Evaluation of a New Treatment Strategy for Geriatric Fragility Fractures of the Posterior Pelvic Ring Using Sensor-Supported Insoles: A Proof-of-Concept Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants and Treatment Principles
2.2. Study Design
2.3. Gait Analysis
2.4. Clinical Questionnaires
2.5. Statistical Analysis
3. Results
3.1. Demographics of the Participants
3.2. Results of the Questionnaires
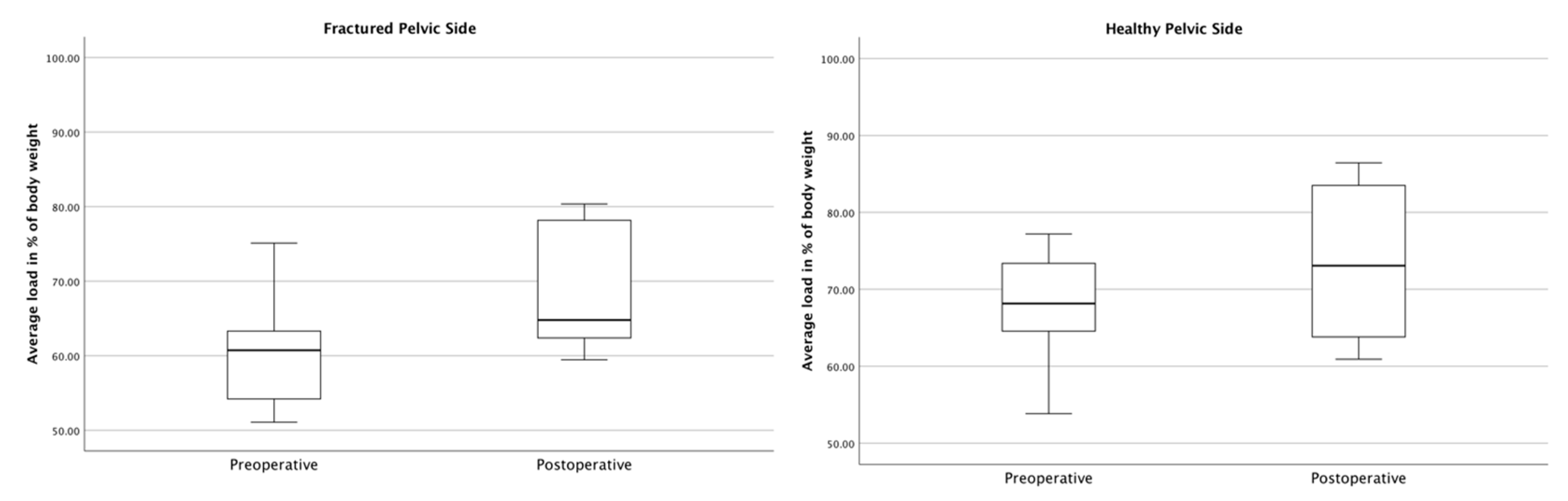
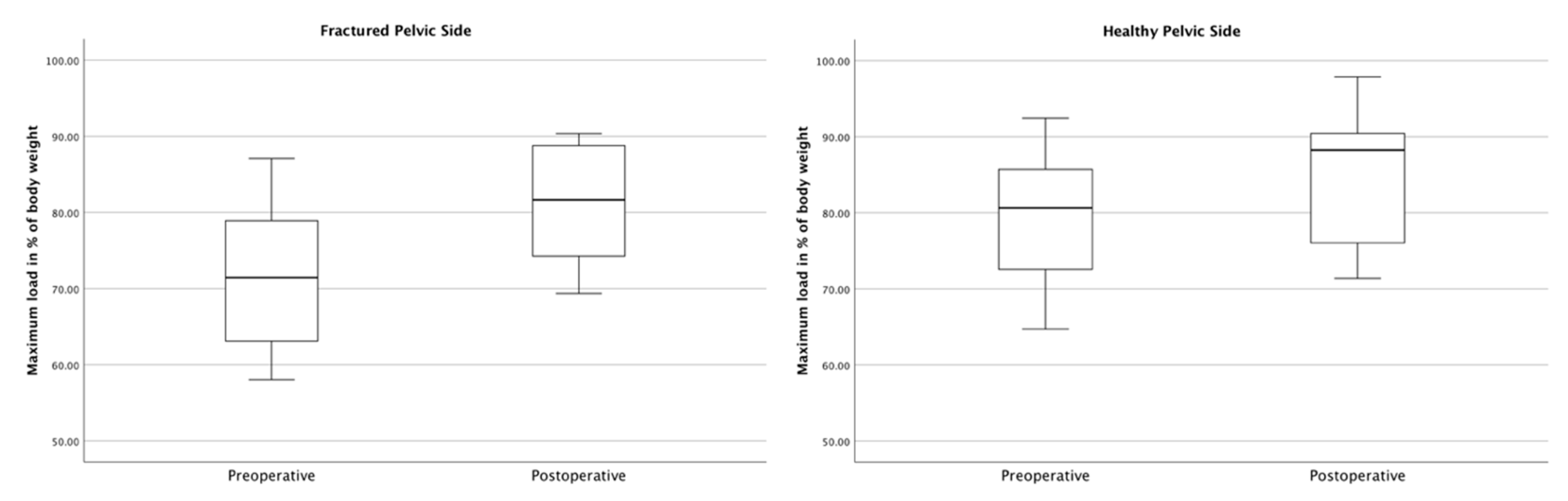
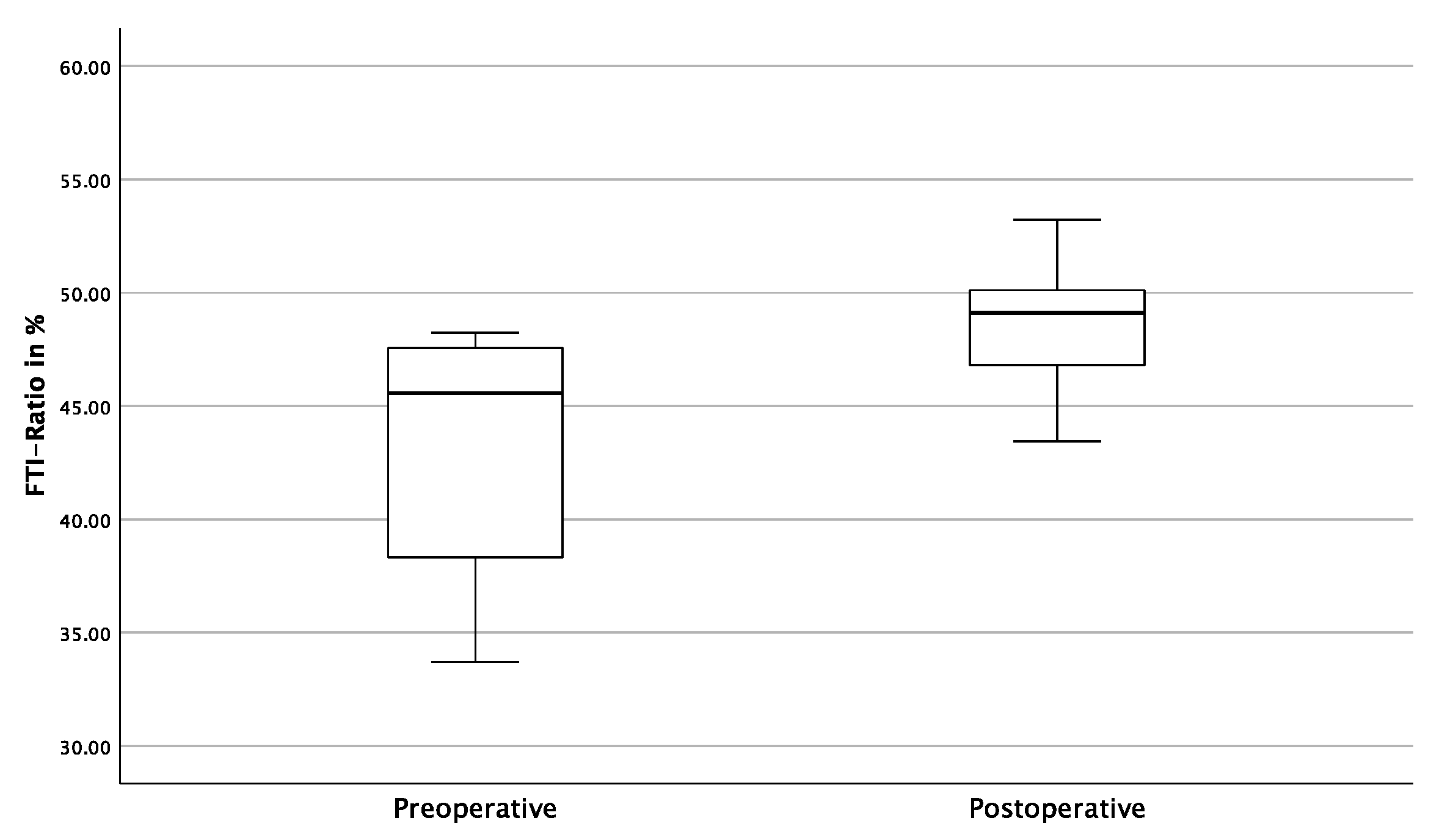
3.3. Gait Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tieland, M.; Trouwborst, I.; Clark, B.C. Skeletal muscle performance and ageing. J. Cachexia Sarcopenia Muscle 2018, 9, 3–19. [Google Scholar] [CrossRef] [PubMed]
- Oberkircher, L.; Ruchholtz, S.; Rommens, P.M.; Hofmann, A.; Bucking, B.; Kruger, A. Osteoporotic Pelvic Fractures. Dtsch. Arztebl. Int. 2018, 115, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Glaser, D.L.; Kaplan, F.S. Osteoporosis. Definition and clinical presentation. Spine 1997, 22 (Suppl. S24), 12S–16S. [Google Scholar] [CrossRef] [PubMed]
- Sterneder, M.; Lang, P.; Riesner, H.J.; Hackenbroch, C.; Friemert, B.; Palm, H.G. Insufficiency Fractures vs. Low-Energy Pelvic Ring Fractures—Epidemiological, Diagnostic and Therapeutic Characteristics of Fragility Fractures of the Pelvic Ring. Z. Orthop. Unfall. 2022, 160, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Wagner, D.; Hofmann, A. Minimal Invasive Surgical Treatment of Fragility Fractures of the Pelvis. Chirurgia 2017, 112, 524–537. [Google Scholar] [CrossRef]
- Rommens, P.M.; Hofmann, A. Comprehensive classification of fragility fractures of the pelvic ring: Recommendations for surgical treatment. Injury 2013, 44, 1733–1744. [Google Scholar] [CrossRef]
- Rommens, P.M.; Drees, P.; Thomczyk, S.; Betz, U.; Wagner, D.; Hofmann, A. Die Fragilitätsfraktur des Beckens ist eine Indikatorfraktur der Osteoporose. Osteologie 2018, 27, 144–153. [Google Scholar] [CrossRef]
- Bornemann, R.; Pflugmacher, R.; Webler, M.; Koch, E.M.; Dengler, J.; Wirtz, D.C.; Frey, S.P. Clinical Trial to Test the iFuse Implant System(R) in Patients with Sacroiliac Joint Syndrome: One Year Results. Z. Orthop. Unfall. 2016, 154, 601–605. [Google Scholar] [CrossRef]
- MacBarb, R.F.; Lindsey, D.P.; Woods, S.A.; Lalor, P.A.; Gundanna, M.I.; Yerby, S.A. Fortifying the Bone-Implant Interface Part 2: An In Vivo Evaluation of 3D-Printed and TPS-Coated Triangular Implants. Int. J. Spine Surg. 2017, 11, 16. [Google Scholar] [CrossRef]
- Torsional Rigidity of the iFuse Implant in a Sawbones Model Report. SI-BONE Technische Studie, 300191-TS. Available online: https://si-bone.de/anwender/losungen/trauma (accessed on 10 April 2023).
- Smith, A.G.; Capobianco, R.; Cher, D.; Rudolf, L.; Sachs, D.; Gundanna, M.; Kleiner, J.; Mody, M.G.; Shamie, A.N. Open versus minimally invasive sacroiliac joint fusion: A multi-center comparison of perioperative measures and clinical outcomes. Ann. Surg. Innov. Res. 2013, 7, 14. [Google Scholar] [CrossRef]
- Ledonio, C.G.; Polly, D.W., Jr.; Swiontkowski, M.F. Minimally invasive versus open sacroiliac joint fusion: Are they similarly safe and effective? Clin. Orthop. Relat. Res. 2014, 472, 1831–1838. [Google Scholar] [CrossRef]
- Ledonio, C.G.; Polly, D.W., Jr.; Swiontkowski, M.F.; Cummings, J.T., Jr. Comparative effectiveness of open versus minimally invasive sacroiliac joint fusion. Med. Devices 2014, 7, 187–193. [Google Scholar] [CrossRef] [PubMed]
- SI-Bone. iFuse Operationstechnik. Available online: https://si-bone.de/anwender/losungen/ifuse/op-technik (accessed on 9 May 2023).
- Pfeufer, D.; Becker, C.A.; Faust, L.; Keppler, A.M.; Stagg, M.; Kammerlander, C.; Bocker, W.; Neuerburg, C. Load-Bearing Detection with Insole-Force Sensors Provides New Treatment Insights in Fragility Fractures of the Pelvis. J. Clin. Med. 2020, 9, 2551. [Google Scholar] [CrossRef] [PubMed]
- Faust, L.M.; Keppler, A.M.; Suero, E.; Gleich, J.; Lisitano, L.; Bocker, W.; Neuerburg, C.; Pfeufer, D. The grade of instability in fragility fractures of the pelvis correlates with impaired early mobilization. Eur. J. Trauma Emerg. Surg. 2022, 48, 4053–4060. [Google Scholar] [CrossRef]
- Mahoney, F.I.; Barthel, D.W. Functional Evaluation: The Barthel Index. Md. State Med. J. 1965, 14, 61–65. [Google Scholar] [PubMed]
- Voeten, S.C.; Nijmeijer, W.S.; Vermeer, M.; Schipper, I.B.; Hegeman, J.H.; DHFA Taskforce Study Group. Validation of the Fracture Mobility Score against the Parker Mobility Score in hip fracture patients. Injury 2020, 51, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Charlson, M.E.; Carrozzino, D.; Guidi, J.; Patierno, C. Charlson Comorbidity Index: A Critical Review of Clinimetric Properties. Psychother. Psychosom. 2022, 91, 8–35. [Google Scholar] [CrossRef]
- Wille, N.; Badia, X.; Bonsel, G.; Burstrom, K.; Cavrini, G.; Devlin, N.; Egmar, A.C.; Greiner, W.; Gusi, N.; Herdman, M.; et al. Development of the EQ-5D-Y: A child-friendly version of the EQ-5D. Qual. Life Res. 2010, 19, 875–886. [Google Scholar] [CrossRef]
- Herteleer, M.; Dejaeger, M.; Nijs, S.; Hoekstra, H.; Laurent, M.R. Epidemiology and secular trends of pelvic fractures in Belgium: A retrospective, population-based, nationwide observational study. Bone 2021, 153, 116141. [Google Scholar] [CrossRef]
- Kannus, P.; Parkkari, J.; Niemi, S.; Sievanen, H. Low-Trauma Pelvic Fractures in Elderly Finns in 1970–2013. Calcif. Tissue Int. 2015, 97, 577–580. [Google Scholar] [CrossRef] [PubMed]
- Andrich, S.; Haastert, B.; Neuhaus, E.; Neidert, K.; Arend, W.; Ohmann, C.; Grebe, J.; Vogt, A.; Jungbluth, P.; Rosler, G.; et al. Epidemiology of Pelvic Fractures in Germany: Considerably High Incidence Rates among Older People. PLoS ONE 2015, 10, e0139078. [Google Scholar] [CrossRef] [PubMed]
- Rommens, P.M.; Hopf, J.C.; Herteleer, M.; Devlieger, B.; Hofmann, A.; Wagner, D. Isolated Pubic Ramus Fractures Are Serious Adverse Events for Elderly Persons: An Observational Study on 138 Patients with Fragility Fractures of the Pelvis Type I (FFP Type I). J. Clin. Med. 2020, 9, 2498. [Google Scholar] [CrossRef] [PubMed]
- Dale, M.; Evans, J.; Carter, K.; O’Connell, S.; Morgan, H.; Carolan-Rees, G. iFuse Implant System for Treating Chronic Sacroiliac Joint Pain: A NICE Medical Technology Guidance. Appl. Health Econ. Health Policy 2020, 18, 363–373. [Google Scholar] [CrossRef]
- Tran, Z.V.; Ivashchenko, A.; Brooks, L. Sacroiliac Joint Fusion Methodology—Minimally Invasive Compared to Screw-Type Surgeries: A Systematic Review and Meta-Analysis. Pain Physician 2019, 22, 29–40. [Google Scholar] [CrossRef]
- Bornemann, R.; Roessler, P.P.; Strauss, A.C.; Sander, K.; Rommelspacher, Y.; Wirtz, D.C.; Pflugmacher, R.; Frey, S.P. Two-year clinical results of patients with sacroiliac joint syndrome treated by arthrodesis using a triangular implant system. Technol. Health Care 2017, 25, 319–325. [Google Scholar] [CrossRef]
- Cederholm, T.; Cruz-Jentoft, A.J.; Maggi, S. Sarcopenia and fragility fractures. Eur. J. Phys. Rehabil. Med. 2013, 49, 111–117. [Google Scholar] [PubMed]
- Yeung SS, Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers CG, M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Rommens, P.M.; Boudissa, M.; Kramer, S.; Kisilak, M.; Hofmann, A.; Wagner, D. Operative treatment of fragility fractures of the pelvis is connected with lower mortality. A single institution experience. PLoS ONE 2021, 16, e0253408. [Google Scholar] [CrossRef]
- Wagner, D.; Kisilak, M.; Porcheron, G.; Kramer, S.; Mehling, I.; Hofmann, A.; Rommens, P.M. Trans-sacral bar osteosynthesis provides low mortality and high mobility in patients with fragility fractures of the pelvis. Sci. Rep. 2021, 11, 14201. [Google Scholar] [CrossRef]
- Hollensteiner, M.; Sandriesser, S.; Bliven, E.; von Ruden, C.; Augat, P. Biomechanics of Osteoporotic Fracture Fixation. Curr. Osteoporos. Rep. 2019, 17, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Duhon, B.S.; Cher, D.J.; Wine, K.D.; Kovalsky, D.A.; Lockstadt, H.; Group, S.S. Triangular Titanium Implants for Minimally Invasive Sacroiliac Joint Fusion: A Prospective Study. Global Spine J. 2016, 6, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Polly, D.W.; Cher, D.J.; Wine, K.D.; Whang, P.G.; Frank, C.J.; Harvey, C.F.; Lockstadt, H.; Glaser, J.A.; Limoni, R.P.; Sembrano, J.N.; et al. Randomized Controlled Trial of Minimally Invasive Sacroiliac Joint Fusion Using Triangular Titanium Implants vs Nonsurgical Management for Sacroiliac Joint Dysfunction: 12-Month Outcomes. Neurosurgery 2015, 77, 674–690; discussion 671–690. [Google Scholar] [CrossRef] [PubMed]
- Cher, D.J.; Reckling, W.C.; Capobianco, R.A. Implant survivorship analysis after minimally invasive sacroiliac joint fusion using the iFuse Implant System((R)). Med. Devices 2015, 8, 485–492. [Google Scholar] [CrossRef]





| Preoperative | Postoperative | p-Value | |
|---|---|---|---|
| Barthel Index | 50.00 ± 12.91 | 70.00 ± 9.72 | p < 0.001 |
| Parker Mobility Score | 1.89 ± 1.17 | 3.89 ± 0.78 | p = 0.011 |
| EQ-5D | 26.00 ± 14.30 | 51.00 ± 13.70 | p < 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebert, L.; Keppler, A.M.; Bruder, J.; Faust, L.; Becker, C.A.; Böcker, W.; Neuerburg, C.; Cavalcanti Kußmaul, A. Evaluation of a New Treatment Strategy for Geriatric Fragility Fractures of the Posterior Pelvic Ring Using Sensor-Supported Insoles: A Proof-of-Concept Study. J. Clin. Med. 2023, 12, 5199. https://doi.org/10.3390/jcm12165199
Lebert L, Keppler AM, Bruder J, Faust L, Becker CA, Böcker W, Neuerburg C, Cavalcanti Kußmaul A. Evaluation of a New Treatment Strategy for Geriatric Fragility Fractures of the Posterior Pelvic Ring Using Sensor-Supported Insoles: A Proof-of-Concept Study. Journal of Clinical Medicine. 2023; 12(16):5199. https://doi.org/10.3390/jcm12165199
Chicago/Turabian StyleLebert, Luca, Alexander Martin Keppler, Jan Bruder, Leon Faust, Christopher Alexander Becker, Wolfgang Böcker, Carl Neuerburg, and Adrian Cavalcanti Kußmaul. 2023. "Evaluation of a New Treatment Strategy for Geriatric Fragility Fractures of the Posterior Pelvic Ring Using Sensor-Supported Insoles: A Proof-of-Concept Study" Journal of Clinical Medicine 12, no. 16: 5199. https://doi.org/10.3390/jcm12165199
APA StyleLebert, L., Keppler, A. M., Bruder, J., Faust, L., Becker, C. A., Böcker, W., Neuerburg, C., & Cavalcanti Kußmaul, A. (2023). Evaluation of a New Treatment Strategy for Geriatric Fragility Fractures of the Posterior Pelvic Ring Using Sensor-Supported Insoles: A Proof-of-Concept Study. Journal of Clinical Medicine, 12(16), 5199. https://doi.org/10.3390/jcm12165199





