- Vandenput, I.; Amant, F.; Vergote, I. Peritoneal recurrences might be less common in advanced stage serous borderline ovarian tumors that were treated by laparotomy. Gynecol. Oncol. 2005, 98, 523–524. [Google Scholar] [CrossRef]
- Denschlag, D.; Von Wolff, M.; Amant, F.; Kesic, V.; Reed, N.; Schneider, A.; Rodolakis, A. Clinical recommendation on fertility preservation in border-line ovarian neoplasm: Ovarian stimulation and oocyte retrieval after conservative surgery. Gynecol. Obstet. Invest. 2010, 70, 160–165. [Google Scholar] [CrossRef]
- Attar, E.; Berkman, S.; Topuz, S.; Baysal, B.; Akhan, S.; Chambers, J.T. Evolutive peritoneal disease after conservative management and the use of infertility drugs in a patient with stage IIIC borderline micro-papillary serous carcinoma (MPSC) of the ovary: Case report. Hum. Reprod. 2004, 19, 1472–1475. [Google Scholar] [CrossRef]
- Marchette, M.D.; Ceppi, L.; Andreano, A.; Bonazzi, C.M.; Buda, A.; Grassi, T.; Giuliani, D.; Sina, F.; Lamanna, M.; Bianchi, T.; et al. Oncologic and fertility impact of surgical approach for borderline ovarian tumours treated with fertility sparing surgery. Eur. J. Cancer 2019, 111, 61–68. [Google Scholar] [CrossRef]
| Characteristics | n or Median | % or Range |
|---|---|---|
| Age at diagnosis (y) | 28 | 16–42 |
| Tumor size (cm) | 7.8 | 2.4–30 |
| CA125 (U/mL) | 294.8 | 27.6–1953 |
| Preoperative pregnancy | ||
| Yes | 27 | 41.5 |
| No | 39 | 58.5 |
| Previous live birth | ||
| Yes | 11 | 16.9 |
| No | 54 | 83.1 |
| Laterality | ||
| Unilateral | 9 | 13.8 |
| Bilateral | 56 | 86.2 |
| FIGO stage | ||
| II | 34 | 52.3 |
| III | 31 | 47.7 |
| Surgical approach | ||
| Laparotomy | 37 | 56.9 |
| Laparoscopy | 29 | 43.1 |
| Micropapillary | ||
| Yes | 23 | 35.4 |
| No | 42 | 64.6 |
| Invasive implants | ||
| Yes | 9 | 13.8 |
| No | 56 | 86.2 |
| Chemotherapy after surgery | ||
| Yes | 15 | 23.1 |
| No | 50 | 76.9 |
| Patient | Age (yr) | FIGO Stage | Surgery | DFS (Months) | CA125 (U/mL) before Invasive Recurrence | Treatment after Recurrence | Adjuvant Treatment | Recurrence Histology | Outcome | Time To Death (Months) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 28 | IIIC | BC | 10.7 | 69.1 | RCRS | TC × 6 | LGSOC | AWD | |
| 2 | 23 | IIIC | USO + CC | 9.1 | 140.6 | FSS→RCRS | TC × 8 | Invasive implants | NED | |
| 3 | 25 | IIIC | USO + CC | 7.1 | 20 | FSS→RCRS | TC × 8 | LGSOC | AWD | |
| 4 | 22 | IIIC | USO + CC | 22.6 | 164 | RCRS | None | Invasive implants | NED | |
| 5 | 36 | IIB | UC | 9.2 | 91.3 | FSS→RCRS | TC × 4 | Invasive implants | NED | |
| 6 | 28 | IIIC | USO + CC | 17.1 | 393.7 | FSS→RCRS | Letrozole | LGSOC, | NED | |
| 7 | 40 | IIB | USO + CC | 21.3 | 1675 | RCRS | TC × 3 | LGSOC, | Death | 57.7 |
| 8 | 29 | IIIB | BC | 6.5 | 35 | RCRS | None | Invasive implants | NED | |
| 9 | 22 | IIB | BC | 3.0 | 303.2 | RCRS | TC × 4 | LGSOC | Death | 81.7 |
| 10 | 30 | IIIC | BC | 6.1 | 216.5 | FSS | None | LGSOC | AWD | |
| 11 | 27 | IIA | USO + CC | 4.2 | 251 | FSS→RCRS | TC × 8 | Invasive implants | AWD | |
| 12 | 33 | IIB | UC | 9.3 | 279.8 | RCRS | None | LGSOC | NED | |
| 13 | 18 | IIIB | BC | 16.3 | 14.5 | FSS | None | Invasive implants | AWD |
| Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Recurrence n (%) | HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 30 y | 0.011 | 0.013 | |||
| Yes | 11 (50.0) | 1 | 1 | ||
| No | 35 (81.4) | 4.375 (1.406–13.612) | 5.390 (1.426–20.370) | ||
| FIGO stage | 0.031 | 0.254 | |||
| II | 20 (58.8) | 1 | 1 | ||
| III | 26 (83.9) | 3.636 (1.123–11.765) | 2.175 (0.572–8.266) | ||
| Laterality | 0.287 | ||||
| Unilateral | 5 (55.5) | 1 | |||
| Bilateral | 41 (71.9) | 2.187 (0.517–9.245) | |||
| Surgical approach | 0.170 | ||||
| Laparoscopy | 18 (62.1) | 1 | |||
| Open | 28 (75.7) | 2.139 (0.722–6.338) | |||
| Surgical procedures | 0. 609 | ||||
| Cystectomy | 25 (73.5) | 1 | |||
| Other | 21 (67.7) | 0.756 (0.259–2.207) | |||
| Lymphadenectomy | 0.876 | ||||
| Yes | 8 (72.7) | 1 | |||
| No | 38 (70.4) | 0.890 (0.209–3.802) | |||
| Micropapillary | 0.680 | ||||
| Yes | 17 (81.0) | 1 | |||
| No | 29 (65.9) | 0.787 (0.252–2.457) | |||
| Invasive implants | 0.279 | ||||
| Yes | 9 (100.0) | 1 | |||
| No | 37 (66.1) | 0.302 (0.034–2.639) | |||
| Adjuvant chemotherapy | 0.139 | ||||
| Yes | 14 (93.3) | 1 | |||
| No | 32 (64.0) | 0.299 (0.060–1.480) | |||
| Residual tumor after surgery | 0.014 | 0.013 | |||
| Yes | 20 (95.2) | 1 | 1 | ||
| No | 26 (59.1) | 0.072 (0.009–0.588) | 0.060 (0.006–0.555) | ||
| Factors | Univariate Analysis | Multivariate Analysis | |||
|---|---|---|---|---|---|
| Median DFS (Months) | HR (95% CI) | p | HR (95% CI) | p | |
| Age ≥ 30 y | 0.032 | 0.076 | |||
| Yes | 58.6 | 1 | 1 | ||
| No | 22.6 | 2.164 (1.070 4.376) | 1.962 (0.932–4.127) | ||
| FIGO stage | 0.056 | ||||
| II | 31.6 | 1 | |||
| III | 22.6 | 1.789 (0.984 3.247) | |||
| Laterality | 0.099 | ||||
| Unilateral | 97.8 | 1 | |||
| Bilateral | 22.8 | 2.389 (0.848–6.734) | |||
| Surgical approach | 0.682 | ||||
| Laparoscopy | 25.4 | 1 | |||
| Open | 23.3 | 1.135 (0.620–2.077) | |||
| Surgical procedures | 0. 136 | ||||
| Cystectomy | 17.2 | 1 | |||
| Other | 33.5 | 0.634 (0.349–1.154) | |||
| Lymphadenectomy | 0.512 | ||||
| Yes | 22.6 | 1 | |||
| No | 72.5 | 0.773 (0.358–1.669) | |||
| Micropapillary | 0.062 | ||||
| Yes | 17.3 | 1 | |||
| No | 31.6 | 0.555 (0.299–1.029) | |||
| Invasive implants | <0.001 | 0.003 | |||
| Yes | 9.3 | 5.252 (2.396–11.516) | 3.764 (1.551–9.132) | ||
| No | 31.6 | 1 | 1 | ||
| Adjuvant chemotherapy | 0.014 | 0.977 | |||
| Yes | 17.2 | 2.224 (1.174–4.214) | 0.988 (0.444–2.200) | ||
| No | 31.6 | 1 | 1 | ||
| Residual tumor after surgery | <0.001 | <0.001 | |||
| Yes | 16.2 | 4.709 (2.395–9.260) | 3.903 (1.895–8.038) | ||
| No | 66.1 | 1 | 1 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cang, W.; Liang, C.; Wang, D.; Xue, X.; Cao, D.; Yang, J.; Pan, L.; Wu, M.; Yang, J.; Xiang, Y. Oncological and Reproductive Outcomes after Fertility-Sparing Surgery in Patients with Advanced-Stage Serous Borderline Ovarian Tumor: A Single-Center Retrospective Study. J. Clin. Med. 2023, 12, 5827. https://doi.org/10.3390/jcm12185827
Cang W, Liang C, Wang D, Xue X, Cao D, Yang J, Pan L, Wu M, Yang J, Xiang Y. Oncological and Reproductive Outcomes after Fertility-Sparing Surgery in Patients with Advanced-Stage Serous Borderline Ovarian Tumor: A Single-Center Retrospective Study. Journal of Clinical Medicine. 2023; 12(18):5827. https://doi.org/10.3390/jcm12185827
Chicago/Turabian StyleCang, Wei, Chao Liang, Dan Wang, Xiaowei Xue, Dongyan Cao, Jiaxin Yang, Lingya Pan, Ming Wu, Junjun Yang, and Yang Xiang. 2023. "Oncological and Reproductive Outcomes after Fertility-Sparing Surgery in Patients with Advanced-Stage Serous Borderline Ovarian Tumor: A Single-Center Retrospective Study" Journal of Clinical Medicine 12, no. 18: 5827. https://doi.org/10.3390/jcm12185827
APA StyleCang, W., Liang, C., Wang, D., Xue, X., Cao, D., Yang, J., Pan, L., Wu, M., Yang, J., & Xiang, Y. (2023). Oncological and Reproductive Outcomes after Fertility-Sparing Surgery in Patients with Advanced-Stage Serous Borderline Ovarian Tumor: A Single-Center Retrospective Study. Journal of Clinical Medicine, 12(18), 5827. https://doi.org/10.3390/jcm12185827






