Impact of Advanced Age on the Incidence of Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stable Coronary Artery Disease in a Real-World Setting in Spain
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Source
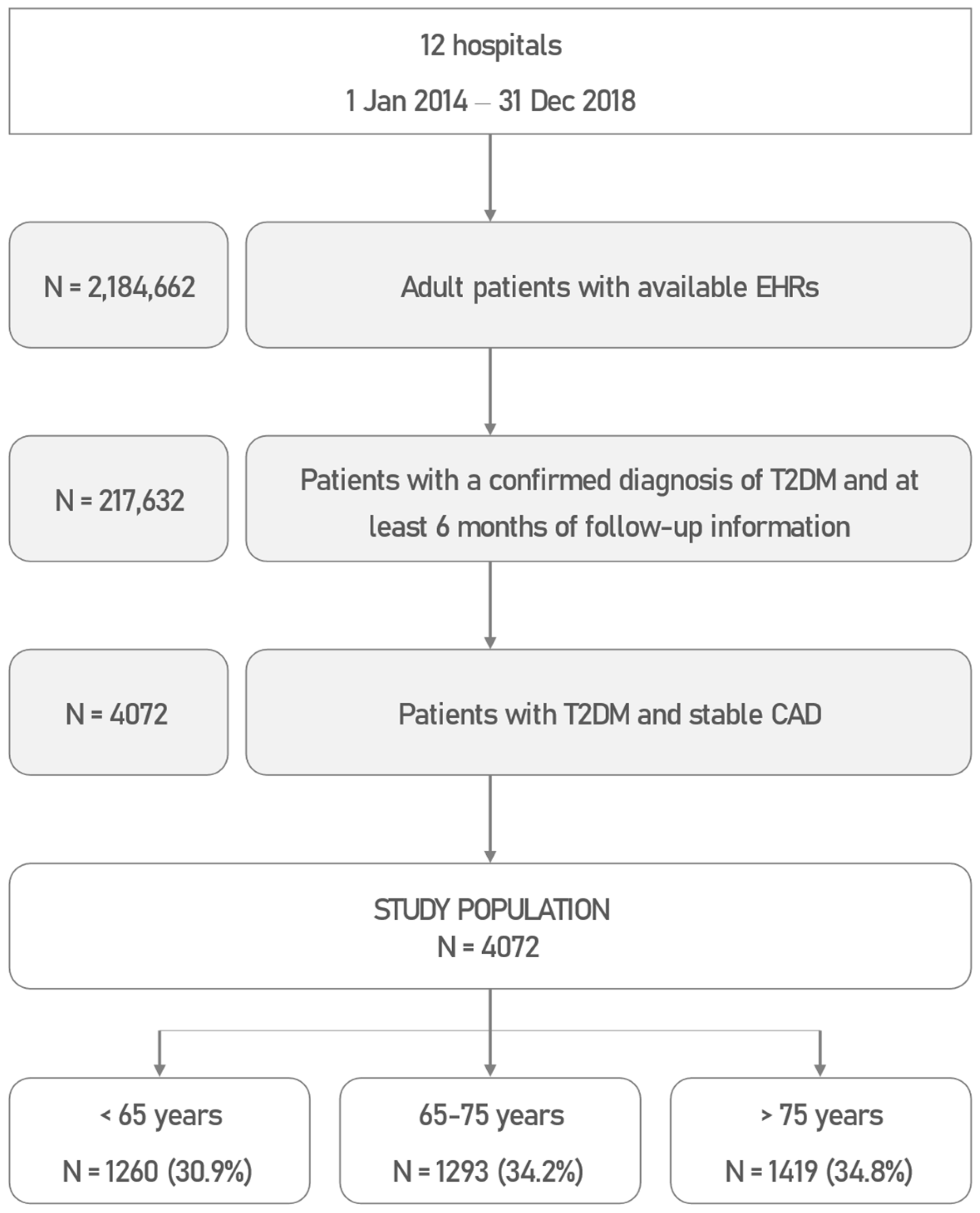
2.3. Study Population
2.4. Extraction of Clinical Data from EHRs
2.5. Statistical Data Analyses
2.6. Ethical Considerations and Study Approval
3. Results
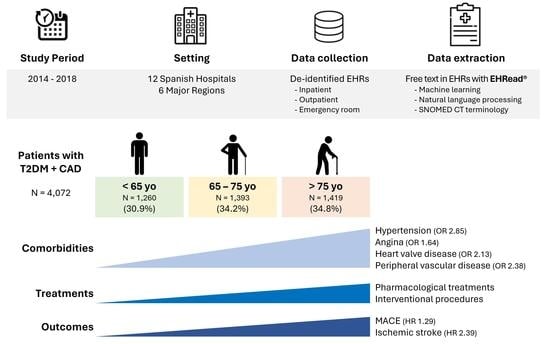
3.1. Study Population
3.2. Patient Demographic and Clinical Characteristics
3.3. Pharmacological and Interventional Disease Management
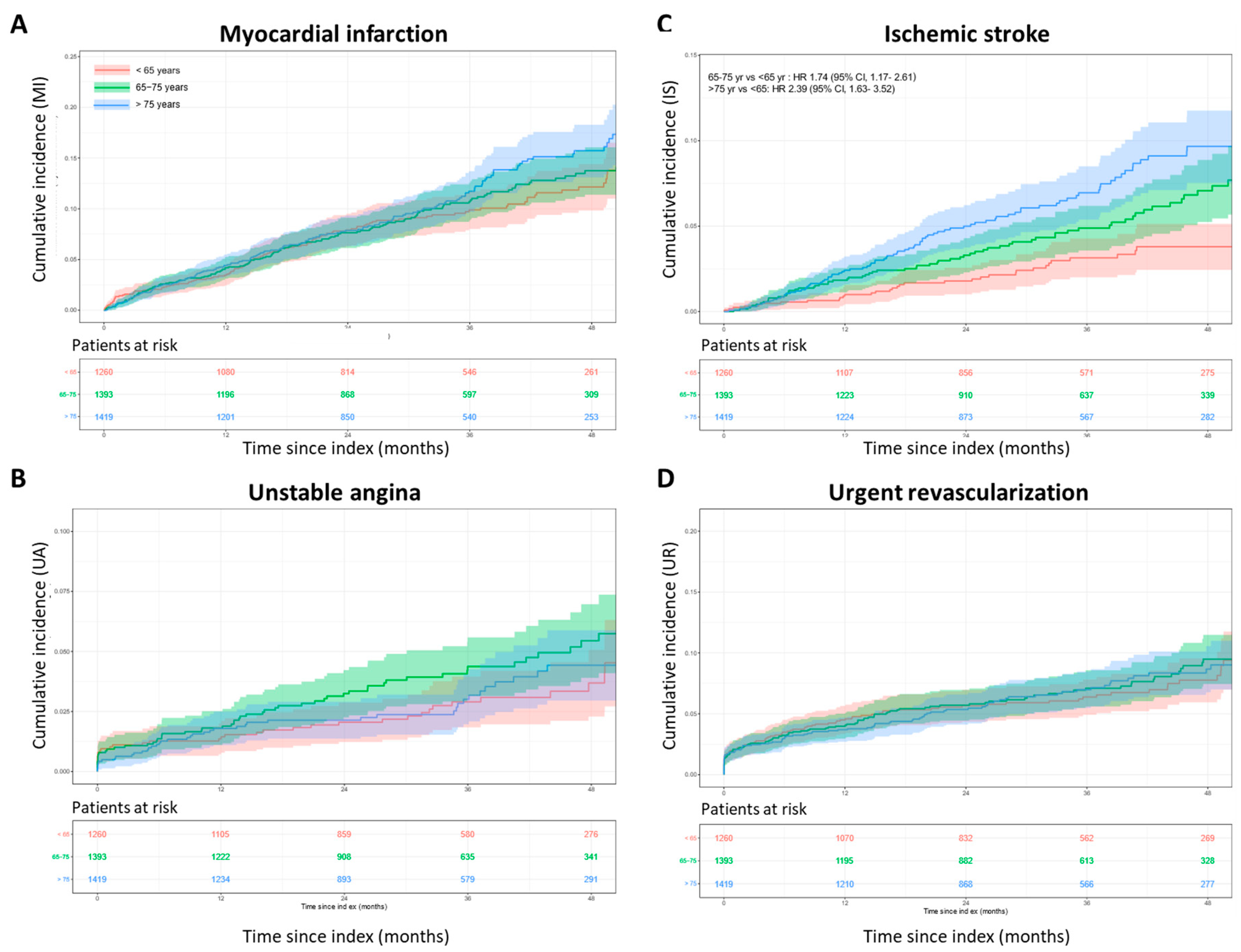
3.4. Cumulative Incidence of MACE
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACE | angiotensin-converting enzyme |
| AEMPS | Agencia Española de Medicamentos y Productos Sanitarios (from Spanish, Agency of Medicines and Health Products) |
| AHA | American Heart Association |
| ARB | angiotensin receptor blocker |
| ASA | acetylsalicylic acid |
| BMI | body mass index |
| CABG | coronary artery bypass grafting |
| CAD | coronary artery disease |
| CI | confidence interval |
| COPD | chronic obstructive pulmonary disease |
| CVD | cardiovascular disease |
| CVOTs | cardiovascular outcome trials |
| DPPi | dipeptidyl peptidase-4 inhibitor |
| EHR | electronic health record |
| EMA | European Medicines Agency |
| FA | fast-acting |
| FDA | Food and Drug Administration |
| GLP1-RA | glucagon-like peptide 1 receptor agonist |
| HbA1c | glycated hemoglobin, type A1c |
| HDL | high-density lipoprotein cholesterol |
| HR | hazard ratio |
| ICD | International Classification of Diseases |
| IDF | International Diabetes Federation |
| IQR | interquartile range |
| IRB | Institutional Review Board |
| iSGLT2 | sodium–glucose cotransporter 2 inhibitor |
| KM | Kaplan–Meier |
| LA | long-acting |
| LDL | low-density lipoprotein cholesterol |
| LVEF | left ventricular ejection fraction |
| MACE | major cardiovascular event |
| MI | myocardial infarction |
| ML | machine learning |
| NLP | natural language processing |
| OR | odds ratio |
| PCI | percutaneous coronary intervention |
| PH | proportional hazards |
| SD | standard deviation |
| T2DM | type 2 diabetes mellitus |
| USD | United States Dollar |
References
- World Health Organization (WHO). Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 20 April 2023).
- Lin, X.; Xu, Y.; Pan, X.; Xu, J.; Ding, Y.; Sun, X.; Song, X.; Ren, Y.; Shan, P.F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: An analysis from 1990 to 2025. Sci. Rep. 2020, 10, 14790. [Google Scholar] [CrossRef]
- Sun, H.; Saeedi, P.; Karuranga, S.; Pinkepank, M.; Ogurtsova, K.; Duncan, B.B.; Stein, C.; Basit, A.; Chan, J.C.N.; Mbanya, J.C.; et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res. Clin. Pract. 2022, 183, 109119. [Google Scholar] [CrossRef]
- International Diabetes Federation (IDF). IDF Diabetes Atlas—10th Edition. Available online: https://diabetesatlas.org/data/en/world/ (accessed on 26 April 2023).
- Nwaneri, C.; Cooper, H.; Bowen-Jones, D. Mortality in type 2 diabetes mellitus: Magnitude of the evidence from a systematic review and meta-analysis. Br. J. Diabetes Vasc. Dis. 2013, 13, 192–207. [Google Scholar] [CrossRef]
- Cannon, A.; Handelsman, Y.; Heile, M.; Shannon, M. Burden of Illness in Type 2 Diabetes Mellitus. J. Manag. Care Spec. Pharm. 2018, 24, S5–S13. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Cuevas, J.; Santos, A.; Armendariz-Borunda, J. Pathophysiological Molecular Mechanisms of Obesity: A Link between MAFLD and NASH with Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 11629. [Google Scholar] [CrossRef]
- Alberti, K.G.; Zimmet, P.Z. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: Diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet. Med. A J. Br. Diabet. Assoc. 1998, 15, 539–553. [Google Scholar] [CrossRef]
- The Emerging Risk Factors, C. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: A collaborative meta-analysis of 102 prospective studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 diabetes mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Shah, A.D.; Langenberg, C.; Rapsomaniki, E.; Denaxas, S.; Pujades-Rodriguez, M.; Gale, C.P.; Deanfield, J.; Smeeth, L.; Timmis, A.; Hemingway, H. Type 2 diabetes and incidence of cardiovascular diseases: A cohort study in 1·9 million people. Lancet. Diabetes Endocrinol. 2015, 3, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Araujo, G.; Nakagami, H. Pathophysiology of cardiovascular disease in diabetes mellitus. Cardiovasc. Endocrinol. Metab. 2018, 7, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Lee, W.L.; Cheung, A.M.; Cape, D.; Zinman, B. Impact of diabetes on coronary artery disease in women and men: A meta-analysis of prospective studies. Diabetes Care 2000, 23, 962–968. [Google Scholar] [CrossRef]
- Peters, S.A.; Huxley, R.R.; Woodward, M. Diabetes as risk factor for incident coronary heart disease in women compared with men: A systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia 2014, 57, 1542–1551. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, A.M.; Grady, D.; Barrett-Connor, E. Explaining the sex difference in coronary heart disease mortality among patients with type 2 diabetes mellitus: A meta-analysis. Arch. Intern. Med. 2002, 162, 1737–1745. [Google Scholar] [CrossRef]
- Rodgers, J.L.; Jones, J.; Bolleddu, S.I.; Vanthenapalli, S.; Rodgers, L.E.; Shah, K.; Karia, K.; Panguluri, S.K. Cardiovascular Risks Associated with Gender and Aging. J. Cardiovasc. Dev. Dis. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). NICE Guideline, No. 28. Type 2 diabetes in adults: Management. In Type 2 Diabetes in Adults: Management; National Institute for Health and Care Excellence (NICE): London, UK, 2022. [Google Scholar]
- White, J.R., Jr. A Brief History of the Development of Diabetes Medications. Diabetes Spectr. A Publ. Am. Diabetes Assoc. 2014, 27, 82–86. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association Professional Practice Committee. 9. Pharmacologic Approaches to Glycemic Treatment: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S125–S143. [Google Scholar] [CrossRef]
- Davies, M.J.; Drexel, H.; Jornayvaz, F.R.; Pataky, Z.; Seferović, P.M.; Wanner, C. Cardiovascular outcomes trials: A paradigm shift in the current management of type 2 diabetes. Cardiovasc. Diabetol. 2022, 21, 144. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Gragnano, F.; Gallinoro, E.; Cesaro, A.; Sardu, C.; Mileva, N.; Foa, A.; Armillotta, M.; Sansonetti, A.; et al. Outcomes in diabetic patients treated with SGLT2-Inhibitors with acute myocardial infarction undergoing PCI: The SGLT2-I AMI PROTECT Registry. Pharmacol. Res. 2023, 187, 106597. [Google Scholar] [CrossRef]
- Paolisso, P.; Bergamaschi, L.; Cesaro, A.; Gallinoro, E.; Gragnano, F.; Sardu, C.; Mileva, N.; Foa, A.; Armillotta, M.; Sansonetti, A.; et al. Impact of SGLT2-inhibitors on contrast-induced acute kidney injury in diabetic patients with acute myocardial infarction with and without chronic kidney disease: Insight from SGLT2-I AMI PROTECT registry. Diabetes Res. Clin. Pract. 2023, 202, 110766. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, M.V.; Gersh, B.J.; Alexander, K.P.; Granger, C.B.; Stone, G.W. Coronary Artery Disease in Patients >/=80 Years of Age. J. Am. Coll. Cardiol. 2018, 71, 2015–2040. [Google Scholar] [CrossRef]
- Dodd, K.S.; Saczynski, J.S.; Zhao, Y.; Goldberg, R.J.; Gurwitz, J.H. Exclusion of older adults and women from recent trials of acute coronary syndromes. J. Am. Geriatr. Soc. 2011, 59, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Carpena-Ruiz, M.; Montero-Errasquín, B.; Sánchez-Castellano, C.; Sánchez-García, E. Exclusion of older adults from ongoing clinical trials about type 2 diabetes mellitus. J. Am. Geriatr. Soc. 2013, 61, 734–738. [Google Scholar] [CrossRef]
- Bourgeois, F.T.; Orenstein, L.; Ballakur, S.; Mandl, K.D.; Ioannidis, J.P.A. Exclusion of Elderly People from Randomized Clinical Trials of Drugs for Ischemic Heart Disease. J. Am. Geriatr. Soc. 2017, 65, 2354–2361. [Google Scholar] [CrossRef]
- Vitale, C.; Fini, M.; Spoletini, I.; Lainscak, M.; Seferovic, P.; Rosano, G.M. Under-representation of elderly and women in clinical trials. Int. J. Cardiol. 2017, 232, 216–221. [Google Scholar] [CrossRef]
- Ruiter, R.; Burggraaf, J.; Rissmann, R. Under-representation of elderly in clinical trials: An analysis of the initial approval documents in the Food and Drug Administration database. Br. J. Clin. Pharmacol. 2019, 85, 838–844. [Google Scholar] [CrossRef]
- González-Juanatey, C.; Anguita-Sánchez, M.; Barrios, V.; Núñez-Gil, I.; Gómez-Doblas, J.J.; García-Moll, X.; Lafuente-Gormaz, C.; Rollán-Gómez, M.J.; Peral-Disdier, V.; Martínez-Dolz, L.; et al. Major Adverse Cardiovascular Events in Coronary Type 2 Diabetic Patients: Identification of Associated Factors Using Electronic Health Records and Natural Language Processing. J. Clin. Med. 2022, 11, 6004. [Google Scholar] [CrossRef] [PubMed]
- Blin, P.; Darmon, P.; Henry, P.; Guiard, E.; Bernard, M.A.; Dureau-Pournin, C.; Maizi, H.; Thomas-Delecourt, F.; Lassalle, R.; Droz-Perroteau, C.; et al. Patients with stable coronary artery disease and type 2 diabetes but without prior myocardial infarction or stroke and THEMIS-like patients: Real-world prevalence and risk of major outcomes from the SNDS French nationwide claims database. Cardiovasc. Diabetol. 2021, 20, 229. [Google Scholar] [CrossRef]
- Choi, B.G.; Rha, S.W.; Yoon, S.G.; Choi, C.U.; Lee, M.W.; Kim, S.W. Association of Major Adverse Cardiac Events up to 5 Years in Patients with Chest Pain without Significant Coronary Artery Disease in the Korean Population. J. Am. Heart Assoc. 2019, 8, e010541. [Google Scholar] [CrossRef]
- Canales, L.; Menke, S.; Marchesseau, S.; D’Agostino, A.; Del Rio-Bermudez, C.; Taberna, M.; Tello, J. Assessing the Performance of Clinical Natural Language Processing Systems: Development of an Evaluation Methodology. JMIR Med. Inform. 2021, 9, e20492. [Google Scholar] [CrossRef] [PubMed]
- González-Juanatey, C.; Anguita-Sá Nchez, M.; Barrios, V.; Núñez-Gil, I.; Gómez-Doblas, J.J.; García-Moll, X.; Lafuente-Gormaz, C.; Rollán-Gómez, M.J.; Peral-Disdie, V.; Martínez-Dolz, L.; et al. Assessment of medical management in Coronary Type 2 Diabetic patients with previous percutaneous coronary intervention in Spain: A retrospective analysis of electronic health records using Natural Language Processing. PLoS ONE 2022, 17, e0263277. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.L.; Almonacid, C.; González, Y.; Del Rio-Bermudez, C.; Ancochea, J.; Cárdenas, R.; Lumbreras, S.; Soriano, J.B. The impact of COVID-19 on patients with asthma. Eur. Respir. J. 2021, 57, 2003142. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, J.L.; Ancochea, J.; Soriano, J.B. Clinical Characteristics and Prognostic Factors for Intensive Care Unit Admission of Patients with COVID-19: Retrospective Study Using Machine Learning and Natural Language Processing. J. Med. Internet Res. 2020, 22, e21801. [Google Scholar] [CrossRef] [PubMed]
- Sheikhalishahi, S.; Miotto, R.; Dudley, J.T.; Lavelli, A.; Rinaldi, F.; Osmani, V. Natural Language Processing of Clinical Notes on Chronic Diseases: Systematic Review. JMIR Med. Inform. 2019, 7, e12239. [Google Scholar] [CrossRef]
- Goldstein, B.A.; Navar, A.M.; Pencina, M.J.; Ioannidis, J.P. Opportunities and challenges in developing risk prediction models with electronic health records data: A systematic review. J. Am. Med. Inform. Assoc. JAMIA 2017, 24, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Thompson, W.K.; Herr, T.M.; Zeng, Z.; Berendsen, M.A.; Jonnalagadda, S.R.; Carson, M.B.; Starren, J. Natural Language Processing for EHR-Based Pharmacovigilance: A Structured Review. Drug Saf. 2017, 40, 1075–1089. [Google Scholar] [CrossRef]
- Ancochea, J.; Izquierdo, J.L.; Soriano, J.B. Evidence of Gender Differences in the Diagnosis and Management of Coronavirus Disease 2019 Patients: An Analysis of Electronic Health Records Using Natural Language Processing and Machine Learning. J. Women’s Health 2021, 30, 393–404. [Google Scholar] [CrossRef]
- Graziani, D.; Soriano, J.B.; Del Rio-Bermudez, C.; Morena, D.; Díaz, T.; Castillo, M.; Alonso, M.; Ancochea, J.; Lumbreras, S.; Izquierdo, J.L. Characteristics and Prognosis of COVID-19 in Patients with COPD. J. Clin. Med. 2020, 9, 3259. [Google Scholar] [CrossRef]
- Espinosa, L.; Tello, J.; Pardo, A.; Medrano, I.; Ureña, A.; Salcedo, I.; Saggion, H. Savana: A global information extraction and terminology expansion framework in the medical domain. Proces. Leng. Nat. 2016, 57, 23–30. [Google Scholar]
- Benson, T. Principles of Health Interoperability HL7 and SNOMED; Springer: London, UK, 2012. [Google Scholar]
- Timmis, A.; Vardas, P.; Townsend, N.; Torbica, A.; Katus, H.; De Smedt, D.; Gale, C.P.; Maggioni, A.P.; Petersen, S.E.; Huculeci, R.; et al. European Society of Cardiology: Cardiovascular disease statistics 2021. Eur. Heart J. 2022, 43, 716–799. [Google Scholar] [CrossRef]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, S49–S73. [Google Scholar] [CrossRef]
- Chia, C.W.; Egan, J.M.; Ferrucci, L. Age-Related Changes in Glucose Metabolism, Hyperglycemia, and Cardiovascular Risk. Circ. Res. 2018, 123, 886–904. [Google Scholar] [CrossRef] [PubMed]
- Halter, J.B.; Musi, N.; McFarland Horne, F.; Crandall, J.P.; Goldberg, A.; Harkless, L.; Hazzard, W.R.; Huang, E.S.; Kirkman, M.S.; Plutzky, J.; et al. Diabetes and cardiovascular disease in older adults: Current status and future directions. Diabetes 2014, 63, 2578–2589. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Dondi, L.; Andreotti, F.; Ronconi, G.; Calabria, S.; Piccinni, C.; Pedrini, A.; Esposito, I.; Martini, N. Prevalence, prescriptions, outcomes and costs of type 2 diabetes patients with or without prior coronary artery disease or stroke: A longitudinal 5-year claims-data analysis of over 7 million inhabitants. Ther. Adv. Chronic Dis. 2021, 12, 20406223211026390. [Google Scholar] [CrossRef] [PubMed]
- Wittbrodt, E.; Bhalla, N.; Sundell, K.A.; Hunt, P.; Wong, N.D.; Kuster, M.; Mellström, C. Assessment of The High risk and unmEt Need in patients with CAD and type 2 diabetes (ATHENA): US healthcare resource use, cost, and burden of illness in a commercially insured population. J. Diabetes Its Complicat. 2021, 35, 107859. [Google Scholar] [CrossRef] [PubMed]
- Naito, R.; Miyauchi, K. Coronary Artery Disease and Type 2 Diabetes Mellitus. Int. Heart J. 2017, 58, 475–480. [Google Scholar] [CrossRef]
- Fihn, S.D.; Gardin, J.M.; Abrams, J.; Berra, K.; Blankenship, J.C.; Dallas, A.P.; Douglas, P.S.; Foody, J.M.; Gerber, T.C.; Hinderliter, A.L.; et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: Executive summary: A report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2012, 126, 3097–3137. [Google Scholar] [CrossRef] [PubMed]
- Dagenais, G.R.; Lu, J.; Faxon, D.P.; Kent, K.; Lago, R.M.; Lezama, C.; Hueb, W.; Weiss, M.; Slater, J.; Frye, R.L. Effects of optimal medical treatment with or without coronary revascularization on angina and subsequent revascularizations in patients with type 2 diabetes mellitus and stable ischemic heart disease. Circulation 2011, 123, 1492–1500. [Google Scholar] [CrossRef]
- Verma, S.; Farkouh, M.E.; Yanagawa, B.; Fitchett, D.H.; Ahsan, M.R.; Ruel, M.; Sud, S.; Gupta, M.; Singh, S.; Gupta, N.; et al. Comparison of coronary artery bypass surgery and percutaneous coronary intervention in patients with diabetes: A meta-analysis of randomised controlled trials. Lancet Diabetes Endocrinol. 2013, 1, 317–328. [Google Scholar] [CrossRef]
- Wang, H.; Wang, H.; Wei, Y.; Li, X.; Jhummun, V.; Ahmed, M.A. Ten-Year Outcomes of Percutaneous Coronary Intervention Versus Coronary Artery Bypass Grafting for Patients with Type 2 Diabetes Mellitus Suffering from Left Main Coronary Disease: A Meta-Analysis. Diabetes Ther. 2021, 12, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Huang, J.; Zhu, K.; Chen, Q. Percutaneous coronary intervention versus coronary artery bypass grafting in patients with coronary heart disease and type 2 diabetes mellitus: Cumulative meta-analysis. Clin. Cardiol. 2021, 44, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Bundhun, P.K.; Wu, Z.J.; Chen, M.H. Coronary artery bypass surgery compared with percutaneous coronary interventions in patients with insulin-treated type 2 diabetes mellitus: A systematic review and meta-analysis of 6 randomized controlled trials. Cardiovasc. Diabetol. 2016, 15, 2. [Google Scholar] [CrossRef]
- Kappetein, A.P.; Head, S.J.; Morice, M.C.; Banning, A.P.; Serruys, P.W.; Mohr, F.W.; Dawkins, K.D.; Mack, M.J. Treatment of complex coronary artery disease in patients with diabetes: 5-year results comparing outcomes of bypass surgery and percutaneous coronary intervention in the SYNTAX trial. Eur. J. Cardio-Thorac. Surg. Off. J. Eur. Assoc. Cardio-Thorac. Surg. 2013, 43, 1006–1013. [Google Scholar] [CrossRef]
- Maggioni, A.P.; Dondi, L.; Andreotti, F.; Ronconi, G.; Calabria, S.; Piccinni, C.; Pedrini, A.; Esposito, I.; Martini, N. Clinical epidemiology and costs of type 2 diabetic patients with or without prior coronary artery disease or stroke. A longitudinal 5-year claims-data analysis of over 7 million inhabitants. Eur. Heart J. 2021, 42, ehab724.2627. [Google Scholar] [CrossRef]
- Lin, P.J.; Kent, D.M.; Winn, A.; Cohen, J.T.; Neumann, P.J. Multiple chronic conditions in type 2 diabetes mellitus: Prevalence and consequences. Am. J. Manag. Care 2015, 21, e23–e34. [Google Scholar]
- Iglay, K.; Hannachi, H.; Joseph Howie, P.; Xu, J.; Li, X.; Engel, S.S.; Moore, L.M.; Rajpathak, S. Prevalence and co-prevalence of comorbidities among patients with type 2 diabetes mellitus. Curr. Med. Res. Opin. 2016, 32, 1243–1252. [Google Scholar] [CrossRef]
- Fortin, M.; Lapointe, L.; Hudon, C.; Vanasse, A.; Ntetu, A.L.; Maltais, D. Multimorbidity and quality of life in primary care: A systematic review. Health Qual. Life Outcomes 2004, 2, 51. [Google Scholar] [CrossRef]
- Gunn, J.M.; Ayton, D.R.; Densley, K.; Pallant, J.F.; Chondros, P.; Herrman, H.E.; Dowrick, C.F. The association between chronic illness, multimorbidity and depressive symptoms in an Australian primary care cohort. Soc. Psychiatry Psychiatr. Epidemiol. 2012, 47, 175–184. [Google Scholar] [CrossRef]
- Piette, J.D.; Kerr, E.A. The impact of comorbid chronic conditions on diabetes care. Diabetes Care 2006, 29, 725–731. [Google Scholar] [CrossRef]
- Gaede, P.; Vedel, P.; Larsen, N.; Jensen, G.V.; Parving, H.H.; Pedersen, O. Multifactorial intervention and cardiovascular disease in patients with type 2 diabetes. N. Engl. J. Med. 2003, 348, 383–393. [Google Scholar] [CrossRef]
- Arnold, S.V.; Bhatt, D.L.; Barsness, G.W.; Beatty, A.L.; Deedwania, P.C.; Inzucchi, S.E.; Kosiborod, M.; Leiter, L.A.; Lipska, K.J.; Newman, J.D.; et al. Clinical Management of Stable Coronary Artery Disease in Patients with Type 2 Diabetes Mellitus: A Scientific Statement From the American Heart Association. Circulation 2020, 141, e779–e806. [Google Scholar] [CrossRef]
- American Diabetes Association. 12. Older Adults: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S168–S179. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J. Management of hypoglycemia in older adults with type 2 diabetes. Postgrad. Med. 2019, 131, 241–250. [Google Scholar] [CrossRef]
- Kumari, S.; Jain, S.; Kumar, S. Effects of Polypharmacy in Elderly Diabetic Patients: A Review. Cureus 2022, 14, e29068. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D. Screening CT Angiography in Asymptomatic Diabetes Mellitus? JACC Cardiovasc. Imaging 2016, 9, 1301–1303. [Google Scholar] [CrossRef] [PubMed]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Steg, P.G.; Bhatt, D.L.; Simon, T.; Fox, K.; Mehta, S.R.; Harrington, R.A.; Held, C.; Andersson, M.; Himmelmann, A.; Ridderstrale, W.; et al. Ticagrelor in Patients with Stable Coronary Disease and Diabetes. N. Engl. J. Med. 2019, 381, 1309–1320. [Google Scholar] [CrossRef]
- Bonaca, M.P.; Bhatt, D.L.; Cohen, M.; Steg, P.G.; Storey, R.F.; Jensen, E.C.; Magnani, G.; Bansilal, S.; Fish, M.P.; Im, K.; et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N. Engl. J. Med. 2015, 372, 1791–1800. [Google Scholar] [CrossRef] [PubMed]

| All (n = 4072) | <65 yr (n = 1260) | 65–75 yr (n = 1393) | >75 yr (n = 1419) | 65–75 vs. <65 | >75 vs. <65 | >75 vs. 65–75 | |
|---|---|---|---|---|---|---|---|
| OR ‡ (95% CI); p-Value | |||||||
| Demographic Characteristics | |||||||
| Male sex, n (%) | 2531 (62.2) | 835 (66.3) | 925 (66.4) | 771 (54.3) | 1.01 (0.86, 1.18) | 0.61 (0.52, 0.71) | 0.60 (0.52, 0.70) |
| p = 0.942 | p < 0.001 * | p < 0.001 * | |||||
| Smoking history, n (%) | 2208 (54.2) | 835 (66.3) | 770 (55.3) | 603 (42.5) | 0.63 (0.54, 0.74) | 0.38 (0.32, 0.44) | 0.60 (0.51, 0.69) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Current smoker | 713 (17.5) | 332 (26.3) | 226 (16.2) | 155 (10.9) | 0.54 (0.45, 0.65) | 0.34 (0.28, 0.42) | 0.63 (0.51, 0.79) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Former smoker | 1495 (36.7) | 503 (39.9) | 544 (39.1) | 448 (31.6) | 0.96 (0.83, 1.13) | 0.69 (0.59, 0.81) | 0.72 (0.62, 0.84) |
| p = 0.648 | p < 0.001 * | p < 0.001 * | |||||
| Never smoker/unknown | 1864 (45.8) | 425 (33.7) | 623 (44.7) | 816 (57.5) | 1.59 (1.36, 1.86) | 2.66 (2.27, 3.11) | 1.67 (1.44, 1.94) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Clinical parameters | |||||||
| BMI, kg/m2 | |||||||
| n (%) | 696 (17.1) | 309 (24.5) | 254 (18.2) | 133 (9.4) | |||
| Median (Q1, Q3) | 30.5 (26.9, 35.4) | 31.6 (27.4, 37) | 30.3 (26.9, 35.4) | 29.3 (26.3, 32) | −1.01 (−2.28, 0.26) † | −2.17 (−3.73, −0.61) † | −1.16 (−2.77, 0.45) † |
| p = 0.119 | p = 0.006 * | p = 0.158 | |||||
| Type of CAD, n (%) | |||||||
| Single-vessel CAD | 813 (20.0) | 216 (17.1) | 276 (19.8) | 321 (22.6) | 1.19 (0.98, 1.46) | 1.41 (1.17, 1.71) | 1.18 (0.99, 1.42) |
| p = 0.077 | p < 0.001 * | p = 0.069 | |||||
| Multivessel CAD | 1602 (39.3) | 444 (35.2) | 561 (40.3) | 597 (42.1) | 1.24 (1.06, 1.45) | 1.33 (1.14, 1.56) | 1.08 (0.93, 1.25) |
| p = 0.008 * | p < 0.001 * | p = 0.332 | |||||
| Left main CAD | 27 (0.7) | 9 (0.7) | 7 (0.5) | 11 (0.8) | 0.70 (0.25, 1.89) | 1.09 (0.45, 2.70) | 1.55 (0.61, 4.21) |
| p = 0.484 | p = 0.855 | p = 0.368 | |||||
| Other/Unknown | 1630 (40.0) | 591 (46.9) | 549 (39.4) | 490 (34.5) | 0.74 (0.63, 0.86) | 0.60 (0.51, 0.70) | 0.81 (0.70, 0.95) |
| p < 0.001 * | p < 0.001 * | p = 0.007 * | |||||
| LVEF, % | |||||||
| n (%) | 365 (9.0) | 106 (8.4) | 126 (9) | 133 (9.4) | |||
| Median (Q1, Q3) | 51 (40, 62) | 50 (35, 60) | 55.5 (40, 65) | 50 (40, 60) | 4.37 (0.38, 8.36) † | 3.23 (−0.71, 7.17) † | −1.14 (−4.90, 2.62) † |
| p = 0.032 *⁋ | p = 0.108 | p = 0.552 | |||||
| All (n = 4072) | <65 yr (n = 1260) | 65–75 yr (n = 1393) | >75 yr (n = 1419) | 65–75 vs. <65 | >75 vs. <65 | >75 vs. 65–75 | |
|---|---|---|---|---|---|---|---|
| OR ‡ (95% CI); p-Value | |||||||
| Analytical parameters | |||||||
| Glucose, mg/dL | |||||||
| n (%) | 2749 (67.5) | 888 (70.5) | 933 (67) | 928 (65.4) | |||
| Median (Q1, Q3) | 135 (113, 168) | 135 (112, 175) | 134 (114, 166) | 134.5 (112, 164) | −3.44 (−9.08, 2.20) † | −4.82 (−10.46, 0.83) † | −1.38 (−6.96, 4.20) † |
| p = 0.232 | p = 0.095 | p = 0.628 | |||||
| HbA1c, % | |||||||
| n (%) | 1987 (48.8) | 694 (55.1) | 677 (48.6) | 616 (43.4) | |||
| Median (Q1, Q3) | 6.9 (6.3, 7.9) | 7.0 (6.3, 8.1) | 6.9 (6.3, 7.8) | 6.9 (6.3, 7.7) | −0.23 (−0.39, −0.08) † | −0.20 (−0.36, −0.05) † | 0.03 (−0.13, 0.19) † |
| p = 0.003* | p = 0.011* | p = 0.731 | |||||
| Total cholesterol, mg/dL | |||||||
| n (%) | 1943 (47.7) | 687 (54.5) | 643 (46.2) | 613 (43.2) | |||
| Median (Q1, Q3) | 160 (133, 193) | 172 (141, 204) | 156 (133, 188) | 151 (126, 182) | −13.11 (−18.11, −8.10) † | −19.49 (−24.55, −14.42) † | −6.38 (−11.52, −1.23) † |
| p < 0.001 * | p < 0.001 * | p = 0.015 * | |||||
| HDL, mg/dL | |||||||
| n (%) | 1958 (48.1) | 699 (55.5) | 647 (46.4) | 612 (43.1) | |||
| Median (Q1, Q3) | 42 (35, 51) | 42 (35, 50) | 42 (35, 50) | 43 (36, 52) | −0.92 (−2.66, 0.82) † | 0.04 (−1.73, 1.81) † | 0.96 (−0.84, 2.76) † |
| p = 0.299 | p = 0.965 | p = 0.294 | |||||
| LDL, mg/dL | |||||||
| n (%) | 1999 (49.1) | 673 (53.4) | 674 (48.4) | 652 (45.9) | |||
| Median (Q1, Q3) | 85 (68, 110) | 92 (71, 120) | 84 (67, 107) | 81 (65, 103) | −9.22 (−13.18, −5.26) † | −11.29 (−15.28, −7.29) † | −2.07 (−6.06, 1.93) † |
| p < 0.001 * | p < 0.001 * | p = 0.311 | |||||
| Triglycerides, mg/dL | |||||||
| n (%) | 2073 (50.9) | 733 (58.2) | 691 (49.6) | 649 (45.7) | |||
| Median (Q1, Q3) | 142 (99, 198) | 155 (106, 216) | 148 (102, 197) | 121 (88, 175) | 1.23 (−69.26, 71.72) † | 10.78 (−60.87, 82.44) † | 9.55 (−63.11, 82.22) † |
| p = 0.973 | p = 0.768 | p = 0.797 | |||||
| Comorbidities, n (%) | |||||||
| Arterial hypertension | 3447 (84.7) | 971 (77.1) | 1191 (85.5) | 1285 (90.6) | 1.75 (1.44, 2.14) | 2.85 (2.29, 3.57) | 1.63 (1.29, 2.05) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Angina | 1646 (40.4) | 420 (33.3) | 587 (42.1) | 639 (45.0) | 1.46 (1.24, 1.71) | 1.64 (1.40, 1.92) | 1.12 (0.97, 1.31) |
| p < 0.001 * | p < 0.001 * | p = 0.122 | |||||
| Heart valve disease | 1568 (38.5) | 381 (30.2) | 505 (36.3) | 682 (48.1) | 1.31 (1.12, 1.54) | 2.13 (1.82, 2.50) | 1.63 (1.40, 1.89) |
| p = 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Peripheral vascular disease | 1513 (37.2) | 339 (26.9) | 511 (36.7) | 663 (46.7) | 1.57 (1.33, 1.86) | 2.38 (2.03, 2.80) | 1.51 (1.30, 1.76) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Heart failure | 936 (23.0) | 249 (19.8) | 267 (19.2) | 420 (29.6) | 0.96 (0.79, 1.17) | 1.71 (1.43, 2.04) | 1.77 (1.49, 2.11) |
| p = 0.699 | p < 0.001 * | p < 0.001 * | |||||
| Atrial fibrillation | 590 (14.5) | 86 (6.8) | 192 (13.8) | 312 (22.0) | 2.18 (1.68, 2.86) | 3.85 (3.00, 4.98) | 1.76 (1.45, 2.15) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Peripheral artery disease | 528 (13.0) | 141 (11.2) | 197 (14.1) | 190 (13.4) | 1.31 (1.04, 1.65) | 1.23 (0.97, 1.55) | 0.94 (0.76, 1.16) |
| p = 0.023* | p = 0.085 | p = 0.563 | |||||
| Hyperlipidemia | 1666 (40.9) | 553 (43.9) | 599 (43.0) | 514 (36.2) | 0.96 (0.83, 1.12) | 0.73 (0.62, 0.85) | 0.75 (0.65, 0.88) |
| p = 0.645 | p < 0.001 * | p < 0.001 * | |||||
| Obesity | 1328 (32.6) | 545 (43.3) | 465 (33.4) | 318 (22.4) | 0.66 (0.56, 0.77) | 0.38 (0.32, 0.45) | 0.58 (0.49, 0.68) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Anemia | 867 (21.3) | 193 (15.3) | 285 (20.5) | 389 (27.4) | 1.42 (1.16, 1.74) | 2.09 (1.72, 2.54) | 1.47 (1.23, 1.75) |
| p = 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Chronic kidney disease | 740 (18.2) | 145 (11.5) | 235 (16.9) | 360 (25.4) | 0.53 (0.32, 0.87) | 0.44 (0.25, 0.73) | 0.82 (0.45, 1.47) |
| p = 0.013 * | p = 0.002 * | p = 0.511 | |||||
| Depression/anxiety | 820 (20.1) | 306 (24.3) | 258 (18.5) | 256 (18.0) | 0.71 (0.59, 0.85) | 0.69 (0.57, 0.83) | 0.97 (0.80, 1.17) |
| p < 0.001 * | p < 0.001 * | p = 0.742 | |||||
| COPD/asthma | 699 (17.2) | 195 (15.5) | 253 (18.2) | 251 (17.7) | 1.21 (0.99, 1.49) | 1.17 (0.96, 1.44) | 0.97 (0.80, 1.17) |
| p = 0.065 | p = 0.125 | p = 0.743 | |||||
| All (n = 4072) | <65 yr (n = 1260) | 65–75 yr (n = 1393) | >75 yr (n = 1419) | 65–75 vs. <65 | >75 vs. < 65 | >75 vs. 65–75 | |
|---|---|---|---|---|---|---|---|
| OR ‡ (95% CI); p-Value | |||||||
| Insulin treatment | 1018 (25.0) | 354 (28.1) | 341 (24.5) | 323 (22.8) | 0.83 (0.70, 0.99) | 0.75 (0.63, 0.90) | 0.91 (0.76, 1.08) |
| p = 0.035 *⁋ | p = 0.002 * | p = 0.284 | |||||
| LA insulin | 795 (19.5) | 285 (22.6) | 257 (18.4) | 253 (17.8) | 0.77 (0.64, 0.93) | 0.74 (0.61, 0.90) | 0.96 (0.79, 1.16) |
| p = 0.008 * | p = 0.002 * | p = 0.670 | |||||
| FA insulin | 345 (8.5) | 155 (12.3) | 110 (7.9) | 80 (5.6) | 0.61 (0.47, 0.79) | 0.43 (0.32, 0.56) | 0.70 (0.52, 0.94) |
| p < 0.001 * | p < 0.001 * | p = 0.018 * | |||||
| Intermediate or LA insulin + FA insulin | 219 (5.4) | 68 (5.4) | 75 (5.4) | 76 (5.4) | >0.99 (0.71, 1.40) | 0.99 (0.71, 1.39) | 0.99 (0.72, 1.38) |
| p = 0.988 | p = 0.963 | p = 0.974 | |||||
| Intermediate-acting insulin | 104 (2.6) | 36 (2.9) | 40 (2.9) | 28 (2.0) | 1.01 (0.64, 1.59) | 0.68 (0.41, 1.13) | 0.68 (0.41, 1.11) |
| p = 0.982 | p = 0.137 | p = 0.123 | |||||
| Oral hypoglycemic agents | 4072 (100.0) | 1260 (100.0) | 1393 (100.0) | 1419 (100.0) | ** | ** | ** |
| Metformin | 3160 (77.6) | 1012 (80.3) | 1086 (78.0) | 1062 (74.8) | 0.87 (0.72, 1.05) | 0.73 (0.61, 0.88) | 0.84 (0.71, <1.01) |
| p = 0.136 | p = 0.001 * | p = 0.052 | |||||
| Sulfonylureas | 881 (21.6) | 223 (17.7) | 327 (23.5) | 331 (23.3) | 1.43 (1.18, 1.73) | 1.41 (1.17, 1.71) | 0.99 (0.83, 1.18) |
| p < 0.001 * | p < 0.001 * | p = 0.926 | |||||
| DPP4i | 848 (20.8) | 235 (18.7) | 283 (20.3) | 330 (23.3) | 1.11 (0.92, 1.35) | 1.32 (1.10, 1.60) | 1.19 (0.99, 1.42) |
| p = 0.280 | p = 0.004 | p = 0.059 | |||||
| Glinidines | 507 (12.5) | 141 (11.2) | 162 (11.6) | 204 (14.4) | 1.04 (0.82, 1.33) | 1.33 (1.06, 1.68) | 1.28 (1.02, 1.59) |
| p = 1.723 | p = 0.014 * | p = 0.031 * | |||||
| GLP1-RA | 212 (5.2) | 128 (10.2) | 66 (4.7) | 18 (1.3) | 0.44 (0.32, 0.60) | 0.11 (0.07, 0.18) | 0.26 (0.15, 0.43) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| iSGLT2 | 106 (2.6) | 58 (4.6) | 32 (2.3) | 16 (1.1) | 0.49 (0.31, 0.75) | 0.24 (0.13, 0.40) | 0.49 (0.26, 0.87) |
| p = 0.001 * | p < 0.001 * | p = 0.019 * | |||||
| Thiazolidinediones | 91 (2.2) | 27 (2.1) | 36 (2.6) | 28 (2) | 1.21 (0.73, 2.02) | 0.92 (0.54, 1.57) | 0.76 (0.46, 1.25) |
| p = 0.456 | p = 0.757 | p = 0.279 | |||||
| Alpha glucosidase | 57 (1.4) | 9 (0.7) | 22 (1.6) | 26 (1.8) | 2.23 (1.06, 5.12) | 2.59 (1.26, 5.88) | 1.16 (0.66, 2.08) |
| p = 0.044 * | p = 0.014 * | p = 0.605 | |||||
| Anticoagulant therapy | 776 (19.1) | 165 (13.1) | 252 (18.1) | 359 (25.3) | 1.47 (1.19, 1.82) | 2.25 (1.84, 2.76) | 1.53 (1.28, 1.84) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Antiplatelet agents | 2843 (69.8) | 778 (61.7) | 1008 (72.4) | 1057 (74.5) | 1.62 (1.38, 1.91) | 1.81 (1.53, 2.13) | 1.12 (0.94, 1.32) |
| p < 0.001 * | p < 0.001 * | p = 0.202 | |||||
| ASA | 2606 (64.0) | 737 (58.5) | 923 (66.3) | 946 (66.7) | 1.39 (1.19, 1.63) | 1.42 (1.21, 1.66) | 1.02 (0.87, 1.19) |
| p < 0.001 * | p < 0.001 * | p = 0.819 | |||||
| Clopidogrel | 1208 (29.7) | 324 (25.7) | 420 (30.2) | 464 (32.7) | 1.25 (1.05, 1.48) | 1.40 (1.19, 1.66) | 1.13 (0.96, 1.32) |
| p = 0.011 * | p < 0.001 * | p = 0.146 | |||||
| Dual antiplatelet therapy | 1027 (25.2) | 300 (23.8) | 362 (26) | 365 (25.7) | 1.12 (0.94, 1.34) | 1.11 (0.93, 1.32) | 0.99 (0.83, 1.17) |
| p = 0.196 | p = 0.253 | p = 0.873 | |||||
| Clopidogrel + ASA | 830 (20.4) | 235 (18.7) | 285 (20.5) | 310 (21.8) | 1.12 (0.93, 1.36) | 1.22 (1.01, 1.47) | 1.09 (0.91, 1.30) |
| p = 0.241 | p = 0.040 *⁋ | p = 0.368 | |||||
| Other cardiovascular therapy | 3922 (96.3) | 1173 (93.1) | 1351 (97.0) | 1398 (98.5) | 2.39 (1.65, 3.51) | 4.94 (3.11, 8.21) | 2.07 (1.23, 3.58) |
| p < 0.001 * | p < 0.001 * | p = 0.007 * | |||||
| ACE inhibitors or ARB | 3282 (80.6) | 930 (73.8) | 1123 (80.6) | 1229 (86.6) | 1.48 (1.23, 1.77) | 2.30 (1.89, 2.80) | 1.56 (1.27, 1.91) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| ACE inhibitors | 2153 (52.9) | 637 (50.6) | 707 (50.8) | 809 (57.0) | 1.01 (0.87, 1.17) | 1.30 (1.11, 1.51) | 1.29 (1.11, 1.49) |
| p = 0.919 | p = 0.001 * | p = 0.001 * | |||||
| ARB | 1895 (46.5) | 475 (37.7) | 674 (48.4) | 746 (52.6) | 1.55 (1.33, 1.81) | 1.83 (1.57, 2.14) | 1.18 (1.02, 1.37) |
| p < 0.001 * | p < 0.001 * | p = 0.026 *⁋ | |||||
| Beta blockers | 2418 (59.4) | 713 (56.6) | 819 (58.8) | 886 (62.4) | 1.09 (0.94, 1.28) | 1.28 (1.09, 1.49) | 1.17 (<1.01, 1.36) |
| p = 0.251 | p = 0.002 * | p = 0.048 *⁋ | |||||
| Calcium channel blockers | 1686 (41.4) | 401 (31.8) | 586 (42.1) | 699 (49.3) | 1.56 (1.33, 1.82) | 2.08 (1.78, 2.44) | 1.34 (1.15, 1.55) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Nitrates | 1327 (32.6) | 319 (25.3) | 440 (31.6) | 568 (40.0) | 1.36 (1.15, 1.61) | 1.97 (1.67, 2.32) | 1.45 (1.24, 1.69) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Diuretics | 1914 (47.0) | 469 (37.2) | 618 (44.4) | 827 (58.3) | 1.34 (1.15, 1.57) | 2.36 (2.02, 2.75) | 1.75 (1.51, 2.03) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| Lipid-lowering drugs | 3386 (83.2) | 1019 (80.9) | 1188 (85.3) | 1179 (83.1) | 1.37 (1.12, 1.68) | 1.16 (0.95, 1.42) | 0.85 (0.69, 1.04) |
| p = 0.002 * | p = 0.136 | p = 0.111 | |||||
| Statins | 3223 (79.2) | 947 (75.2) | 1140 (81.8) | 1136 (80.1) | 1.49 (1.24, 1.80) | 1.33 (1.11, 1.59) | 0.89 (0.74, 1.08) |
| p < 0.001 * | p = 0.002 * | p = 0.229 | |||||
| Other lipid-lowering drugs | 885 (21.7) | 361 (28.7) | 314 (22.5) | 210 (14.8) | 0.72 (0.61, 0.86) | 0.43 (0.36, 0.52) | 0.60 (0.49, 0.72) |
| p < 0.001 * | p < 0.001 * | p < 0.001 * | |||||
| All (n = 4072) | <65 yr (n = 1260) | 65–75 yr (n = 1393) | >75 yr (n = 1419) | 65–75 vs. <65 | >75 vs. <65 | >75 vs. 65–75 | |
|---|---|---|---|---|---|---|---|
| OR ‡ (95% CI); p-Value | |||||||
| Revascularization treatment | 2016 (49.5) | 564 (44.8) | 710 (51) | 742 (52.3) | 1.28 (1.10, 1.49) | 1.35 (1.16, 1.58) | 1.05 (0.91, 1.22) |
| p = 0.001 * | p <0.001 * | p = 0.483 | |||||
| PCI | 1579 (38.8) | 458 (36.3) | 558 (40.1) | 563 (39.7) | 1.17 (<1.01, 1.37) | 1.15 (0.98, 1.35) | 0.98 (0.85, 1.14) |
| p = 0.050 *⁋ | p = 0.077 | p = 0.836 | |||||
| CABG | 585 (14.4) | 147 (11.7) | 218 (15.6) | 220 (15.5) | 1.40 (1.12, 1.76) | 1.39 (1.11, 1.74) | 0.99 (0.81, 1.21) |
| p = 0.003 * | p = 0.004 * | p = 0.915 | |||||
| 12 Months | 24 Months | 36 Months | 48 Months | 65–75 vs. <65 | >75 vs. <65 | >75 vs. 65–75 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <65 yr | 65–75 yr | >75 yr | <65 yr | 65–75 yr | >75 yr | <65 yr | 65–75 yr | >75 yr | <65 yr | 65–75 yr | >75 yr | HR ‡ (95% CI); p-Value | |||
| MACEs | 9.34 | 10.85 | 10.55 | 14.55 | 16.93 | 17.32 | 18.23 | 21.91 | 23.19 | 23.08 | 27.26 | 30.75 | 1.18 (>0.99, 1.40) | 1.29 (1.09, 1.52) | 1.09 (0.93, 1.27) |
| p = 0.051 | p = 0.003 * | p = 0.301 | |||||||||||||
| Myocardial infarction | 3.48 | 4.19 | 4.41 | 7.92 | 7.64 | 7.86 | 9.89 | 10.72 | 11.72 | 12.14 | 13.75 | 15.73 | 1.07 (0.84, 1.36) | 1.20 (0.94, 1.51) | 1.12 (0.89, 1.40) |
| p = 0.585 | p = 0.139 | p = 0.334 | |||||||||||||
| Stroke | 1.97 | 3.37 | 3.49 | 3.68 | 5.67 | 6.82 | 5.91 | 8.24 | 9.82 | 8.62 | 11.01 | 14.19 | 1.39 (1.04, 1.87) | 1.77 (1.33, 2.35) | 1.27 (0.99, 1.63) |
| p = 0.027 *⁋ | p < 0.001 * | p = 0.063 | |||||||||||||
| Ischemic stroke | 0.99 | 1.83 | 2.41 | 1.79 | 3.29 | 5.00 | 3.14 | 4.89 | 6.95 | 3.79 | 7.08 | 9.67 | 1.74 (1.17, 2.61) | 2.39 (1.63, 3.52) | 1.37 (1.01, 1.87) |
| p = 0.007 * | p < 0.001 * | p = 0.045 *⁋ | |||||||||||||
| Unstable angina | 1.36 | 1.81 | 1.80 | 2.05 | 3.24 | 2.14 | 2.90 | 4.37 | 3.01 | 3.69 | 5.45 | 4.43 | 1.52 (>0.99, 2.31) | 1.10 (0.70, 1.73) | 0.73 (0.49, 1.08) |
| p = 0.051 | p = 0.672 | p = 0.113 | |||||||||||||
| Urgent revascularization | 4.50 | 3.98 | 3.69 | 5.66 | 5.70 | 5.35 | 6.38 | 7.10 | 6.98 | 7.76 | 9.49 | 8.67 | 1.04 (0.79, 1.39) | 0.97 (0.73, 1.30) | 0.93 (0.70, 1.23) |
| p = 0.763 | p = 0.838 | p = 0.603 | |||||||||||||
| THEMIS Trial | PEGASUS-TIMI 54 Trial | ACORDE Study | ||||
|---|---|---|---|---|---|---|
| Placebo (n = 9601) | Ticagrelor (n = 9619) | Placebo (n = 7067) | Ticagrelor, 60 mg (n = 7045) | Ticagrelor, 90 mg (n = 7050) | All (n = 4072) | |
| Age, years | ||||||
| Mean (SD) | — | — | 65.4 ± 8.4 | 65.2 ± 8.4 | 65.4 ± 8.3 | 70 ± 11.3 |
| Median (IQR) | 66.0 (61.0–72.0) | 66.0 (61.0–72.0) | — | — | — | 70.7 (62.9–78.1) |
| Male sex, n (%) | 6613 (68.9) | 6576 (68.4) | 5385 (76.2) | 5384 (76.4) | 5333 (75.6) | 2531 (62.2) |
| Median BMI, kg/m2 (IQR) | 29.1 (26.0–32.8) | 29.0 (26.1–32.6) | — | — | — | 30.5 (26.9–35.4) ‡ |
| Weight, Kg | — | — | 82.0 ± 16.7 | 82.0 ± 17.0 | 81.8 ± 16.6 | — |
| Current smoker, n (%) | 1038 (10.8) | 1056 (11.0) | 1187 (16.8) | 1206 (17.1) | 1143 (16.2) | 713 (17.5) |
| Comorbidities, n (%) | ||||||
| Hypertension | 8867 (92.4) | 8909 (92.6) | 5462 (77.5) | 5461 (77.5) | 5484 (77.6) | 3447 (84.7) |
| Dyslipidemia | 8367 (87.1) | 8386 (87.2) | — | — | — | — |
| Hyperlipidemia | — | — | 5410 (76.7) | 5380 (76.4) | 5451 (77.1) | 1666 (40.9) |
| Cardiovascular events, n (incidence) | ||||||
| MACEs | 818 (8.5) | 736 (7.7) | 493 (7.85) | 487 (7.77) | 578 (9.04) | 858 (21.1) |
| Cardiovascular death | 357 (3.7) | 364 (3.8) | 182 (2.94) | 174 (2.86) | 210 (3.39) | — |
| Myocardial infarction | 328 (3.4) | 274 (2.8) | 275 (4.40) | 285 (4.53) | 338 (5.25) | 424 (10.4) |
| Ischemic stroke | 191 (2.0) | 152 (1.6) | 88 (1.41) | 78 (1.28) | 103 (1.65) | 198 (4.9) |
| Coronary arterial revascularization | 879 (9.2) | 828 (8.6) | 74 (1.16) | 62 (0.95) | 76 (1.13) | 2016 (49.5) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Juanatey, C.; Anguita-Sánchez, M.; Barrios, V.; Núñez-Gil, I.; Gómez-Doblas, J.J.; García-Moll, X.; Lafuente-Gormaz, C.; Rollán-Gómez, M.J.; Peral-Disdier, V.; Martínez-Dolz, L.; et al. Impact of Advanced Age on the Incidence of Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stable Coronary Artery Disease in a Real-World Setting in Spain. J. Clin. Med. 2023, 12, 5218. https://doi.org/10.3390/jcm12165218
González-Juanatey C, Anguita-Sánchez M, Barrios V, Núñez-Gil I, Gómez-Doblas JJ, García-Moll X, Lafuente-Gormaz C, Rollán-Gómez MJ, Peral-Disdier V, Martínez-Dolz L, et al. Impact of Advanced Age on the Incidence of Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stable Coronary Artery Disease in a Real-World Setting in Spain. Journal of Clinical Medicine. 2023; 12(16):5218. https://doi.org/10.3390/jcm12165218
Chicago/Turabian StyleGonzález-Juanatey, Carlos, Manuel Anguita-Sánchez, Vivencio Barrios, Iván Núñez-Gil, Juan José Gómez-Doblas, Xavier García-Moll, Carlos Lafuente-Gormaz, María Jesús Rollán-Gómez, Vicente Peral-Disdier, Luis Martínez-Dolz, and et al. 2023. "Impact of Advanced Age on the Incidence of Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stable Coronary Artery Disease in a Real-World Setting in Spain" Journal of Clinical Medicine 12, no. 16: 5218. https://doi.org/10.3390/jcm12165218
APA StyleGonzález-Juanatey, C., Anguita-Sánchez, M., Barrios, V., Núñez-Gil, I., Gómez-Doblas, J. J., García-Moll, X., Lafuente-Gormaz, C., Rollán-Gómez, M. J., Peral-Disdier, V., Martínez-Dolz, L., Rodríguez-Santamarta, M., Viñolas-Prat, X., Soriano-Colomé, T., Muñoz-Aguilera, R., Plaza, I., Curcio-Ruigómez, A., Orts-Soler, E., Segovia-Cubero, J., Fanjul, V., ... SAVANA Research Group. (2023). Impact of Advanced Age on the Incidence of Major Adverse Cardiovascular Events in Patients with Type 2 Diabetes Mellitus and Stable Coronary Artery Disease in a Real-World Setting in Spain. Journal of Clinical Medicine, 12(16), 5218. https://doi.org/10.3390/jcm12165218