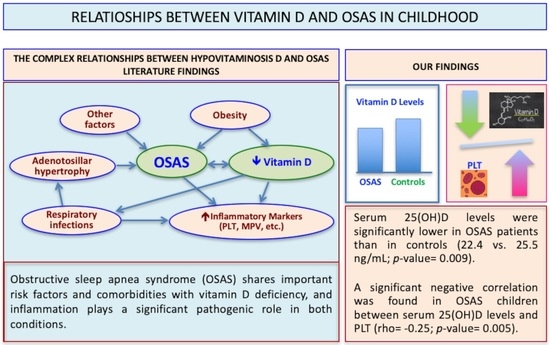
Relationships between 25-Hydroxyvitamin D Levels and Obstructive Sleep Apnea Severity in Children: An Observational Study
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OSAS | obstructive sleep apnea syndrome |
| AHI | apnea–hypopnea index |
| ODI | oxygen desaturation index |
| PG | polygraphy |
| HRP | home cardio-respiratory polygraphy |
| 25(OH)D | serum 25-hydroxyvitamin D |
| MPV | mean platelet volume |
| PLT | platelet count |
| BMI | body mass index |
| IQR | interquartile range |
References
- Ferrante, G.; Fasola, S.; Piazza, M.; Tenero, L.; Zaffanello, M.; La Grutta, S.; Piacentini, G. Vitamin D and Healthcare Service Utilization in Children: Insights from a Machine Learning Approach. J. Clin. Med. 2022, 11, 7157. [Google Scholar] [CrossRef]
- Zaffanello, M.; Ferrante, G.; Fasola, S.; Piazza, M.; Piacentini, G.; La Grutta, S. Personal and Environmental Risk Factors at Birth and Hospital Admission: Direct and Vitamin D-Mediated Effects on Bronchiolitis Hospitalization in Italian Children. Int. J. Environ. Res. Public Health 2021, 18, 747. [Google Scholar] [CrossRef] [PubMed]
- Prono, F.; Bernardi, K.; Ferri, R.; Bruni, O. The Role of Vitamin D in Sleep Disorders of Children and Adolescents: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 1430. [Google Scholar] [CrossRef] [PubMed]
- McCarty, D.E.; Chesson, A.L., Jr.; Jain, S.K.; Marino, A.A. The link between vitamin D metabolism and sleep medicine. Sleep Med. Rev. 2014, 18, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Kim, B.G.; Kim, B.Y.; Kim, S.W.; Kim, S.W.; Kim, H. Is there an association between vitamin D deficiency and adenotonsillar hypertrophy in children with sleep-disordered breathing? BMC Pediatr. 2018, 18, 196. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.L.; Gozal, D.; Kheirandish-Gozal, L. The Status of Pediatric Obstructive Sleep Apnea in 2015: Progress? YES!! More Questions? Definitely YES!! Curr. Sleep Med. Rep. 2016, 2, 20–30. [Google Scholar] [CrossRef]
- Narkiewicz, K.; Somers, V.K. Sympathetic nerve activity in obstructive sleep apnoea. Acta Physiol. Scand. 2003, 177, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, A.; Yokoyama, K.; Yokoo, T.; Urashima, M. Role of vitamin D in diabetes mellitus and chronic kidney disease. World J. Diabetes 2016, 7, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.; Kikuta, J.; Ishii, M. The Effects of Vitamin D on Immune System and Inflammatory Diseases. Biomolecules 2021, 11, 1624. [Google Scholar] [CrossRef]
- Antonucci, R.; Locci, C.; Clemente, M.G.; Chicconi, E.; Antonucci, L. Vitamin D deficiency in childhood: Old lessons and current challenges. J. Pediatr. Endocrinol. Metabol. 2018, 31, 247–260. [Google Scholar] [CrossRef]
- Chun, R.F.; Liu, P.T.; Modlin, R.L.; Adams, J.S.; Hewison, M. Impact of vitamin D on immune function: Lessons learned from genome-wide analysis. Front. Physiol. 2014, 5, 151. [Google Scholar] [CrossRef]
- Lang, J.E. Contribution of comorbidities to obesity-related asthma in children. Paediatr. Respir. Rev. 2021, 37, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Herr, C.; Greulich, T.; Koczulla, R.A.; Meyer, S.; Zakharkina, T.; Branscheidt, M.; Eschmann, R.; Bals, R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir. Res. 2011, 12, 31. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.; Morton, R.; Salkeld, L.; Bartley, J. Vitamin D and tonsil disease--preliminary observations. Int. J. Pediatr. Otorhinolaryngol. 2011, 75, 261–264. [Google Scholar] [CrossRef] [PubMed]
- Igelström, H.; Emtner, M.; Lindberg, E.; Asenlöf, P. Physical activity and sedentary time in persons with obstructive sleep apnea and overweight enrolled in a randomized controlled trial for enhanced physical activity and healthy eating. Sleep Breath. 2013, 17, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Ozgurhan, G.; Vehapoglu, A.; Vermezoglu, O.; Temiz, R.N.; Guney, A.; Hacihamdioglu, B. Risk assessment of obstructive sleep apnea syndrome in pediatric patients with vitamin D deficiency: A questionnaire-based study. Medicine 2016, 95, e4632. [Google Scholar] [CrossRef] [PubMed]
- Archontogeorgis, K.; Nena, E.; Papanas, N.; Steiropoulos, P. The role of vitamin D in obstructive sleep apnoea syndrome. Breathe 2018, 14, 206–215. [Google Scholar] [CrossRef]
- Varol, E.; Ozturk, O.; Gonca, T.; Has, M.; Ozaydin, M.; Erdogan, D.; Akkaya, A. Mean platelet volume is increased in patients with severe obstructive sleep apnea. Scand. J. Clin. Lab. Investig. 2010, 70, 497–502. [Google Scholar] [CrossRef]
- Zicari, A.M.; Occasi, F.; Di Mauro, F.; Lollobrigida, V.; Di Fraia, M.; Savastano, V.; Loffredo, L.; Nicita, F.; Spalice, A.; Duse, M. Mean Platelet Volume, Vitamin D and C Reactive Protein Levels in Normal Weight Children with Primary Snoring and Obstructive Sleep Apnea Syndrome. PLoS ONE 2016, 11, e0152497. [Google Scholar] [CrossRef]
- Kheirandish-Gozal, L.; Peris, E.; Gozal, D. Vitamin D levels and obstructive sleep apnoea in children. Sleep Med. 2014, 15, 459–463. [Google Scholar] [CrossRef]
- Bhatt, S.P.; Guleria, R.; Kabra, S.K. Metabolic alterations and systemic inflammation in over-weight/obese children with obstructive sleep apnea. PLoS ONE 2021, 16, e0252353. [Google Scholar] [CrossRef] [PubMed]
- Cacciari, E.; Milani, S.; Balsamo, A.; Spada, E.; Bona, G.; Cavallo, L.; Cerutti, F.; Gargantini, L.; Greggio, N.; Tonini, G.; et al. Italian cross-sectional growth charts for height, weight and BMI (2 to 20 yr). J. Endocrinol. Investig. 2006, 29, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Barlow, S.E. Expert Committee. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: Summary report. Pediatrics 2007, 120, S164–S192. [Google Scholar] [CrossRef]
- Alonso-Álvarez, M.L.; Terán-Santos, J.; Ordax Carbajo, E.; Cordero-Guevara, J.A.; Navazo-Egüia, A.I.; Kheirandish-Gozal, L.; Gozal, D. Reliability of home respiratory polygraphy for the diagnosis of sleep apnea in children. Chest 2015, 147, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Marcus, C.L.; Brooks, L.J.; Draper, K.A.; Gozal, D.; Halbower, A.C.; Jones, J.; Schechter, M.S.; Sheldon, S.H.; Spruyt, K.; Ward, S.D.; et al. American Academy of Pediatrics. Diagnosis and management of childhood obstructive sleep apnea syndrome. Pediatrics 2012, 130, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events. Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IO, USA, 2012; p. 176. [Google Scholar]
- French, D.; Gorgi, A.W.; Ihenetu, K.U.; Weeks, M.A.; Lynch, K.L.; Wu, A.H. Vitamin D status of county hospital patients assessed by the DiaSorin LIAISON® 25-hydroxyvitamin D assay. Clin. Chim. Acta 2011, 412, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Munns, C.F.; Shaw, N.; Kiely, M.; Specker, B.L.; Thacher, T.D.; Ozono, K.; Michigami, T.; Tiosano, D.; Mughal, M.Z.; Mäkitie, O.; et al. Global consensus recommendations on prevention and management of nutritional rickets. J. Clin. Endocrinol. Metab. 2016, 101, 394–415. [Google Scholar] [CrossRef]
- Alsubie, H.S.; BaHammam, A.S. Obstructive Sleep Apnoea: Children are not little Adults. Paediatr. Respir. Rev. 2017, 21, 72–79. [Google Scholar] [CrossRef]
- Al-Shawwa, B.; Ehsan, Z.; Ingram, D.G. Vitamin D and sleep in children. J. Clin. Sleep Med. 2020, 16, 1119–1123. [Google Scholar] [CrossRef]
- Galluzzi, F.; Pignataro, L.; Gaini, R.M.; Garavello, W. Drug induced sleep endoscopy in the decision-making process of children with obstructive sleep apnea. Sleep Med. 2015, 16, 331–335. [Google Scholar] [CrossRef]
- Zaffanello, M.; Piacentini, G.; La Grutta, S. The cardiovascular risk in paediatrics: The paradigm of the obstructive sleep apnoea syndrome. Blood Transfus. 2020, 18, 217–225. [Google Scholar]
- Kang, K.T.; Weng, W.C.; Lee, P.L.; Hsu, W.C. Age- and gender-related characteristics in pediatric obstructive sleep apnea. Pediatr. Pulmonol. 2022, 57, 1520–1526. [Google Scholar] [CrossRef]
- Barja-Fernández, S.; Aguilera, C.M.; Martínez-Silva, I.; Vazquez, R.; Gil-Campos, M.; Olza, J.; Bedoya, J.; Cadarso-Suárez, C.; Gil, Á.; Seoane, L.M.; et al. 25-Hydroxyvitamin D levels of children are inversely related to adiposity assessed by body mass index. J. Physiol. Biochem. 2018, 74, 111–1118. [Google Scholar] [CrossRef]
- Cengiz, C.; Erhan, Y.; Murat, T.; Ercan, A.; Ibrahim, S.; Ihsan, G.; Ertap, A. Values of mean platelet volume in patients with chronic tonsillitis and adenoid hypertrophy. Pak. J. Med. Sci. 2013, 29, 569–572. [Google Scholar] [PubMed]
- Onder, S.; Caypinar, B.; Sahin-Yilmaz, A.; Toros, S.Z.; Oysu, C. Relation of mean platelet volume with obstructive adenoid hypertrophy in children. Int. J. Pediatr. Otorhinolaryngol. 2014, 78, 1449–1451. [Google Scholar] [CrossRef] [PubMed]
- Soyalıç, H.; Somuk, B.T.; Doğru, S.; Gürbüzler, L.; Göktaş, G.; Eyibilen, A. Evaluation of mean platelet volume and its ratio over platelet count in children with obstructive sleep apnea syndrome. Kulak Burun Bogaz Ihtis Derg. 2015, 25, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Di Sessa, A.; Messina, G.; Bitetti, I.; Falanga, C.; Farello, G.; Verrotti, A.; Carotenuto, M. Cardiometabolic risk profile in non-obese children with obstructive sleep apnea syndrome. Eur. J. Pediatr. 2022, 181, 1689–1697. [Google Scholar] [CrossRef] [PubMed]
- Aryan, Z.; Rezaei, N.; Camargo, C.A., Jr. Vitamin D status, aeroallergen sensitization, and allergic rhinitis: A systematic review and meta-analysis. Int. Rev. Immunol. 2017, 36, 41–53. [Google Scholar] [CrossRef]


| OSAS Cases, n | 127 |
|---|---|
| Males, n (%) | 77/127 (60.6) |
| Median (IQR) age, years (n = 127) | 5 (4–7) |
| Median (IQR) weight, kg (n = 127) | 18.9 (15.3–26.0) |
| Median (IQR) height, cm (n = 127) | 112 (102–123) |
| Median (IQR) BMI, kg/m2 (n = 127) | 15.4 (14.4–17.4) |
| Median (IQR) BMI percentile (n = 127) | 30.5 (12–72.5) |
| Mean (SD) 25(OH)D, ng/mL | 22.4 (7.7) |
| Median (IQR) IgE total, UI/mL (n = 118) | 38.5 (10–168) |
| Median (IQR) MPV, fL (n = 127) | 6.9 (6.6–7.4) |
| Median (IQR) PLT ×103/mcL (n = 127) | 320 (258–383) |
| Median (IQR) AHI, events/h (n = 127) | 7.2 (5.1–12.1) |
| Tonsil size grading III–IV, n (%) | 78/127 (61.4) |
| Friedman palate position III–IV, n (%) | 51 (40.2) |
| Median (IQR) oxygen saturation, % (SpO2 nadir) (n = 127) | 92 (89–93) |
| Oral breathing, n (%) | 78/127 (61.4) |
| Nasal airway patency, n (%) | 104/127 (81.9) |
| Snoring, n (%) | 112/127 (88.2) |
| Allergen Sensitization, n (%) | 32/127 (25.2) |
| Asthma, n (%) | 22/127 (17.3) |
| Rhinitis, n (%) | 53/127 (41.7) |
| HC (n = 96) | OSAS (n = 96) | p-Value | |
|---|---|---|---|
| Males, n (%) | 48 (50.0) | 60 (62.5) | 0.08 |
| Median (IQR) age, years (n = 192) | 6 (4–8) | 6 (4–8) | 0.86 |
| Median (IQR) weight, kg (n= 192) | 18.9 (15.7–25.5) | 20 (16–28.4) | 0.20 |
| Median (IQR) height, cm (n = 192) | 112.8 (102.5–127.0) | 116 (105–127) | 0.34 |
| Median (IQR) BMI, kg/m2 (n = 192) | 15.5 (14.3–16.8) | 15.7 (14.4–18) | 0.19 |
| Median (IQR) BMI percentile (n = 192) | 29.5 (9.5–61.5) | 29.1 (10.5–74.0) | 0.33 |
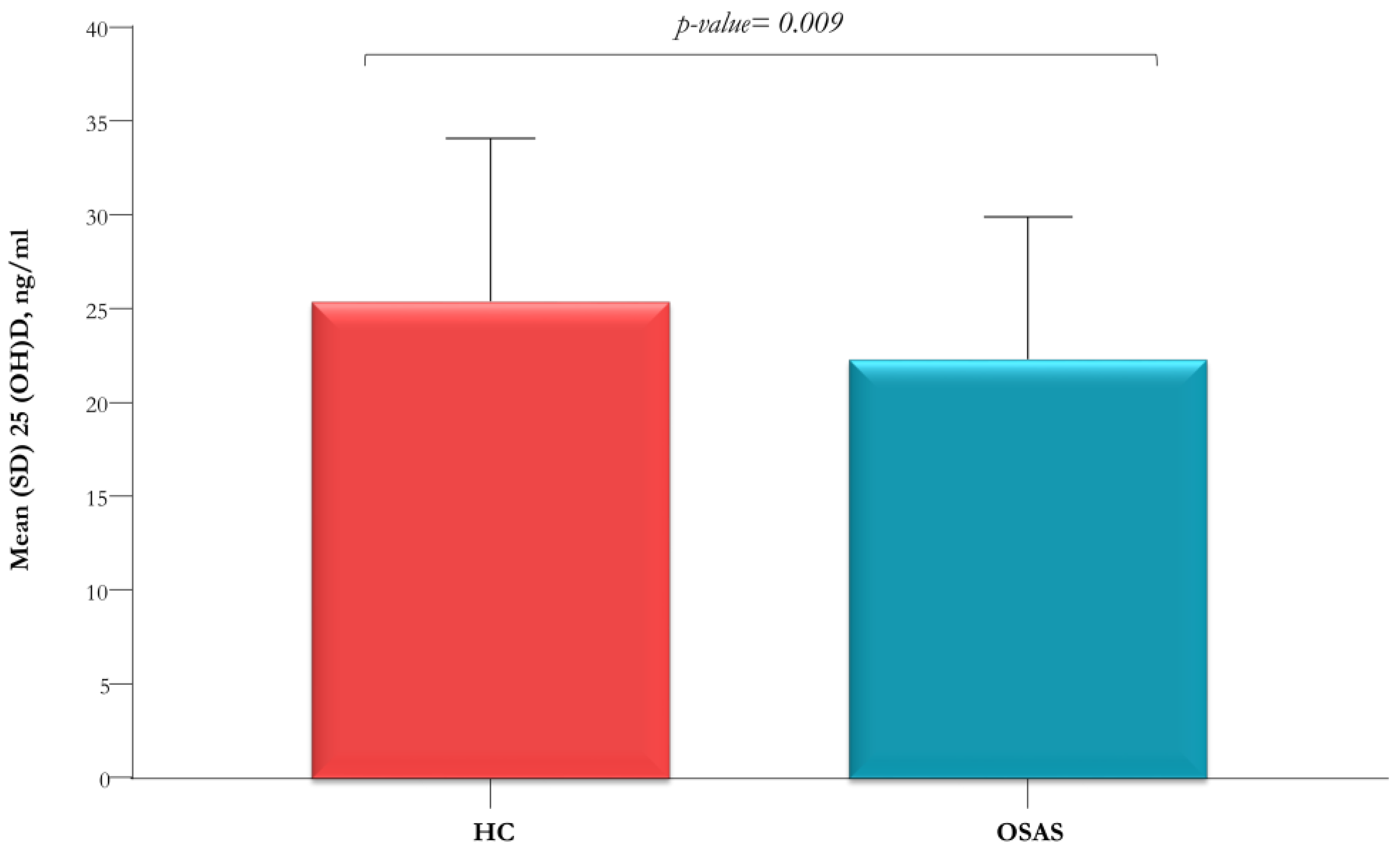
| Mean (SD) 25(OH)D, ng/mL | 25.5 (8.7) | 22.4 (7.6) | 0.009 |
| 25(OH)D ≥ 20 ng/mL (n = 83) | 25(OH)D < 20 ng/mL (n = 44) | p-Value | |
|---|---|---|---|
| Median (IQR) BMI percentile | 38 (12–74) | 23.3 (9.8–59.8) | 0.22 |
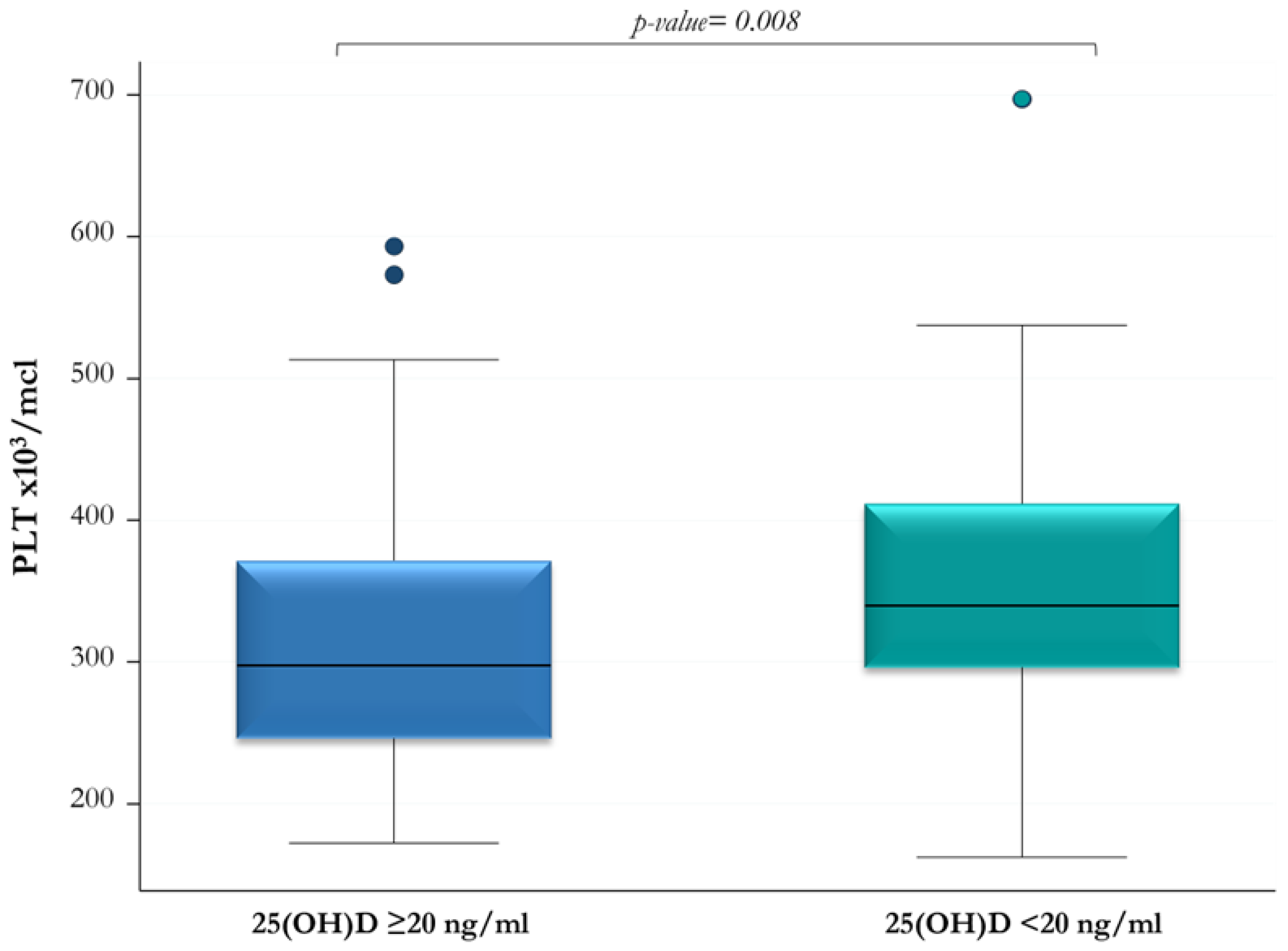
| Median (IQR) PLT ×103/mcL | 297 (246–371) | 339.5 (296–411.5) | 0.008 |
| Median (IQR) MPV, fL | 6.9 (6.6–7.4) | 6.9 (6.4–7.4) | 0.35 |
| Median (IQR) AHI, events/h | 6.5 (5.1–10.8) | 9.3 (5.4–13.2) | 0.10 |
| Tonsil size grading III–IV, n (%) | 47 (56.6) | 31 (70.5) | 0.12 |
| Median (IQR) SpO2 nadir, % | 92 (90–94) | 91 (86.5–92.5) | 0.08 |
| 25(OH)D, ng/mL | |
|---|---|
| rho (p-Value) | |
| BMI percentile | 0.05 (0.58) |
| PLT ×103/mcL | −0.25 (0.005) |
| MPV, fL | 0.06 (0.49) |
| AHI, events/h | −0.14 (0.11) |
| SpO2 nadir, % | 0.14 (0.11) |
| Allergen Sensitization | p-Value | |||
|---|---|---|---|---|
| NO (n = 95) | YES (n = 32) | |||
| AHI, n (%) | AHI < 5 events/h | 19 (20.0) | 8 (25.0) | 0.83 |
| 5≤ AHI < 10 events/h | 42 (44.2) | 13 (40.6) | ||
| AHI ≥ 10 events/h | 34 (35.8) | 11 (34.4) | ||
| Median (IQR) AHI, events/h | 7.3 (5.3–12.8) | 6.9 (4.9–10.9) | 0.56 | |
| 25(OH)D < 20 ng/mL, n (%) | 30 (31.6) | 14 (43.8) | 0.21 | |
| Mean (SD) 25(OH)D, ng/mL | 22.7 (7.9) | 21.4 (7.3) | 0.43 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Locci, C.; Ruiu, A.; Saderi, L.; Sotgiu, G.; Bassu, S.; Zaffanello, M.; Antonucci, R. Relationships between 25-Hydroxyvitamin D Levels and Obstructive Sleep Apnea Severity in Children: An Observational Study. J. Clin. Med. 2023, 12, 1242. https://doi.org/10.3390/jcm12031242
Locci C, Ruiu A, Saderi L, Sotgiu G, Bassu S, Zaffanello M, Antonucci R. Relationships between 25-Hydroxyvitamin D Levels and Obstructive Sleep Apnea Severity in Children: An Observational Study. Journal of Clinical Medicine. 2023; 12(3):1242. https://doi.org/10.3390/jcm12031242
Chicago/Turabian StyleLocci, Cristian, Antonella Ruiu, Laura Saderi, Giovanni Sotgiu, Stefania Bassu, Marco Zaffanello, and Roberto Antonucci. 2023. "Relationships between 25-Hydroxyvitamin D Levels and Obstructive Sleep Apnea Severity in Children: An Observational Study" Journal of Clinical Medicine 12, no. 3: 1242. https://doi.org/10.3390/jcm12031242
APA StyleLocci, C., Ruiu, A., Saderi, L., Sotgiu, G., Bassu, S., Zaffanello, M., & Antonucci, R. (2023). Relationships between 25-Hydroxyvitamin D Levels and Obstructive Sleep Apnea Severity in Children: An Observational Study. Journal of Clinical Medicine, 12(3), 1242. https://doi.org/10.3390/jcm12031242