High Prevalence of Persistent Measurable Postoperative Knee Joint Laxity in Patients with Tibial Plateau Fractures Treated by Open Reduction and Internal Fixation (ORIF)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Analysis of Laxity
2.3. Statistical Analysis
3. Results
3.1. Participants
3.2. Measurements
3.3. Complex vs. Simple Fractures
3.4. Ligament Repair vs. No Ligament Repair
3.5. AP Translation ≥2 mm vs. <2 mm
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Wennergren, D.; Bergdahl, C.; Ekelund, J.; Juto, H.; Sundfeldt, M.; Möller, M. Epidemiology and incidence of tibia fractures in the Swedish Fracture Register. Injury 2018, 49, 2068–2074. [Google Scholar] [CrossRef]
- Rupp, M.; Walter, N.; Pfeifer, C.; Lang, S.; Kerschbaum, M.; Krutsch, W.; Baumann, F.; Alt, V. The incidence of fractures among the adult population of Germany. Deutsch. Ärztebl. Int. 2021, 118, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Bormann, M.; Neidlein, C.; Gassner, C.; Keppler, A.M.; Bogner-Flatz, V.; Ehrnthaller, C.; Prall, W.C.; Böcker, W.; Fürmetz, J. Changing patterns in the epidemiology of tibial plateau fractures: A 10-year review at a level-I trauma center. Eur. J. Trauma Emerg. Surg. 2022, 49, 401–409. [Google Scholar] [CrossRef]
- Elsoe, R.; Larsen, P.; Nielsen, N.P.H.; Swenne, J.; Rasmussen, S.; Ostgaard, S.E. Population-Based Epidemiology of Tibial Plateau Fractures. Orthopedics 2015, 38, e780–e786. [Google Scholar] [CrossRef]
- Herteleer, M.; Van Brandt, C.; Vandoren, C.; Nijs, S.; Hoekstra, H. Tibial plateau fractures in Belgium: Epidemiology, financial burden and costs curbing strategies. Eur. J. Trauma Emerg. Surg. 2022, 48, 3643–3650. [Google Scholar] [CrossRef] [PubMed]
- Castiglia, M.T.; Nogueira-Barbosa, M.H.; Messias, A.M.V.; Salim, R.; Fogagnolo, F.; Schatzker, J.; Kfuri, M. The Impact of Computed Tomography on Decision Making in Tibial Plateau Fractures. J. Knee Surg. 2018, 31, 1007–1014. [Google Scholar] [CrossRef]
- Kfuri, M.; Schatzker, J. Revisiting the Schatzker classification of tibial plateau fractures. Injury 2018, 49, 2252–2263. [Google Scholar] [CrossRef]
- Krause, M.; Frosch, K.-H. Wandel in der Behandlung der Tibiakopffraktur. Die Unfallchirurgie 2022, 125, 527–534. [Google Scholar] [CrossRef]
- Millar, S.C.; Arnold, J.B.; Thewlis, D.; Fraysse, F.; Solomon, L.B. A systematic literature review of tibial plateau fractures: What classifications are used and how reliable and useful are they? Injury 2018, 49, 473–490. [Google Scholar] [CrossRef]
- Bala, A.; Penrose, C.T.; Seyler, T.M.; Mather, R.C.; Wellman, S.S.; Bolognesi, M.P. Outcomes after Total Knee Arthroplasty for post-traumatic arthritis. Knee 2015, 22, 630–639. [Google Scholar] [CrossRef] [PubMed]
- Elsoe, R.; Johansen, M.; Larsen, P. Tibial plateau fractures are associated with a long-lasting increased risk of total knee arthroplasty a matched cohort study of 7,950 tibial plateau fractures. Osteoarthr. Cartil. 2019, 27, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Larsen, P.; Elsoe, R. Characteristics of patients requiring early total knee replacement after surgically treated lateral tibial plateau fractures—A comparative cohort study. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Kester, B.S.; Minhas, S.V.; Vigdorchik, J.M.; Schwarzkopf, R. Total Knee Arthroplasty for Posttraumatic Osteoarthritis: Is it Time for a New Classification? J. Arthroplast. 2016, 31, 1649–1653.e1. [Google Scholar] [CrossRef] [PubMed]
- Oladeji, L.O.; Worley, J.R.; Crist, B.D. Age-Related Variances in Patients with Tibial Plateau Fractures. J. Knee Surg. 2020, 33, 611–615. [Google Scholar] [CrossRef]
- Saleh, H.; Yu, S.; Vigdorchik, J.; Schwarzkopf, R. Total knee arthroplasty for treatment of post-traumatic arthritis: Systematic review. World J. Orthop. 2016, 7, 584–591. [Google Scholar] [CrossRef]
- Scott, B.L.; Lee, C.S.; Strelzow, J.A. Five-Year Risk of Conversion to Total Knee Arthroplasty After Operatively Treated Periarticular Knee Fractures in Patients Over 40 Years of Age. J. Arthroplast. 2020, 35, 2084–2089.e1. [Google Scholar] [CrossRef] [PubMed]
- Wasserstein, D.; Henry, P.; Paterson, J.M.; Kreder, H.J.; Jenkinson, R. Risk of Total Knee Arthroplasty After Operatively Treated Tibial Plateau Fracture: A Matched-Population-Based Cohort Study. J. Bone Jt. Surg. 2014, 96, 144–150. [Google Scholar] [CrossRef]
- Aurich, M.; Koenig, V.; Hofmann, G. Comminuted intraarticular fractures of the tibial plateau lead to posttraumatic osteoarthritis of the knee: Current treatment review. Asian J. Surg. 2018, 41, 99–105. [Google Scholar] [CrossRef]
- Houdek, M.T.; Watts, C.D.; Shannon, S.F.; Wagner, E.R.; Sems, S.A.; Sierra, R.J. Posttraumatic Total Knee Arthroplasty Continues to Have Worse Outcome Than Total Knee Arthroplasty for Osteoarthritis. J. Arthroplast. 2016, 31, 118–123. [Google Scholar] [CrossRef]
- Iliopoulos, E.; Galanis, N. Physiotherapy after tibial plateau fracture fixation: A systematic review of the literature. SAGE Open Med. 2020, 8, 205031212096531. [Google Scholar] [CrossRef]
- Davis, J.T.; Rudloff, M.I. Posttraumatic Arthritis After Intra-Articular Distal Femur and Proximal Tibia Fractures. Orthop. Clin. N. Am. 2019, 50, 445–459. [Google Scholar] [CrossRef] [PubMed]
- Schenker, M.L.; Mauck, R.L.; Ahn, J.; Mehta, S. Pathogenesis and Prevention of Posttraumatic Osteoarthritis After Intra-articular Fracture. J. Am. Acad. Orthop. Surg. 2014, 22, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-J.; Zeng, N.; Yan, Z.-P.; Li, J.-T.; Ni, G.-X. Post-traumatic osteoarthritis following ACL injury. Thromb. Haemost. 2020, 22, 57. [Google Scholar] [CrossRef]
- Mthethwa, J.; Chikate, A. A review of the management of tibial plateau fractures. Musculoskelet. Surg. 2018, 102, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Singleton, N.; Sahakian, V.; Muir, D. Outcome After Tibial Plateau Fracture: How Important Is Restoration of Articular Congruity? J. Orthop. Trauma 2017, 31, 158–163. [Google Scholar] [CrossRef]
- Adams, J.D.; Loeffler, M.F. Soft Tissue Injury Considerations in the Treatment of Tibial Plateau Fractures. Orthop. Clin. N. Am. 2020, 51, 471–479. [Google Scholar] [CrossRef]
- Deutsche Gesellschaft für Orthopädie und Unfallchirurgie e.V. (DGOU). Tibiakopffrakturen; Version 1.0 (29.10.2021); Berninger, M.T., Schüttrumpf, J., Krause, M., Eds.; Deutsche Gesellschaft für Orthopädie und Unfallchirurgie e.V. (DGOU): Berlin, Germany, 2022. [Google Scholar]
- Alm, L.; Drenck, T.C.; Frings, J.; Krause, M.; Korthaus, A.; Krukenberg, A.; Frosch, K.-H.; Akoto, R. Lower Failure Rates and Improved Patient Outcome Due to Reconstruction of the MCL and Revision ACL Reconstruction in Chronic Medial Knee Instability. Orthop. J. Sports Med. 2021, 9, 232596712198931. [Google Scholar] [CrossRef]
- Ayeni, O.R.; Chahal, M.; Tran, M.N.; Sprague, S. Pivot shift as an outcome measure for ACL reconstruction: A systematic review. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 767–777. [Google Scholar] [CrossRef]
- Magnussen, R.A.; Reinke, E.K.; Huston, L.J.; Briskin, I.; Cox, C.L.; Dunn, W.R.; Flanigan, D.C.; Jones, M.H.; Kaeding, C.C.; Matava, M.J.; et al. Neither Residual Anterior Knee Laxity Up to 6 mm nor a Pivot Glide Predict Patient-Reported Outcome Scores or Subsequent Knee Surgery between 2 and 6 Years After ACL Reconstruction. Am. J. Sports Med. 2021, 49, 2631–2637. [Google Scholar] [CrossRef]
- Mayr, H.O.; Hoell, A.; Bernstein, A.; Hube, R.; Zeiler, C.; Kalteis, T.; Suedkamp, N.P.; Stoehr, A. Validation of a Measurement Device for Instrumented Quantification of Anterior Translation and Rotational Assessment of the Knee. Arthrosc. J. Arthrosc. Relat. Surg. 2011, 27, 1096–1104. [Google Scholar] [CrossRef]
- Pugh, L.; Mascarenhas, R.; Arneja, S.; Chin, P.Y.K.; Leith, J.M. Current Concepts in Instrumented Knee-Laxity Testing. Am. J. Sports Med. 2009, 37, 199–210. [Google Scholar] [CrossRef] [PubMed]
- Schatzker, J.; McBroom, R.; Bruce, D. The tibial plateau fracture: The Toronto experience 1968–1975. Clin. Orthop. Relat. Res. 1979, 138, 94–104. [Google Scholar]
- Moore, T.M. Fracture-Dislocation of the knee. Clin. Orthop. Relat. Res. 1981, 156, 128–140. [Google Scholar] [CrossRef]
- Schuster, A.J.; Mcnicholas, M.J.; Wachtl, S.W.; McGurty, D.W.; Jakob, R.P. A New Mechanical Testing Device for Measuring Anteroposterior Knee Laxity. Am. J. Sports Med. 2004, 32, 1731–1735. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, F.; Figueroa, D.; Putnis, S.; Guiloff, R.; Caro, P.; Espregueira-Mendes, J. Posterolateral corner knee injuries: A narrative review. EFORT Open Rev. 2021, 6, 676–685. [Google Scholar] [CrossRef]
- Stannard, J.; Lopez, R.; Volgas, D. Soft Tissue Injury of the Knee after Tibial Plateau Fractures. J. Knee Surg. 2010, 23, 187–192. [Google Scholar] [CrossRef]
- Brockmeyer, M.; Orth, P.; Höfer, D.; Seil, R.; Paulsen, F.; Menger, M.D.; Kohn, D.; Tschernig, T. The anatomy of the anterolateral structures of the knee—A histologic and macroscopic approach. Knee 2019, 26, 636–646. [Google Scholar] [CrossRef]
- Golan, E.J.; Tisherman, R.; Byrne, K.; Diermeier, T.; Vaswani, R.; Musahl, V. Anterior Cruciate Ligament Injury and the Anterolateral Complex of the Knee—Importance in Rotatory Knee Instability? Curr. Rev. Musculoskelet. Med. 2019, 12, 472–478. [Google Scholar] [CrossRef]
- Guenther, D.; Griffith, C.; Lesniak, B.; Lopomo, N.; Grassi, A.; Zaffagnini, S.; Fu, F.H.; Musahl, V. Anterolateral rotatory instability of the knee. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 2909–2917. [Google Scholar] [CrossRef]
- Herbst, E.; Albers, M.; Burnham, J.M.; Shaikh, H.S.; Naendrup, J.-H.; Fu, F.H.; Musahl, V. The anterolateral complex of the knee: A pictorial essay. Knee Surg. Sports Traumatol. Arthrosc. 2017, 25, 1009–1014. [Google Scholar] [CrossRef]
- Kittl, C.; Inderhaug, E.; Williams, A.; Amis, A.A. Biomechanics of the Anterolateral Structures of the Knee. Clin. Sports Med. 2018, 37, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Musahl, V.; Herbst, E.; Burnham, J.M.; Fu, F.H. The Anterolateral Complex and Anterolateral Ligament of the Knee. J. Am. Acad. Orthop. Surg. 2018, 26, 261–267. [Google Scholar] [CrossRef]
- Sonnery-Cottet, B.; Daggett, M.; Fayard, J.-M.; Ferretti, A.; Helito, C.P.; Lind, M.; Monaco, E.; de Pádua, V.B.C.; Thaunat, M.; Wilson, A.; et al. Anterolateral Ligament Expert Group consensus paper on the management of internal rotation and instability of the anterior cruciate ligament-deficient knee. J. Orthop. Traumatol. 2017, 18, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Terry, G.C.; Hughston, J.C.; Norwood, L.A. The anatomy of the iliopatellar band and iliotibial tract. Am. J. Sports Med. 1986, 14, 39–45. [Google Scholar] [CrossRef]
- Hissnauer, T.-N.; Krause, M.; Frings, J.; Frosch, K.-H. Chirurgische Zugänge zum Tibiakopf. OP-J. 2019, 34, 107–116. [Google Scholar] [CrossRef]
- Maclean, J.; Kandemir, U. Surgical Approaches for Tibial Plateau Fractures. J. Knee Surg. 2013, 27, 21–30. [Google Scholar] [CrossRef]
- Korthaus, A.; Krause, M.; Pagenstert, G.; Warncke, M.; Brembach, F.; Frosch, K.-H.; Kolb, J.P. Tibial slope in the posterolateral quadrant with and without ACL injury. Arch. Orthop. Trauma Surg. 2021, 142, 3917–3925. [Google Scholar] [CrossRef]
- Reahl, G.B.; Marinos, D.; O’Hara, N.N.; Howe, A.; Degani, Y.; Wise, B.; Maceroli, M.; O’Toole, R.V. Risk Factors for Knee Stiffness Surgery After Tibial Plateau Fracture Fixation. J. Orthop. Trauma 2018, 32, e339–e343. [Google Scholar] [CrossRef]
- Haller, J.M.; Holt, D.C.; McFadden, M.L.; Higgins, T.F.; Kubiak, E.N. Arthrofibrosis of the knee following a fracture of the tibial plateau. Bone Jt. J. 2015, 97, 109–114. [Google Scholar] [CrossRef]
- Middleton, A.H.; Perlewitz, M.A.; Edelstein, A.I.; Vetter, C.S. Knee Arthrofibrosis following Tibial Plateau Fracture Treated with Arthroscopic Lysis of Adhesions with Manipulation. J. Knee Surg. 2022, 35, 816–820. [Google Scholar] [CrossRef]
- Nannaparaju, M.; Mortada, S.; Wiik, A.; Khan, W.; Alam, M. Posterolateral corner injuries: Epidemiology, anatomy, biomechanics and diagnosis. Injury 2018, 49, 1024–1031. [Google Scholar] [CrossRef] [PubMed]
- Stephen, J.M.; Halewood, C.; Kittl, C.; Bollen, S.R.; Williams, A.; Amis, A.A. Posteromedial Meniscocapsular Lesions Increase Tibiofemoral Joint Laxity with Anterior Cruciate Ligament Deficiency, and Their Repair Reduces Laxity. Am. J. Sports Med. 2016, 44, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mayr, H.O.; Hellbruegge, G.; Haasters, F.; Ipach, B.; Schmal, H.; Prall, W.C. Laxity measurement of internal knee rotation after primary anterior cruciate ligament rupture versus rerupture. Arch. Orthop. Trauma Surg. 2021, 142, 2839–2847. [Google Scholar] [CrossRef] [PubMed]
- Bates, N.A.; Nesbitt, R.J.; Shearn, J.T.; Myer, G.D.; Hewett, T.E. The influence of internal and external tibial rotation offsets on knee joint and ligament biomechanics during simulated athletic tasks. Clin. Biomech. 2018, 52, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, A.; Röttgerkamp, H.; Bobrowitsch, E.; Leichtle, C.I.; Leichtle, U.G. Tibial rotation influences anterior knee stability—A robot-aided in-vitro study. Clin. Biomech. 2016, 32, 131–137. [Google Scholar] [CrossRef]
- Drogset, J.O.; Grøntvedt, T.; Robak, O.R.; Mølster, A.; Viset, A.T.; Engebretsen, L. A Sixteen-Year Follow-up of Three Operative Techniques for the Treatment of Acute Ruptures of the Anterior Cruciate Ligament. J. Bone Jt. Surg. 2006, 88, 944–952. [Google Scholar] [CrossRef]
- Gagliardi, A.G.; Carry, P.M.; Parikh, H.B.; Traver, J.L.; Howell, D.R.; Albright, J.C. ACL Repair with Suture Ligament Augmentation Is Associated with a High Failure Rate Among Adolescent Patients. Am. J. Sports Med. 2019, 47, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Heitmann, M.; Akoto, R.; Krause, M.; Hepp, P.; Schöpp, C.; Gensior, T.J.; Bartl, C.; Lill, H.; Frosch, K.-H. Management of acute knee dislocations: Anatomic repair and ligament bracing as a new treatment option—Results of a multicentre study. Knee Surg. Sports Traumatol. Arthrosc. 2019, 27, 2710–2718. [Google Scholar] [CrossRef]
- Otto, A.; Helal, A.; Imhoff, F.B.; Mehl, J.; Herbst, E.; Achtnich, A.E.; Forkel, P.; Imhoff, A.B.; Schmitt, A. Promising clinical and magnetic resonance imaging results after internal bracing of acute posterior cruciate ligament lesions in multiple injured knees. Knee Surg. Sports Traumatol. Arthrosc. 2020, 28, 2543–2550. [Google Scholar] [CrossRef]
- Taylor, S.A.; Khair, M.M.; Roberts, T.R.; DiFelice, G.S. Primary Repair of the Anterior Cruciate Ligament: A Systematic Review. Arthrosc. J. Arthrosc. Relat. Surg. 2015, 31, 2233–2247. [Google Scholar] [CrossRef]
- Barcellona, M.G.; Morrissey, M.C.; Milligan, P.; Amis, A.A. The effect of thigh muscle activity on anterior knee laxity in the uninjured and anterior cruciate ligament-injured knee. Knee Surg. Sports Traumatol. Arthrosc. 2014, 22, 2821–2829. [Google Scholar] [CrossRef] [PubMed]
- Keizer, M.N.J.; Hijmans, J.M.; Gokeler, A.; Benjaminse, A.; Otten, E. Healthy subjects with lax knees use less knee flexion rather than muscle control to limit anterior tibia translation during landing. J. Exp. Orthop. 2020, 7, 32. [Google Scholar] [CrossRef] [PubMed]
- Olmstead, T.; Wevers, H.; Bryant, J.; Gouw, G. Effect of muscular activity on valgus/varus laxity and stiffness of the knee. J. Biomech. 1986, 19, 565–577. [Google Scholar] [CrossRef] [PubMed]
- Shultz, S.J.; Pye, M.L.; Montgomery, M.M.; Schmitz, R.J. Associations between Lower Extremity Muscle Mass and Multiplanar Knee Laxity and Stiffness: A Potential Explanation for Sex Differences in Frontal and Trans-verse Plane Knee Laxity. Am. J. Sports Med. 2012, 40, 2836–2844. [Google Scholar] [CrossRef]

| Criteria | Total Collective (n = 54) | p-Value |
|---|---|---|
| Male vs. female (%) | 40.7% vs. 59.3% | <0.05 |
| Mean age at surgery (years) | 47 ± 11.9 | - |
| Mean age at follow-up (years) | 51 ± 11.9 | - |
| Schatzker (n) | ||
| I | 0 | - |
| II | 25 | - |
| III | 3 | - |
| IV | 3 | - |
| V | 0 | - |
| VI | 23 | - |
| Cause of accident (%) | ||
| Falls | 33.4% | - |
| Traffic | 20.4% | - |
| Ski | 20.4% | - |
| Bicycle | 18.5% | - |
| Fall from height | 0% | - |
| other | 7.4% | - |
| ROM flexion (°) 1 | 127.1 ± 11.2 vs. 129.7 ± 5.3 | <0.05 |
| ROM extension (°) 1 | 2.1 ± 1.7 vs. 2.7 ± 0.8 | <0.05 |
| Initial imaging (%) | ||
| X-ray | 74.1% | - |
| Computed tomography | 98.1% | - |
| Magnetic resonance imaging | 20.4% | - |
| Initial treatment | ||
| Brace | 81.5% | - |
| External fixator | 18.5% | - |
| Surgery time (minutes) | 165.1 ± 76.8 | - |
| ASA score | 1.8 ± 0.5 | - |
| Mean difference BMI 2 | 24.2 ± 3.1 vs. 24.9 ± 3.7 | n.s. |
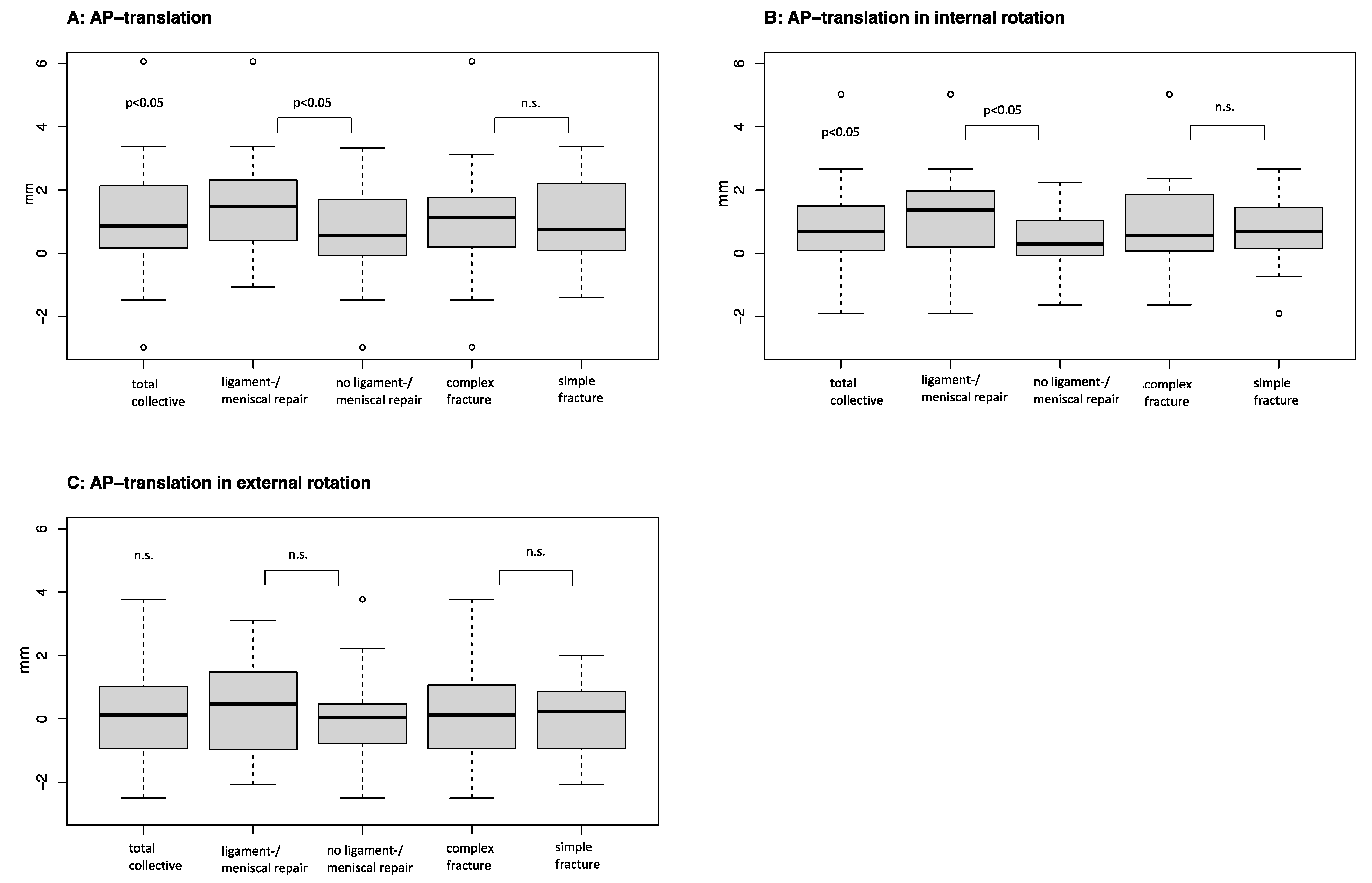
| AP translation 3 | 1.02 (0.6 to 1.5) | <0.05 |
| AP translation in internal rotation 3 | 0.71 (0.5 to 1.1) | n.s. |
| Internal rotation angle 3 | 1.6° ± 10.6° | n.s. |
| AP translation in external rotation 3 | 0.08 (−0.1 to 0.5) | n.s. |
| External rotation angle | −5.1° ± 10.1 | <0.05 |
| Medial deviation 3 | 0.45 | n.s. |
| Criteria | Complex Fractures (n = 26) | Simple Fractures (n = 28) | p-Value |
|---|---|---|---|
| Male vs. female (%) | 38.5% vs. 61.5% | 42.9% vs. 57.1% | n.s. |
| Mean age at surgery (years) | 48 ± 11.9 | 44.5 ± 12 | n.s. |
| Mean age at follow-up (years) | 51.6 ± 11.9 | 50.5 ± 12 | |
| Schatzker (n) | |||
| I | 0 | 0 | - |
| II | 0 | 25 | - |
| III | 0 | 3 | - |
| IV | 3 | 0 | - |
| V | 0 | 0 | - |
| VI | 23 | 0 | - |
| Cause of accident (%) | |||
| Falls | 23.1% | 42.9% | <0.05 |
| Traffic | 26.9% | 14.3% | <0.05 |
| Ski | 19.2% | 21.4% | n.s. |
| Bicycle | 26.9% | 10.7% | <0.05 |
| Fall from height | 0% | 0% | n.s. |
| Other | 3.8% | 10.7% | n.s. |
| ROM flexion (°) 1 | 126.3 ± 14.1 vs. 129.8 ± 7.8 | 127.8 ± 7.8 vs. 129.6 ± 5 | n.s. |
| ROM extension (°) 1 | 2.3 ± 1.8 vs. 2.9 ± 0.7 | 1.8 ± 1.6 vs. 2.6 ± 0.9 | n.s. |
| Initial imaging (%) | |||
| X-ray | 69.2% | 78.6% | <0.05 |
| Computed tomography | 100% | 96.4% | n.s. |
| Magnetic resonance imaging | 19.2% | 21.4% | n.s. |
| Initial treatment | |||
| Brace | 65.4% | 96.4% | <0.01 |
| External fixator | 34.6% | 3.6% | <0.01 |
| Surgery time (minutes) | 173 ± 91 | 157.8 ± 62.1 | n.s. |
| ASA score | 2 ± 0.5 | 1.6 ± 0.5 | - |
| Mean difference BMI 2 | 24.8 ± 2.6 vs. 24.8 ± 4.4 | 23.6 ± 3.5 vs. 24.7 ± 3.8 | n.s. |
| AP translation 3 | 1.12 (0.4 to 1.8) | 1.02 (0.5 to 1.5) | n.s. |
| AP translation in internal rotation 3 | 0.83 (0.3 to 1.4) | 0.71 (0.3 to 1.1) | n.s. |
| Internal rotation angle 3 | 1.2° ± 9.6 | 2.9° ± 11.6 | n.s. |
| AP translation in external rotation 3 | 0.35 (−0.2 to 1) | 0.08 (−0.3 to 0.5) | n.s. |
| External rotation angle | −7.4° ± 11.2 | −3.1° ± 15.1 | <0.05 |
| Medial deviation 3 | 0.67 | 0.25 | n.s. |
| Criteria | Ligament Repair (n = 24) | No Ligament Repair (n = 30) | p-Value |
|---|---|---|---|
| Male vs. female (%) | 41.7% vs. 58.3% | 40% vs. 60% | n.s. |
| Mean age at surgery (years) | 48.2 ± 11.6 | 46.2 ± 12.2 | n.s. |
| Mean age at follow-up (years) | 52.5 ± 11.6 | 49.8 ± 12.2 | |
| Schatzker (n) | |||
| I | 0 | 0 | - |
| II | 10 | 15 | - |
| III | 1 | 2 | - |
| IV | 0 | 3 | - |
| V | 0 | 0 | - |
| VI | 13 | 10 | - |
| Cause of accident (%) | |||
| Falls | 33.3% | 33.3% | n.s. |
| Traffic | 20.8% | 20% | n.s. |
| Ski | 20.8% | 20% | n.s. |
| Bicycle | 20.8% | 16.7% | n.s. |
| Fall from height | 0% | 0% | n.s. |
| other | 4.2% | 10% | n.s. |
| ROM flexion (°) 1 | 126.2 ± 14 vs. 129.5 ± 6.9 | 127.8 ± 8.4 vs. 129.8 ± 4.2 | <0.05 |
| ROM extension (°) 1 | 2.3 ± 1.7 vs. 2.7 ± 0.6 | 1.9 ± 1.7 vs. 2.7 ± 1 | n.s. |
| Initial imaging (%) | |||
| X-ray | 79.2% | 70% | n.s. |
| Computed tomography | 100% | 96.7% | <0.05 |
| Magnetic resonance imaging | 20.8% | 20% | n.s. |
| Initial treatment | |||
| Brace | 75% | 86.7% | <0.05 |
| External fixator | 25% | 13.3% | n.s. |
| Surgery time (minutes) | 160 ± 72.7 | 169.1 ± 80.7 | <0.05 |
| ASA score | 1.8 ± 0.4 | 1.8 ± 0.6 | - |
| Mean difference BMI 2 | 24.4 ± 3.7 vs. 24.2 ± 4.1 | 24 ± 2.7 vs. 25.5 ± 3.2 | n.s. |
| AP translation 3 | 1.51 (0.9 to 2.1) | 0.71 (0.1 to 1.2) | <0.05 |
| AP translation in internal rotation 3 | 1.19 (0.6 to 1.8) | 0.44 (0.1 to 0.8) | <0.05 |
| Internal rotation angle 3 | −0.3° ± 12.4 | 3.8° ± 8.5 | <0.05 |
| AP translation in external rotation 3 | 0.38 (−0.2 to 1) | 0.07 (−0.3 to 0.6) | n.s. |
| External rotation angle | −7.5° ± 10.3 | −3.1° ± 9.6 | <0.05 |
| Medial deviation 3 | 0.25 | 0.61 | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bormann, M.; Neidlein, C.; Neidlein, N.; Ehrl, D.; Jörgens, M.; Berthold, D.P.; Böcker, W.; Holzapfel, B.M.; Fürmetz, J. High Prevalence of Persistent Measurable Postoperative Knee Joint Laxity in Patients with Tibial Plateau Fractures Treated by Open Reduction and Internal Fixation (ORIF). J. Clin. Med. 2023, 12, 5580. https://doi.org/10.3390/jcm12175580
Bormann M, Neidlein C, Neidlein N, Ehrl D, Jörgens M, Berthold DP, Böcker W, Holzapfel BM, Fürmetz J. High Prevalence of Persistent Measurable Postoperative Knee Joint Laxity in Patients with Tibial Plateau Fractures Treated by Open Reduction and Internal Fixation (ORIF). Journal of Clinical Medicine. 2023; 12(17):5580. https://doi.org/10.3390/jcm12175580
Chicago/Turabian StyleBormann, Markus, Claas Neidlein, Niels Neidlein, Dennis Ehrl, Maximilian Jörgens, Daniel P. Berthold, Wolfgang Böcker, Boris Michael Holzapfel, and Julian Fürmetz. 2023. "High Prevalence of Persistent Measurable Postoperative Knee Joint Laxity in Patients with Tibial Plateau Fractures Treated by Open Reduction and Internal Fixation (ORIF)" Journal of Clinical Medicine 12, no. 17: 5580. https://doi.org/10.3390/jcm12175580
APA StyleBormann, M., Neidlein, C., Neidlein, N., Ehrl, D., Jörgens, M., Berthold, D. P., Böcker, W., Holzapfel, B. M., & Fürmetz, J. (2023). High Prevalence of Persistent Measurable Postoperative Knee Joint Laxity in Patients with Tibial Plateau Fractures Treated by Open Reduction and Internal Fixation (ORIF). Journal of Clinical Medicine, 12(17), 5580. https://doi.org/10.3390/jcm12175580







