Characteristics, Treatment Strategies and Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: A Contemporary Dutch Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Variable Selection
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
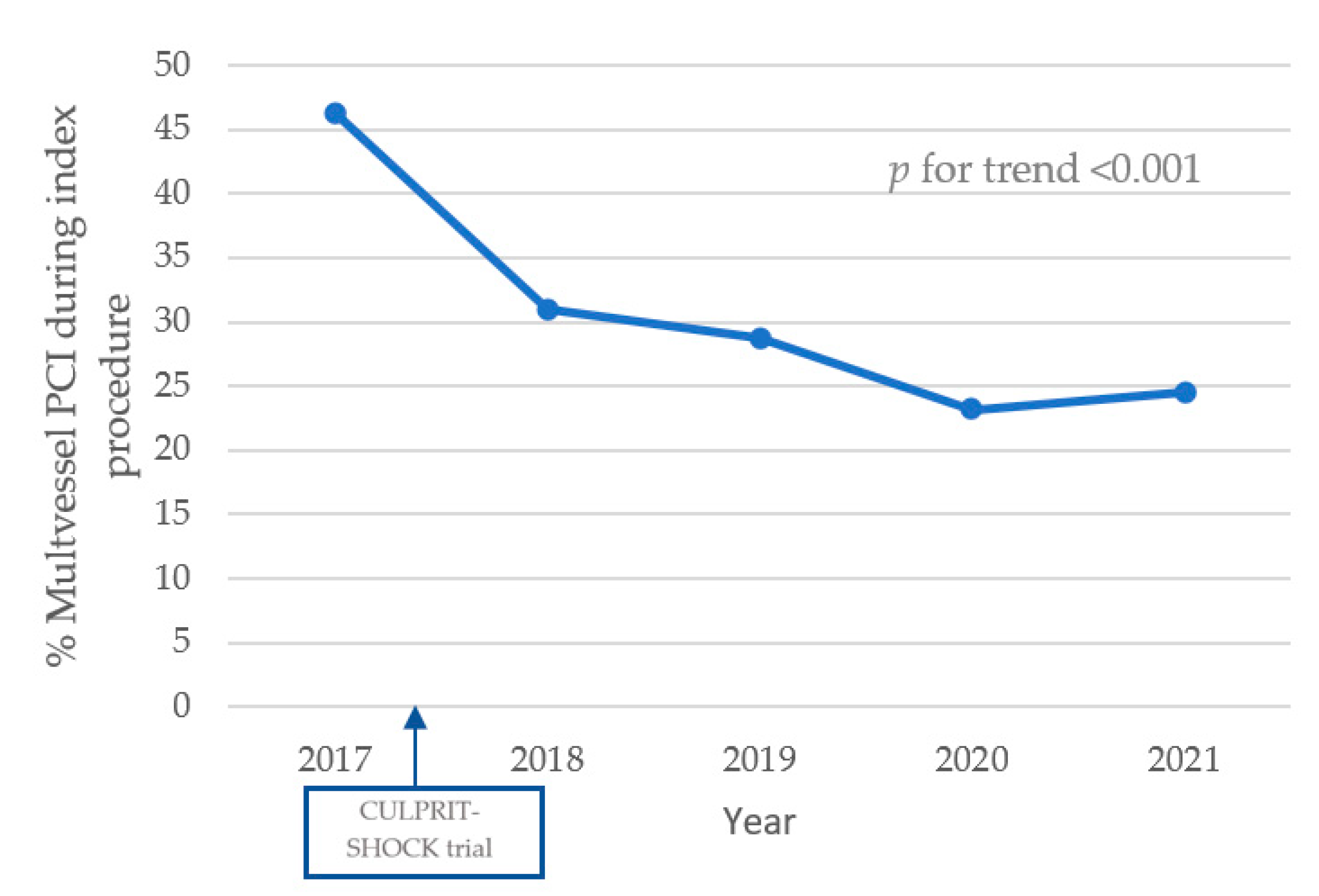
3.2. Angiographic Features
3.3. Mechanical and Pharmacological Support
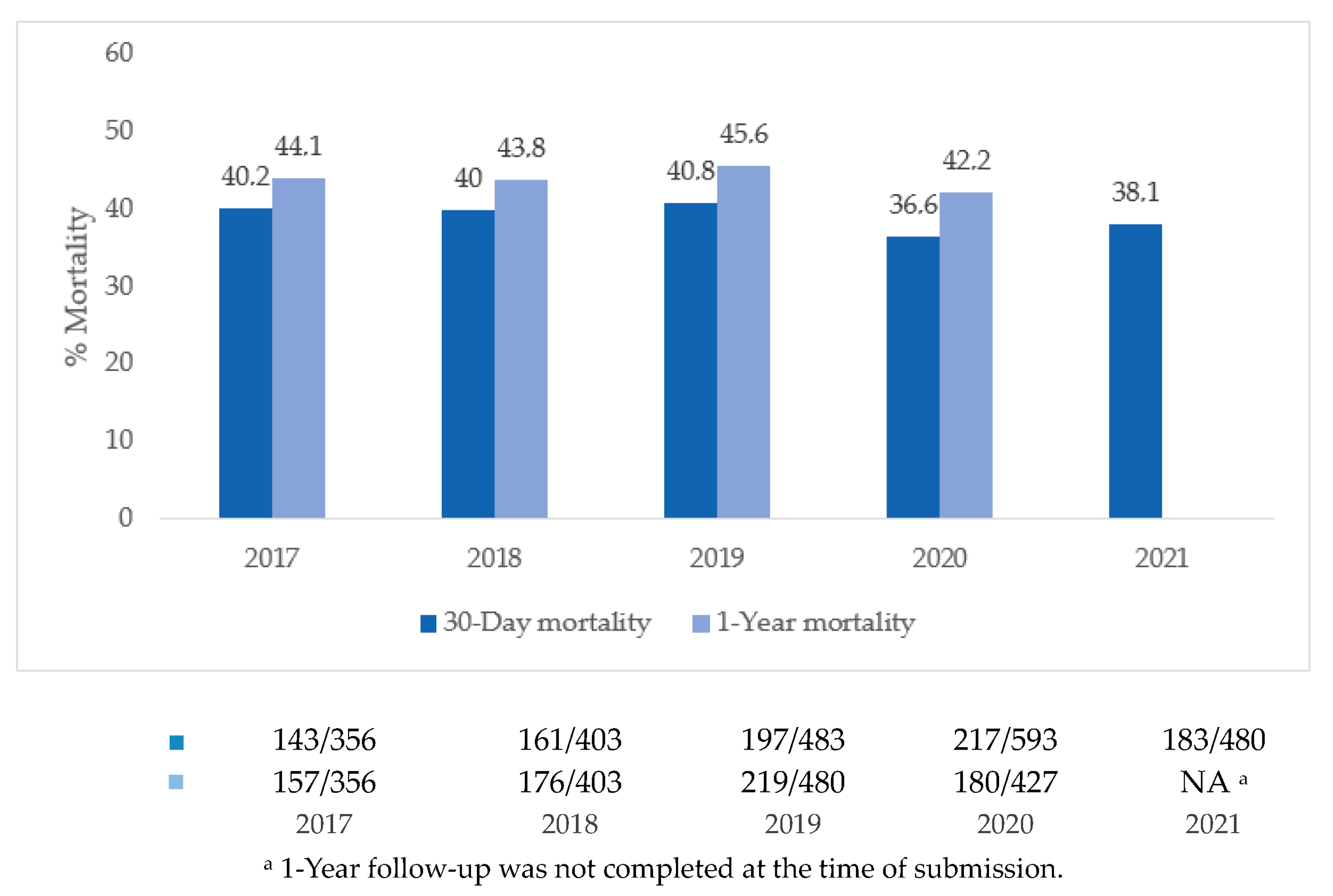
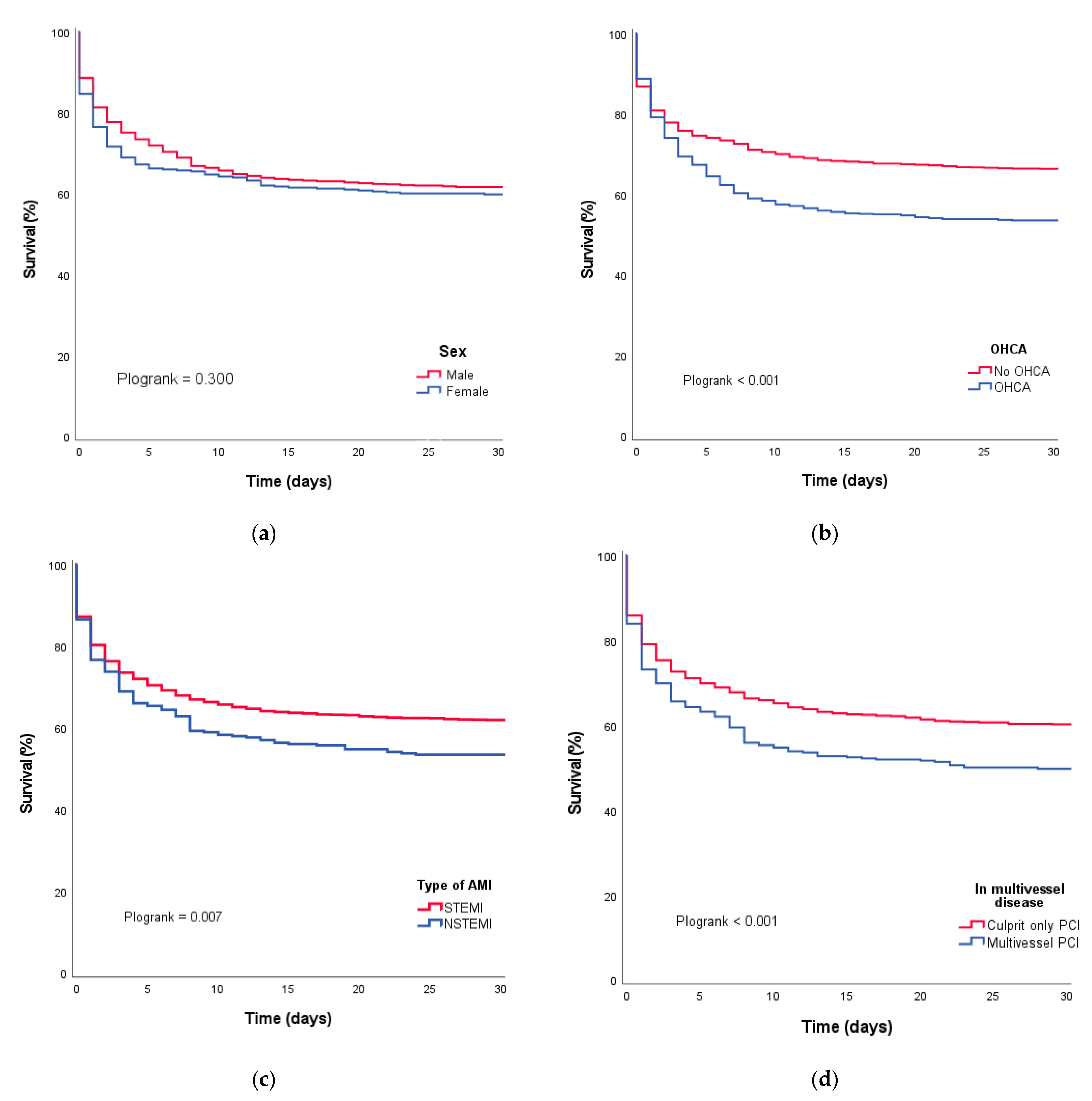
3.4. Survival
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Variable | Outcome | Missing No. (%) |
|---|---|---|
| Age Difference between date of birth and date of intervention. | Continuous | 0 (0) |
| Sex | Male Female | 0 (0) |
| Creatinine—µmol/L Last measured concentration of creatinine (measured no longer than 3 months prior to the intervention or on the day of the intervention). | Continuous (1–2000) | 191 (8.2) |
| Diabetes mellitus Indicate the most intensive therapy that was used to treat diabetes | None Diabetes, treatment unknown Diabetes, no treatment Diabetes, diet Diabetes, oral medication Diabetes, insulin Diabetes, other | 109 (4.7) |
| LVEF—% Fraction of blood ejected from the left ventricle with each contraction (expressed as percentage; registered no more than 6 months prior to the intervention). | Continuous (1–99) | 1627 (69.9) |
| Dialysis Chronic hemodialysis or peritoneal dialysis due to renal failure at the time of the current admission. | No Yes | 318 (13.7) |
| Multivessel disease Presence of multivessel disease during the current intervention. For first interventions: stenosis of ≥70% in ≥2 native vessels with a diameter of at least 1.5 mm. In patients with a prior coronary intervention: ≥70% stenosis in ≥1 native coronary arteries that have not yet been treated and/or multivessel disease during previous intervention. | No Yes | 21 (0.9) |
| Prior MI Patient had at least one documented prior myocardial infarction (excluding infarctions occurring during the same admission that were the reason for the current intervention). | No Yes | 75 (3.2) |
| Indication of PCI Status of the patient during the current intervention: NSTEMI: presence of acute chest pain in the absence of ST elevation (including stable angina); STEMI: presence of acute chest pain and (>20 mm) ST elevation. | NSTEMI STEMI | 24 (1.1) |
| Cardiogenic shock The presence of hypotension (systolic blood pressure (SBP) ≤ 90 mmHg for ≥ 30 min or support to maintain SBP ≥ 90 mmHg) and end-organ hypoperfusion (cold extremities and/or oliguria < 30 mL/hour and/or tachycardia ≥ 60 beats per minute (bpm)). | No Yes | 0 (0) |
| OHCA Patients who were defibrillated (and received chest compressions) outside the hospital (prior to and related to the reason for the current intervention). | No Yes, treatment unknown Yes, defibrillation only Yes, defibrillation and compressions | 11 (0.5) |
| Prior PCI Patient underwent PCI prior to current intervention. | No Yes | 194 (8.3) |
| Prior CABG Patient underwent coronary artery bypass graft surgery prior to current intervention. | No Yes | 42 (1.8) |
| PCI vascular access site Vascular access site used for current intervention. | Radial Femoral Brachial Ulnar Other | 275 (11.8) |
| PCI-treated vessel Name of dilated coronary artery: LM: left main LAD: left coronary artery RCX: circumflex artery AL/IM: anterolateral / intermediate branch RCA: right coronary artery Venous graft Arterial graft | LM LAD RCX AL/IM RCA Venous graft Arterial graft | 214 (9.2) |
| Survival status Survival status (as determined after verification of the personal records database or date of last contact). | Alive Deceased | 7 (0.3) |
| Date of survival status Days between PCI and either verification of survival status (alive patients) or date of death (deceased patients). | Continuous | 6 (0.3) |
Appendix B
| Hospital | Physician |
|---|---|
| Amphia Ziekenhuis | Dr. M. Meuwissen |
| Amsterdam Universitair Medische Centra, AMC | Prof. Dr. J.P. Henriques |
| Amsterdam Universitair Medische Centra, VU | Dr. K.M.J. Marques |
| Catharina Ziekenhuis | Dr. K. Teeuwen |
| Erasmus Medisch Centrum | Dr. J. Daemen |
| HagaZiekenhuis | Dhr. C.E. Schotborgh |
| Isala (ziekenhuis) | Dr. V. Roolvink |
| Leids Universitair Medisch Centrum | Dr. R. Scherptong |
| Medisch Centrum Leeuwarden | Dhr. J. Brouwer |
| Noordwest Ziekenhuisgroep | Dr. A. Dedic |
| Radboud Universitair Medisch Centrum | Dhr. C. Camaro |
| Rijnstate Ziekenhuis | Dr. P.W. Danse |
| Universitair Medisch Centrum Groningen | Dr. E. Lipšic |
| Universitair Medisch Centrum Utrecht | Dr. A.O. Kraaijeveld |
Appendix C. Detailed Description of Variable Selection Process
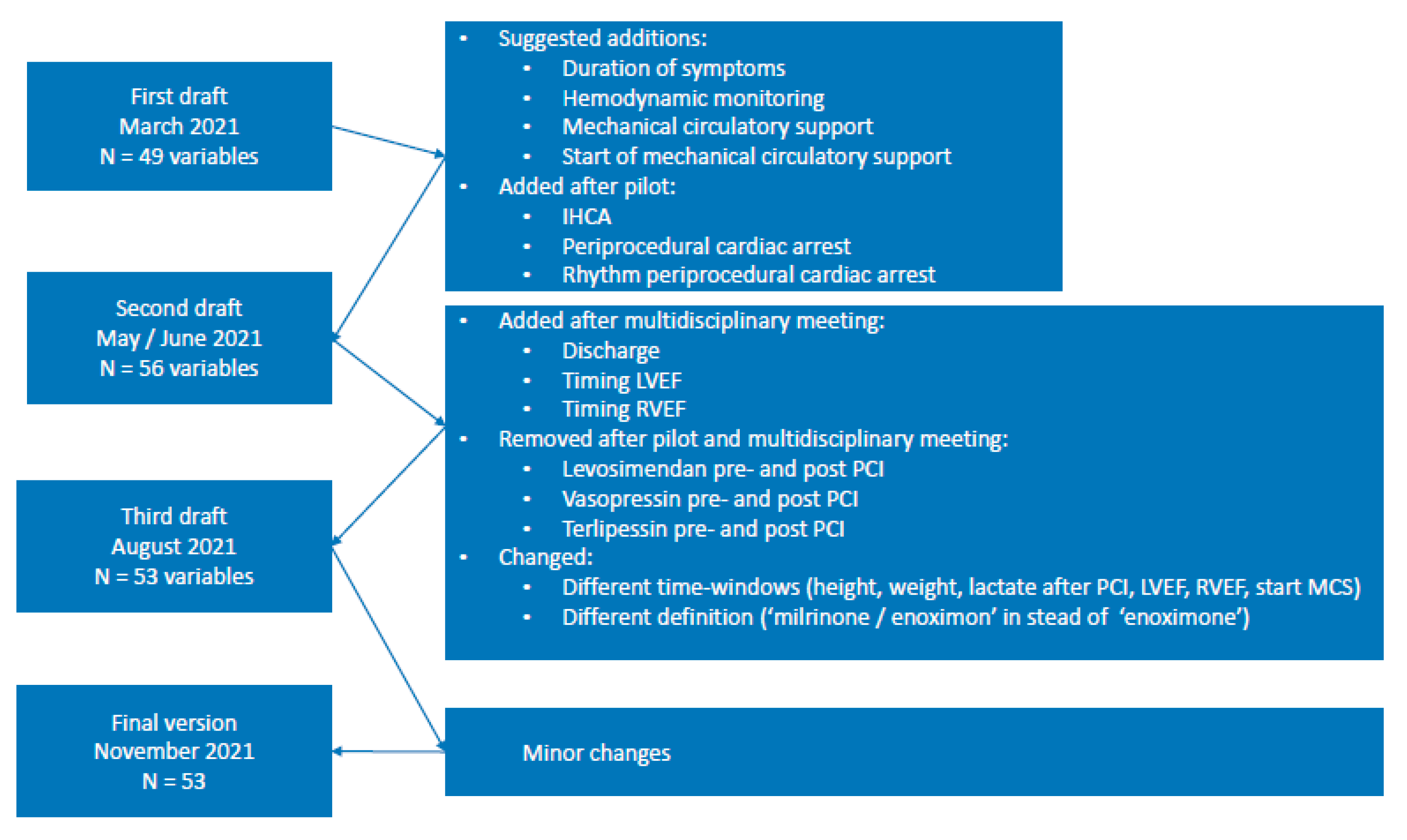
Appendix D
| Variable | Outcome | Missing No. (%) |
|---|---|---|
| Start of cardiogenic shock Timing of cardiogenic shock: Pre-PCI: up to and including the first pressure registration; During or post-PCI: after first pressure registration but before leaving the cath lab; After leaving cath lab: could be identified by linkage of index admission to catecholamine use. | Pre-PCI During or post-PCI After leaving cathlab | 57 (2.4) |
| Duration of symptoms Amount of time between start of symptoms and hospital presentation. | >24 h >12 h, ≤24 h >6 h, ≤12 h >3 h, ≤6 h ≤3 h | 337 (14.5) |
| Systolic blood pressure—mmHg Systolic blood pressure according to first in-hospital measurement pre-PCI. In case of absence of an in-hospital measurement, a measurement by the emergency medical team can be used. | Continuous (0–300) | 279 (12.0) |
| Diastolic blood pressure—mmHg Diastolic blood pressure according to first in-hospital measurement pre-PCI. In case of absence of an in-hospital measurement, a measurement by the emergency medical team can be used. | Continuous (0–300) | 309 (13.3) |
| Heart rate—bpm Heart rate according to first in-hospital measurement pre-PCI. In case of absence of an in-hospital measurement, a measurement by the emergency medical team can be used. | Continuous (0–300) | 329 (14.1) |
| OHCA witnessed Ambulance witnessed: emergency medical team witnessed collapse and acted accordingly; Layperson witnessed: someone (other than emergency medical team) saw or heard collapse and acted accordingly; Unwitnessed: no one saw or heard collapse; No OHCA: patient was not defibrillated (nor received chest compressions) prior outside the hospital, prior to and related to the reason for the current intervention; Unknown; unknown whether collapse was witnessed. | Ambulance witnessed Layperson witnessed Unwitnessed No OHCA | 48 (2.1) |
| OHCA duration Time to return of spontaneous circulation. | ≥30 min <30 min No OHCA | 106 (4.6) |
| IHCA Patient was defibrillated (and received chest compressions) in the hospital before entering the cath lab. | No Yes | 22 (1.0) |
| Height—kg Most recently reported height (measured during index admission). When height is not measured during index admission, the most recently reported height (up to one year old) can be used. | Continuous (20–270) | 398 (17.1) |
| Weight—cm Most recently reported weight (measured during index admission). When weight is not measured during index admission, the most recently reported weight (up to one year old) can be used. | Continuous (0.3–250) | 320 (13.7) |
| Lactate on admission—mmol/L First measured blood lactate level on admission (±1 h around PCI). | Continuous (0.0–40.0) | 802 (34.5) |
| Hemoglobin on admission—mmol/L First measured hemoglobin level on admission (±1 h around PCI). | Continuous (0.0–15.0) | 139 (6.0) |
| Glucose on admission— mmol/L First measured glucose level on admission (±1 h around PCI). | Continuous (1.0–40.0) | 283 (12.2) |
| Creatinine on admission—µmol/L First measured creatinine level on admission (±1 h around PCI). | Continuous (1.0–2000.0) | 233 (10.0) |
| CK-MB max—U/L Highest creatinine kinase-MB level during index admission (up to 3 days after PCI). | Continuous (0–10,000) | 1196 (51.4) |
| hs-Troponin-T—µg/L Highest high-sensitive troponin-T level during index admission (up to 3 days after PCI). | Continuous (0–150,000) | 412 (17.7) |
| Left ventricular ejection fraction (LVEF)—% Fraction of blood ejected from the left ventricle with each contraction (expressed as percentage; measured during shock). The most recent measure is to be used (up to 2 h before and 24 h after intervention). If more than one ejection fraction is available, the lowest registered value should be registered. | Continuous (1–99) | 1102 (47.3) |
| Timing LVEF Timing of echo that measured left ejection fraction. If more than one ejection fraction is available, the timing of the lowest registered ejection fraction should be registered. | 2 h prior to PCI until leaving cathlab ≤3 h after leaving cathlab >3 and ≤6 h after leaving cathlab >6 and ≤ 12 h after leaving cathlab >12 and ≤ 24 h after leaving cathlab | 193 (8.3) |
| Right ventricular ejection fraction (RVEF)—% Fraction of blood ejected from the right ventricle with each contraction (expressed as percentage; measured during shock). The most recent measure is to be used (up to 2 h before and 24 h after intervention). If more than one ejection fraction is available, the lowest registered value should be registered. | Continuous (1–99) | 1523 (65.4) |
| Timing RVEF Timing of echo that measured right ejection fraction. If more than one ejection fraction is available, the timing of the lowest registered ejection fraction should be registered. | 2 h prior to PCI until leaving cathlab ≤3 h after leaving cathlab >3 and ≤6 h after leaving cathlab >6 and ≤ 12 h after leaving cathlab >12 and ≤ 24 h after leaving cathlab | 236 (10.1) |
| Admission Days between date of admission and date PCI was performed. | Continuous | 19 (0.8) |
| Intubation before PCI Patient was intubated prior to PCI (up to first pressure registration). | No Yes | 21 (0.9) |
| Intubated when leaving HCK Patient was intubated when leaving the cathlab. | No Yes | 26 (1.1) |
| Mechanical circulatory support Type of mechanical circulatory support that was initiated during index admission. | None IABP Impella ECMO IABP + ECMO Impella + ECMO IABP + Impella Other | 24 (1.0) |
| Start of mechanical circulatory support Moment that mechanical circulatory support was initiated. | None Prior to HCK In HCK (before first i.c. measurement) After first measurement After leaving HCK, <24 h After leaving HCK > 24 h | 69 (3.0) |
| Hemodynamical monitoring Whether or not patient was hemodynamically monitored with Swan-Ganz or PiCCO catheter during index admission. | None Swann-Ganz PiCCO | 196 (8.4) |
| Periprocedural cardiac arrest Whether or not a cardiac arrest occurred during stay in the cathlab. | No Yes, prior to first measurement Yes, after first measurement | 22 (0.9) |
| Rhythm periprocedural cardiac arrest Initial rhythm of periprocedural cardiac arrest. | None VF/VT PEA/asystole | 74 (3.2) |
| TIMI flow grade pre-PCI TIMI flow measured pre-PCI. | 0 1 2 3 | 385 (16.5) |
| TIMI flow grade post-PCI TIMI flow measured post-PCI. | 0 1 2 3 | 329 (14.1) |
| Norepinephrine prior to PCI Whether or not a patient received norepinephrine pre-PCI (up to first pressure registration). | No Yes | 49 (2.1) |
| Norepinephrine after PCI Norepinephrine use in the first 24 h after PCI. | No Yes, continued Yes, initiated in HCK Yes, initiated <24 h after leaving HCK | 55 (2.4) |
| Dobutamine prior to PCI Whether or not a patient received dobutamine pre-PCI (up to first pressure registration). | No Yes | 42 (1.8) |
| Dobutamine after PCI Dobutamine use in the first 24 h after PCI. | No Yes, continued Yes, initiated in HCK Yes, initiated <24 h after leaving HCK | 65 (2.8) |
| Enoximone or milrinone prior to PCI Whether or not a patient received enoximone/milrinone pre-PCI (up to first pressure registration). | No Yes | 35 (1.5) |
| Enoximone of milrinone after PCI Enoximone/milrinone use in the first 24 h after PCI. | No Yes, continued Yes, initiated in HCK Yes, initiated <24 h after leaving HCK | 52 (2.2) |
| Adrenaline prior to PCI Whether or not a patient received adrenaline pre-PCI (up to first pressure registration). | No Yes | 80 (3.4) |
| Adrenaline after PCI Adrenaline use in the first 24 h after PCI. | No Yes, continued Yes, initiated in HCK Yes, initiated <24 h after leaving HCK | 75 (3.2) |
| Dopamine prior to PCI Whether or not a patient received dopamine pre-PCI (up to first pressure registration). | Yes No | 34 (1.5) |
| Dopamine after PCI Dopamine use in the first 24 h after PCI. | No Yes, continued Yes, initiated in HCK Yes, initiated <24 h after leaving HCK | 54 (2.3) |
| Lactate after PCI—mmol/L Blood lactate level measured 6–24 h after PCI. When more than one measurement is available, the highest value should be registered. | Continuous (0.0–40.0) | 866 (37.2) |
| SOFA score on admission Sequential organ failure assessment score on ICU admission. | Continuous (6–24) | 1888 (81.1) |
| SOFA score after 24 h Sequential organ failure assessment score 24 h after ICU admission. | Continuous (6–24) | 1954 (83.9) |
| Discharge Days between admission date and discharge date. | Continuous | 542 (23.3) |
| Heart transplant Patient received a heart transplant or a heart–lung transplant. | No Yes | 0 (0) |
| Days after PCI Days between PCI and heart transplant/heart–lung transplant. | Continuous | 2 (0.1) |
| VAD Patient received a permanent ventricular assist device. | No Yes | 0 (0) |
| Days after PCI Days between PCI and implantation of permanent ventricular assist device. | Continuous | 4 (0.2) |
| Cause of death (ARC-2) [25] Cardiovascular death: death resulting from cardiovascular causes. The following categories may be collected: 1. Death caused by acute MI; 2. Death caused by sudden cardiac (including unwitnessed) death; 3. Death resulting from heart failure; 4. Death caused by stroke; 5. Death caused by cardiovascular procedures; 6. Death resulting from cardiovascular hemorrhage; 7. Death resulting from other cardiovascular cause. Non-cardiovascular death: death that is not thought to be the results of a cardiovascular cause. The following categories may be collected: 1. Death resulting from malignancy; 2. Death resulting from pulmonary causes; 3. Death caused by infection (includes sepsis); 4. Death resulting from gastrointestinal causes; 5. Death resulting from accident/trauma; 6. Death caused by other non-cardiovascular organ failure; 7. Death resulting from other non-cardiovascular cause. | Unknown Cardiovascular death Non-cardiovascular death | 96 (4.1) |
References
- Goldberg, R.J.; Samad, N.A.; Yarzebski, J.; Gurwitz, J.; Bigelow, C.; Gore, J.M. Temporal trends in cardiogenic shock complicating acute myocardial infarction. N. Engl. J. Med. 1999, 340, 1162–1168. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M.J.; Rueda, F.; Labata, C.; Oliveras, T.; Montero, S.; Ferrer, M.; El Ouaddi, N.; Serra, J.; Lupon, J.; Bayes-Genis, A.; et al. Non-STEMI vs. STEMI Cardiogenic Shock: Clinical Profile and Long-Term Outcomes. J. Clin. Med. 2022, 11, 3558. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Koganti, S.; Iqbal, M.B.; Jain, A.K.; Kalra, S.S.; Astroulakis, Z.; Lim, P.; Rakhit, R.; Dalby, M.C.; Lockie, T.; et al. Contemporary trends in cardiogenic shock: Incidence, intra-aortic balloon pump utilisation and outcomes from the London Heart Attack Group. Eur. Heart J. Acute Cardiovasc. Care 2018, 7, 16–27. [Google Scholar] [CrossRef]
- Helgestad, O.K.L.; Josiassen, J.; Hassager, C.; Jensen, L.O.; Holmvang, L.; Sorensen, A.; Frydland, M.; Lassen, A.T.; Udesen, N.L.J.; Schmidt, H.; et al. Temporal trends in incidence and patient characteristics in cardiogenic shock following acute myocardial infarction from 2010 to 2017: A Danish cohort study. Eur. J. Heart Fail. 2019, 21, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- OECD. Health at a Glance: Europe 2020; OECD: Paris, France, 2020. [Google Scholar] [CrossRef]
- De Luca, L.; Olivari, Z.; Farina, A.; Gonzini, L.; Lucci, D.; Di Chiara, A.; Casella, G.; Chiarella, F.; Boccanelli, A.; Di Pasquale, G.; et al. Temporal trends in the epidemiology, management, and outcome of patients with cardiogenic shock complicating acute coronary syndromes. Eur. J. Heart Fail. 2015, 17, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Kunadian, V.; Qiu, W.; Ludman, P.; Redwood, S.; Curzen, N.; Stables, R.; Gunn, J.; Gershlick, A.; National Institute for Cardiovascular Outcomes Research. Outcomes in patients with cardiogenic shock following percutaneous coronary intervention in the contemporary era: An analysis from the BCIS database (British Cardiovascular Intervention Society). JACC Cardiovasc. Interv. 2014, 7, 1374–1385. [Google Scholar] [CrossRef]
- Timmermans, M.J.C.; Houterman, S.; Daeter, E.D.; Danse, P.W.; Li, W.W.; Lipsic, E.; Roefs, M.M.; van Veghel, D. Using real-world data to monitor and improve quality of care in coronary artery disease: Results from the Netherlands Heart Registration. Neth. Heart J. 2022, 30, 546–556. [Google Scholar] [CrossRef]
- Houterman, S.; van Dullemen, A.; Versteegh, M.; Aengevaeren, W.; Danse, P.; Brinkman, E.; Schuurman, D.; van Veghel, D. Data quality and auditing within the Netherlands Heart Registration: Using the PCI registry as an example. Neth. Heart J. 2023. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.N.; Kaier, K.; Zotzmann, V.; Stachon, P.; Pottgiesser, T.; von Zur Muehlen, C.; Zehender, M.; Duerschmied, D.; Schmid, B.; Bode, C.; et al. Cardiogenic shock: Incidence, survival and mechanical circulatory support usage 2007-2017-insights from a national registry. Clin. Res. Cardiol. 2021, 110, 1421–1430. [Google Scholar] [CrossRef] [PubMed]
- Kolte, D.; Khera, S.; Aronow, W.S.; Mujib, M.; Palaniswamy, C.; Sule, S.; Jain, D.; Gotsis, W.; Ahmed, A.; Frishman, W.H.; et al. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J. Am. Heart Assoc. 2014, 3, e000590. [Google Scholar] [CrossRef]
- Thiele, H.; Akin, I.; Sandri, M.; Fuernau, G.; de Waha, S.; Meyer-Saraei, R.; Nordbeck, P.; Geisler, T.; Landmesser, U.; Skurk, C.; et al. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N. Engl. J. Med. 2017, 377, 2419–2432. [Google Scholar] [CrossRef] [PubMed]
- Ostenfeld, S.; Lindholm, M.G.; Kjaergaard, J.; Bro-Jeppesen, J.; Moller, J.E.; Wanscher, M.; Hassager, C. Prognostic implication of out-of-hospital cardiac arrest in patients with cardiogenic shock and acute myocardial infarction. Resuscitation 2015, 87, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Poss, J.; Koster, J.; Fuernau, G.; Eitel, I.; de Waha, S.; Ouarrak, T.; Lassus, J.; Harjola, V.P.; Zeymer, U.; Thiele, H.; et al. Risk Stratification for Patients in Cardiogenic Shock After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2017, 69, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Arias, F.G.; Alonso-Fernandez-Gatta, M.; Dominguez, M.P.; Martinez, J.M.; Veloso, P.R.; Bermejo, R.M.A.; Alvarez, D.I.; Merchan-Gomez, S.; Diego-Nieto, A.; Casas, C.A.J.; et al. Predictive Model and Risk Score for In-Hospital Mortality in Patients with All-Cause Cardiogenic Shock. Int. Heart J. 2022, 63, 1034–1040. [Google Scholar] [CrossRef]
- Castillo Costa, Y.; Delfino, F.; Mauro, V.; D’Imperio, H.; Barrero, C.; Charask, A.; Zoni, R.; Macín, S.; Perna, E.; Gagliardi, J. Clinical characteristics and evolution of patients with cardiogenic shock in Argentina in the context of an acute myocardial infarction with ST segment elevation. Data from the nationwide ARGEN-IAM-ST Registry. Curr. Probl. Cardiol. 2022, 48, 101468. [Google Scholar] [CrossRef] [PubMed]
- Hochman, J.S.; Sleeper, L.A.; Webb, J.G.; Sanborn, T.A.; White, H.D.; Talley, J.D.; Buller, C.E.; Jacobs, A.K.; Slater, J.N.; Col, J.; et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N. Engl. J. Med. 1999, 341, 625–634. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.L.; Peterson, E.D.; Peng, S.A.; Wang, T.Y.; Ohman, E.M.; Bhatt, D.L.; Saucedo, J.F.; Roe, M.T. Differences in the profile, treatment, and prognosis of patients with cardiogenic shock by myocardial infarction classification: A report from NCDR. Circ. Cardiovasc. Qual. Outcomes 2013, 6, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Van Diepen, S.; Katz, J.N.; Albert, N.M.; Henry, T.D.; Jacobs, A.K.; Kapur, N.K.; Kilic, A.; Menon, V.; Ohman, E.M.; Sweitzer, N.K.; et al. Contemporary Management of Cardiogenic Shock A Scientific Statement From the American Heart Association. Circulation 2017, 136, E232–E268. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed]
- Rusnak, J.; Schupp, T.; Weidner, K.; Ruka, M.; Egner-Walter, S.; Forner, J.; Bertsch, T.; Kittel, M.; Mashayekhi, K.; Tajti, P.; et al. Differences in Outcome of Patients with Cardiogenic Shock Associated with In-Hospital or Out-of-Hospital Cardiac Arrest. J. Clin. Med. 2023, 12, 2604. [Google Scholar] [CrossRef]
- Thiele, H.; Zeymer, U.; Neumann, F.J.; Ferenc, M.; Olbrich, H.G.; Hausleiter, J.; Richardt, G.; Hennersdorf, M.; Empen, K.; Fuernau, G.; et al. Intraaortic Balloon Support for Myocardial Infarction with Cardiogenic Shock. N. Engl. J. Med. 2012, 367, 1287–1296. [Google Scholar] [CrossRef] [PubMed]
- Von Lewinski, D.; Herold, L.; Stoffel, C.; Pätzold, S.; Fruhwald, F.; Altmanninger-Sock, S.; Kolesnik, E.; Wallner, M.; Rainer, P.; Bugger, H.; et al. PRospective REgistry of PAtients in REfractory cardiogenic shock-The PREPARE CardShock registry. Catheter. Cardiovasc. Interv. 2022, 100, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Adelsheimer, A.; Wang, J.; Lu, D.Y.; Elbaum, L.; Krishnan, U.; Cheung, J.W.; Feldman, D.N.; Wong, S.C.; Horn, E.M.; Sobol, I.; et al. Impact of Socioeconomic Status on Mechanical Circulatory Device Utilization and Outcomes in Cardiogenic Shock. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100027. [Google Scholar] [CrossRef]
- Garcia-Garcia, H.M.; McFadden, E.P.; Farb, A.; Mehran, R.; Stone, G.W.; Spertus, J.; Onuma, Y.; Morel, M.A.; van Es, G.A.; Zuckerman, B.; et al. Standardized End Point Definitions for Coronary Intervention Trials: The Academic Research Consortium-2 Consensus Document. Eur. Heart J. 2018, 39, 2192–2207. [Google Scholar] [CrossRef] [PubMed]

| All Patients (n = 2328) | Alive at 30 Days (n = 1414) | Dead at 30 Days (n = 901) | p-Value | ||
|---|---|---|---|---|---|
| Patient characteristics | |||||
| Male | 1696 (72.9) | 1036 (73.3) | 649 (72.0) | 0.515 | |
| Age—years | 66.4 (±12.3) | 64.8 (±12.1) | 69.0 (±12.1) | <0.001 | |
| BMI—kg/cm2 | 26.1 (23.9–29.1) | 25.9 (23.7–28.8) | 26.2 (24.2–29.4) | 0.024 | |
| Indication of PCI | 0.005 | ||||
| STEMI | 1941/2254 (86.1) | 1193/1359 (87.8) | 737/882 (83.6) | ||
| NSTEMI | 313/2254 (13.9) | 166/1359 (12.2) | 145/882 (16.4) | ||
| Out-of-hospital cardiac arrest | 934/2317 (40.3) | 497/1405 (35.4) | 432/899 (48.1) | <0.001 | |
| In-hospital cardiac arrest | 295 /2308 (12.8) | 130/1401 (9.3) | 165/894 (18.5) | <0.001 | |
| Onset of AMI symptoms—hours | <0.001 | ||||
| <3 | 1166/1991 (58.6) | 745/1233 (60.4) | 416/746 (55.8) | ||
| 3–12 | 375/1991 (18.8) | 245/1233 (19.9) | 128/746 (17.2) | ||
| 12–24 | 113/1991 (5.7) | 67/1233 (5.4) | 44/746 (5.9) | ||
| >24 | 337/1991 (16.9) | 176/1233 (14.3) | 158/746 (21.2) | ||
| Intubation pre-PCI | 1030/2307 (44.6) | 500/1404 (35.6) | 524/893 (58.7) | <0.001 | |
| Monitoring via PA catheter | 118/2119 (5.6) | 68/1287 (5.3) | 49/832 (5.9) | 0.613 | |
| Medical history | |||||
| Diabetes | 463/2219 (20.9) | 227/1365 (16.6) | 232/841 (27.6) | <0.001 | |
| Prior coronary event | 631/2153 (29.3) | 361/1310 (27.6) | 265/831 (31.9) | 0.032 | |
| Prior MI | 482/2253 (21.4) | 276/1374 (20.1) | 202/867 (23.3) | 0.071 | |
| Prior PCI | 396/2134 (18.6) | 239/1299 (18.4) | 153/822 (18.6) | 0.901 | |
| Prior CABG | 139/2286 (6.1) | 74/1390 (5.3) | 65/833 (7.4) | 0.048 | |
| Hemodynamics on admission | |||||
| Systolic blood pressure—mmHg | 100 (80–125) | 103 (83–127) | 95 (80–118) | <0.001 | |
| Diastolic blood pressure—mmHg | 61 (50–77) | 64 (50–80) | 60 (48–75) | <0.001 | |
| Mean blood pressure—mmHg | 75 (60–93) | 77 (63–95) | 72 (58–89) | <0.001 | |
| Heart rate—bpm | 82 (63–101) | 80 (60–100) | 89 (70–108) | <0.001 | |
| Shock index | 0.76 (0.58–1.0) | 0.72 (0.56–0.95) | 0.86 (0.64–1.14) | <0.001 | |
| Number of vasoactive agents pre-PCI | <0.001 | ||||
| None | 1147/2215 (51.8) | 833/1356 (61.4) | 309/846 (36.5) | ||
| 1 | 590/2215 (26.6) | 320/1356 (23.6) | 267/846 (31.6) | ||
| 2 | 376/2215 (17.0) | 171/1356 (12.6) | 201/846 (23.8) | ||
| ≥3 | 102/2215 (4.6) | 32/1356 (2.3) | 69/846 (8.1) | ||
| Laboratory values on admission | |||||
| Lactate—mmol/L | 5.5 (2.6–9.4) | 4.2 (2.1–7.2) | 7.8 (3.9–11.4) | <0.001 | |
| Creatinine—µmol/L | 100 (82–123) | 94 (78–113) | 110 (91–140) | <0.001 | |
| eGFR—mL/min | 61 (48–75) | 65 (53–80) | 54 (40–67) | <0.001 | |
| Hemoglobin—mmol/L | 8.3 (±1.4) | 8.4 (±1.3) | 8.1 (±1.5) | <0.001 | |
| Glucose—mmol/L | 12.2 (8.8–17.1) | 10.8 (8.3–14.9) | 14.8 (10.4–19.9) | <0.001 | |
| Peak hs-troponin-T—ng/L a | 3534 (828–10000) | 3292 (831–10000) | 3954 (772–10000) | 0.095 | |
| Peak CK-MB—U/L a | 222 (70–510) | 203 (67–446) | 269 (77–600) | 0.013 | |
| Angiographic features | |||||
| Multivessel disease | 1402/2307 (60.8) | 791 / 1399 (56.5) | 603 / 895 (67.4) | <0.001 | |
| Number of treated vessels | <0.001 | ||||
| 1 | 1749/2114 (82.7) | 1115/1295 (86.1) | 623/806 (77.3) | ||
| ≥2 | 365/2114 (17.3) | 1801295 (13.9) | 183/806 (22.7) | ||
| Treated vessel | |||||
| Left main | 292/2114 (13.8) | 142/1295 (11.0) | 149/806 (18.5) | <0.001 | |
| Left anterior descending | 970/2114 (45.9) | 576/1295 (44.5) | 388/806 (48.1) | 0.102 | |
| Circumflex artery | 479/2114 (22.7) | 250/1295 (19.3) | 226/806 (28.0) | <0.001 | |
| Right coronary artery | 794/2114 (37.6) | 534/1295 (41.2) | 254/806 (31.5) | <0.001 | |
| Venous or arterial graft | 30/2114 (1.4) | 14/1295 (1.1) | 16/806 (2.0) | 0.103 | |
| TIMI flow before PCI | 0.721 | ||||
| 0/1 | 1487/1943 (76.5) | 905/1189 (76.1) | 575/744 (77.3) | ||
| 2 | 208/1943 (10.7) | 132/1189 (11.1) | 74/744 (9.9) | ||
| 3 | 248/1943 (12.8) | 152/1189 (12.8) | 95/744 (12.8) | ||
| TIMI flow after PCI | <0.001 | ||||
| 0/1 | 182/1999 (9.1) | 54/1255 (4.3) | 128/735 (17.4) | ||
| 2 | 193/1999 (9.7) | 111/1255 (8.8) | 81/735 (11.0) | ||
| 3 | 1624/1999 (81.3) | 1090/1255 (86.9) | 526/735 (71.6) | ||
| Arterial access | <0.001 | ||||
| Radial | 1013/2053 (49.3) | 718/1242 (57.8) | 288/798 (36.1) | ||
| Femoral | 1032/2053 (50.3) | 521/1242 (41.9) | 505/798 (63.3) | ||
| Other | 8/2040 (0.3) | 3/1242 (0.3) | 5/798 (0.7) | ||
| Outcome | |||||
| Length of hospital stay—days | 5 (1–12) | 10 (2–24) | 2 (0–6) | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, E.J.; Berg, S.t.; Bogerd, M.; Timmermans, M.J.C.; Kraaijeveld, A.O.; Bunge, J.J.H.; Teeuwen, K.; Lipsic, E.; Sjauw, K.D.; Geuns, R.-J.M.v.; et al. Characteristics, Treatment Strategies and Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: A Contemporary Dutch Cohort. J. Clin. Med. 2023, 12, 5221. https://doi.org/10.3390/jcm12165221
Peters EJ, Berg St, Bogerd M, Timmermans MJC, Kraaijeveld AO, Bunge JJH, Teeuwen K, Lipsic E, Sjauw KD, Geuns R-JMv, et al. Characteristics, Treatment Strategies and Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: A Contemporary Dutch Cohort. Journal of Clinical Medicine. 2023; 12(16):5221. https://doi.org/10.3390/jcm12165221
Chicago/Turabian StylePeters, Elma J., Sanne ten Berg, Margriet Bogerd, Marijke J. C. Timmermans, Adriaan O. Kraaijeveld, Jeroen J. H. Bunge, Koen Teeuwen, Erik Lipsic, Krischan D. Sjauw, Robert-Jan M. van Geuns, and et al. 2023. "Characteristics, Treatment Strategies and Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: A Contemporary Dutch Cohort" Journal of Clinical Medicine 12, no. 16: 5221. https://doi.org/10.3390/jcm12165221
APA StylePeters, E. J., Berg, S. t., Bogerd, M., Timmermans, M. J. C., Kraaijeveld, A. O., Bunge, J. J. H., Teeuwen, K., Lipsic, E., Sjauw, K. D., Geuns, R.-J. M. v., Dedic, A., Dubois, E. A., Meuwissen, M., Danse, P., Verouden, N. J. W., Bleeker, G., Cabezas, J. M. M., Ferreira, I. A., Engström, A. E., ... on behalf of the Participating Centers of the PCI Registration Committee of the Netherlands Heart Registration. (2023). Characteristics, Treatment Strategies and Outcome in Cardiogenic Shock Complicating Acute Myocardial Infarction: A Contemporary Dutch Cohort. Journal of Clinical Medicine, 12(16), 5221. https://doi.org/10.3390/jcm12165221







