Treatment of Myopia with Atropine 0.125% Once Every Night Compared with Atropine 0.125% Every Other Night: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Grouping
2.5. Myopia Monitoring Policy in the ATCFU Database
2.6. Ophthalmic Examinations
2.7. Outcome Measurements
2.8. Statistical Analysis
3. Results
3.1. Subject Enrollment, Comparison of Demographic Data, and Clinical Characteristics of the Two Groups
3.2. Baseline Age Is Negatively Correlated with Annual AL Changes
3.3. Male Sex Is the Only Factor Correlated with Slower Annual SEq Changes toward Myopia
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rudnicka, A.R.; Kapetanakis, V.V.; Wathern, A.K.; Logan, N.S.; Gilmartin, B.; Whincup, P.H.; Cook, D.G.; Owen, C.G. Global variations and time trends in the prevalence of childhood myopia, a systematic review and quantitative meta-analysis: Implications for aetiology and early prevention. Br. J. Ophthalmol. 2016, 100, 882–890. [Google Scholar] [CrossRef] [PubMed]
- Galvis, V.; Tello, A.; Otero, J.; Serrano, A.A.; Gómez, L.M.; Camacho, P.A.; López-Jaramillo, J.P. Prevalence of refractive errors in Colombia: MIOPUR study. Br. J. Ophthalmol. 2018, 102, 1320–1323. [Google Scholar] [CrossRef] [PubMed]
- Flitcroft, D.I.; He, M.; Jonas, J.B.; Jong, M.; Naidoo, K.; Ohno-Matsui, K.; Rahi, J.; Resnikoff, S.; Vitale, S.; Yannuzzi, L. IMI—Defining and Classifying Myopia: A Proposed Set of Standards for Clinical and Epidemiologic Studies. Investig. Ophthalmol. Vis. Sci. 2019, 60, M20–M30. [Google Scholar] [CrossRef] [PubMed]
- Holden, B.A.; Fricke, T.R.; Wilson, D.A.; Jong, M.; Naidoo, K.S.; Sankaridurg, P.; Wong, T.Y.; Naduvilath, T.J.; Resnikoff, S. Global Prevalence of Myopia and High Myopia and Temporal Trends from 2000 through 2050. Ophthalmology 2016, 123, 1036–1042. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Flitcroft, D.I.; Gifford, K.L.; Jong, M.; Jones, L.; Klaver, C.C.W.; Logan, N.S.; Naidoo, K.; Resnikoff, S.; Sankaridurg, P.; et al. IMI—Myopia Control Reports Overview and Introduction. Investig. Ophthalmol. Vis. Sci. 2019, 60, M1–M19. [Google Scholar] [CrossRef]
- Hou, W.; Norton, T.T.; Hyman, L.; Gwiazda, J. Axial Elongation in Myopic Children and its Association with Myopia Progression in the Correction of Myopia Evaluation Trial. Eye Contact Lens 2018, 44, 248–259. [Google Scholar] [CrossRef]
- Wolffsohn, J.S.; Kollbaum, P.S.; Berntsen, D.A.; Atchison, D.A.; Benavente, A.; Bradley, A.; Buckhurst, H.; Collins, M.; Fujikado, T.; Hiraoka, T.; et al. IMI—Clinical Myopia Control Trials and Instrumentation Report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M132–M160. [Google Scholar] [CrossRef]
- Gifford, K.L.; Richdale, K.; Kang, P.; Aller, T.A.; Lam, C.S.; Liu, Y.M.; Michaud, L.; Mulder, J.; Orr, J.B.; Rose, K.A.; et al. IMI—Clinical Management Guidelines Report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M184–M203. [Google Scholar] [CrossRef]
- Galvis, V.; Tello, A.; Rey, J.J.; Serrano Gomez, S.; Prada, A.M. Estimation of ocular axial length with optometric parameters is not accurate. Cont. Lens Anterior. Eye 2022, 45, 101448. [Google Scholar] [CrossRef]
- Wildsoet, C.F.; Chia, A.; Cho, P.; Guggenheim, J.A.; Polling, J.R.; Read, S.; Sankaridurg, P.; Saw, S.M.; Trier, K.; Walline, J.J.; et al. IMI—Interventions Myopia Institute: Interventions for Controlling Myopia Onset and Progression Report. Investig. Ophthalmol. Vis. Sci. 2019, 60, M106–M131. [Google Scholar] [CrossRef]
- Chia, A.; Chua, W.H.; Wen, L.; Fong, A.; Goon, Y.Y.; Tan, D. Atropine for the treatment of childhood myopia: Changes after stopping atropine 0.01%, 0.1% and 0.5%. Am. J. Ophthalmol. 2014, 157, 451–457.e451. [Google Scholar] [CrossRef]
- Yam, J.C.; Li, F.F.; Zhang, X.; Tang, S.M.; Yip, B.H.K.; Kam, K.W.; Ko, S.T.; Young, A.L.; Tham, C.C.; Chen, L.J.; et al. Two-Year Clinical Trial of the Low-Concentration Atropine for Myopia Progression (LAMP) Study: Phase 2 Report. Ophthalmology 2020, 127, 910–919. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Lu, W.Y.; You, J.Y.; Kuo, H.Y. Peripheral Refraction in Myopic Children with and without Atropine Usage. J. Ophthalmol. 2020, 2020, 4919154. [Google Scholar] [CrossRef] [PubMed]
- Yen, M.Y.; Liu, J.H.; Kao, S.C.; Shiao, C.H. Comparison of the effect of atropine and cyclopentolate on myopia. Ann. Ophthalmol. 1989, 21, 180–182, 187. [Google Scholar] [PubMed]
- Galvis, V.; Tello, A.; Rodriguez, C.J.; Rey, J.J. Atropine dose to treat myopia. Ophthalmology 2012, 119, 1718–1719. [Google Scholar] [CrossRef]
- Foo, L.; Htoon, H.; Farooqui, S.; Chia, A. Part-time use of 1% atropine eye drops for prevention of myopia progression in children. Int. Ophthalmol. 2020, 40, 1857–1862. [Google Scholar] [CrossRef]
- Zhu, Q.; Tang, Y.; Guo, L.; Tighe, S.; Zhou, Y.; Zhang, X.; Zhang, J.; Zhu, Y.; Hu, M. Efficacy and Safety of 1% Atropine on Retardation of Moderate Myopia Progression in Chinese School Children. Int. J. Med. Sci. 2020, 17, 176–181. [Google Scholar] [CrossRef]
- Liu, C.F.; Chen, S.C.; Chen, K.J.; Liu, L.; Chen, Y.P.; Kang, E.Y.; Liu, P.K.; Yeung, L.; Wu, W.C.; Lai, C.C.; et al. Higher HbA1c may reduce axial length elongation in myopic children: A comparison cohort study. Acta Diabetol. 2021, 58, 779–786. [Google Scholar] [CrossRef]
- Cooper, J.; Tkatchenko, A.V. A Review of Current Concepts of the Etiology and Treatment of Myopia. Eye Contact Lens 2018, 44, 231–247. [Google Scholar] [CrossRef]
- Kao, P.H.; Chuang, L.H.; Lai, C.C.; Chen, S.Y.; Lin, K.K.; Lee, J.S.; Hou, C.H.; Chen, C.T.; Kuo, Y.K.; Sun, C.C.; et al. Evaluation of axial length to identify the effects of monocular 0.125% atropine treatment for pediatric anisometropia. Sci. Rep. 2021, 11, 21511. [Google Scholar] [CrossRef]
- Ying, G.S.; Maguire, M.G.; Glynn, R.J.; Rosner, B. Tutorial on Biostatistics: Longitudinal Analysis of Correlated Continuous Eye Data. Ophthalmic Epidemiol. 2021, 28, 3–20. [Google Scholar] [CrossRef]
- Kuo, Y.K.; Chen, Y.T.; Chen, H.M.; Wu, P.C.; Sun, C.C.; Yeung, L.; Lin, K.K.; Chen, H.C.; Chuang, L.H.; Lai, C.C.; et al. Efficacy of Myopia Control and Distribution of Corneal Epithelial Thickness in Children Treated with Orthokeratology Assessed Using Optical Coherence Tomography. J. Pers. Med. 2022, 12, 278. [Google Scholar] [CrossRef]
- Liu, C.F.; Lee, J.S.; Sun, C.C.; Lin, K.K.; Hou, C.H.; Yeung, L.; Peng, S.Y. Correlation between pigmented arc and epithelial thickness (COPE) study in orthokeratology-treated patients using OCT measurements. Eye 2020, 34, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.W.; Yeung, L.; Sun, C.C.; Chen, H.M.; Peng, S.Y.; Chen, Y.T.; Liu, C.F. Correlation of corneal pigmented arc with wide epithelial thickness map in orthokeratology-treated children using optical coherence tomography measurements. Cont. Lens Anterior. Eye 2020, 43, 238–243. [Google Scholar] [CrossRef]
- Chia, A.; Chua, W.H.; Cheung, Y.B.; Wong, W.L.; Lingham, A.; Fong, A.; Tan, D. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (Atropine for the Treatment of Myopia 2). Ophthalmology 2012, 119, 347–354. [Google Scholar] [CrossRef]
- Lin, L.; Lan, W.; Liao, Y.; Zhao, F.; Chen, C.; Yang, Z. Treatment outcomes of myopic anisometropia with 1% atropine: A pilot study. Optom. Vis. Sci. 2013, 90, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Tran, H.D.M.; Tran, Y.H.; Tran, T.D.; Jong, M.; Coroneo, M.; Sankaridurg, P. A Review of Myopia Control with Atropine. J. Ocul. Pharmacol. Ther. 2018, 34, 374–379. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Ang, M.; Cho, P.; Guggenheim, J.A.; He, M.G.; Jong, M.; Logan, N.S.; Liu, M.; Morgan, I.; Ohno-Matsui, K.; et al. IMI Prevention of Myopia and Its Progression. Investig. Ophthalmol. Vis. Sci. 2021, 62, 6. [Google Scholar] [CrossRef]
- Ho, M.C.; Hsieh, Y.T.; Shen, E.P.; Hsu, W.C.; Cheng, H.C. Short-term refractive and ocular parameter changes after topical atropine. Taiwan J. Ophthalmol. 2020, 10, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Kumaran, A.; Htoon, H.M.; Tan, D.; Chia, A. Analysis of Changes in Refraction and Biometry of Atropine- and Placebo-Treated Eyes. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5650–5655. [Google Scholar] [CrossRef]
- Chia, A.; Lu, Q.S.; Tan, D. Five-Year Clinical Trial on Atropine for the Treatment of Myopia 2: Myopia Control with Atropine 0.01% Eyedrops. Ophthalmology 2016, 123, 391–399. [Google Scholar] [CrossRef]
- Donovan, L.; Sankaridurg, P.; Ho, A.; Naduvilath, T.; Smith, E.L., 3rd; Holden, B.A. Myopia progression rates in urban children wearing single-vision spectacles. Optom. Vis. Sci. 2012, 89, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Hyman, L.; Gwiazda, J.; Hussein, M.; Norton, T.T.; Wang, Y.; Marsh-Tootle, W.; Everett, D. Relationship of age, sex, and ethnicity with myopia progression and axial elongation in the correction of myopia evaluation trial. Arch. Ophthalmol. 2005, 123, 977–987. [Google Scholar] [CrossRef]
- Zhao, J.; Mao, J.; Luo, R.; Li, F.; Munoz, S.R.; Ellwein, L.B. The progression of refractive error in school-age children: Shunyi district, China. Am. J. Ophthalmol. 2002, 134, 735–743. [Google Scholar] [CrossRef]
- Gwiazda, J.; Hyman, L.; Dong, L.M.; Everett, D.; Norton, T.; Kurtz, D.; Manny, R.; Marsh-Tootle, W.; Scheiman, M. Factors associated with high myopia after 7 years of follow-up in the Correction of Myopia Evaluation Trial (COMET) Cohort. Ophthalmic. Epidemiol. 2007, 14, 230–237. [Google Scholar] [CrossRef] [PubMed]
- Saw, S.M.; Nieto, F.J.; Katz, J.; Schein, O.D.; Levy, B.; Chew, S.J. Factors related to the progression of myopia in Singaporean children. Optom. Vis. Sci. 2000, 77, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Li, S.M.; Li, H.; Li, S.Y.; Liu, L.R.; Kang, M.T.; Wang, Y.P.; Zhang, F.; Zhan, S.Y.; Gopinath, B.; Mitchell, P.; et al. Time Outdoors and Myopia Progression Over 2 Years in Chinese Children: The Anyang Childhood Eye Study. Investig. Ophthalmol. Vis. Sci. 2015, 56, 4734–4740. [Google Scholar] [CrossRef]

| Parameter | Total | QON | HS | p Value | |
|---|---|---|---|---|---|
| Eye number | [n] | 168 | 38 | 130 | |
| Male sex | (%) | 48.8 | 52.6 | 47.7 | 0.592 † |
| Baseline age (median) | (yr) | 10.3 ± 2.6 (10.1) | 10.6 ± 2.4 (11.1) | 10.2 ± 2.6 (9.7) | 0.320 a |
| Total follow-up period ‡ | (m) | 16.9 ± 9.1 | 15.6 ± 11.4 | 17.3 ± 8.3 | 0.392 a |
| Baseline AL | (mm) | 23.93 ± 1.01 | 23.81 ± 0.96 | 23.97 ± 1.02 | 0.396 a |
| Baseline SEq | (D) | −1.46 ± 1.46 | −1.56 ± 1.65 | −1.43 ± 1.41 | 0.633 a |
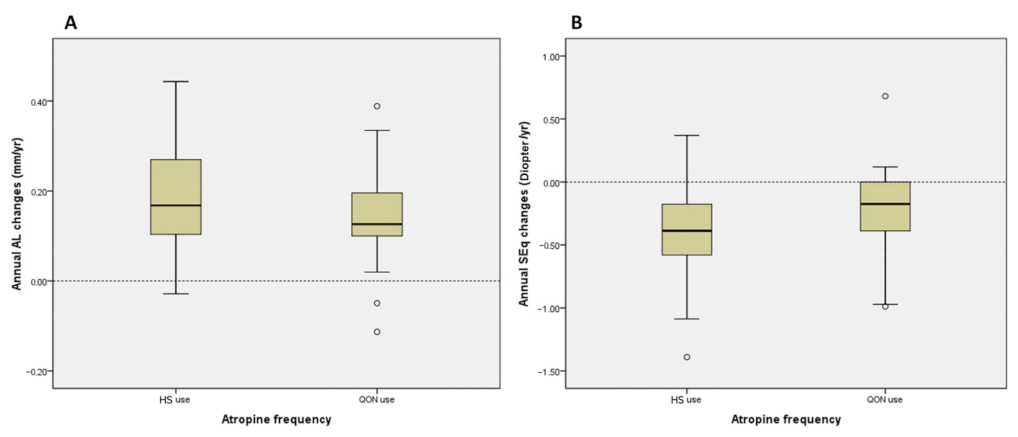
| Annual AL changes § | (mm/yr) | 0.18 ± 0.12 | 0.16 ± 0.10 | 0.18 ± 0.12 | 0.155 a |
| Annual SEq changes ¶ | (D/yr) | −0.33 ± 0.38 | −0.29 ± 0.44 | −0.34 ± 0.36 | 0.486 a |
| Parameter | Univariate | |||
|---|---|---|---|---|
| 95% CI | ||||
| B | Lower | Upper | Sig. | |
| Male sex | −0.032 | −0.083 | 0.018 | 0.211 |
| Baseline age (yr) | −0.020 | −0.028 | −0.013 | <0.001 * |
| Atropine QON use | −0.032 | −0.084 | 0.020 | 0.233 |
| Total follow-up period (m) † | −0.002 | −0.004 | 0.001 | 0.061 |
| Baseline AL (mm) | −0.007 | −0.035 | 0.022 | 0.654 |
| Baseline SEq (D) | −0.003 | −0.018 | 0.013 | 0.735 |
| Parameter | Univariate | |||
|---|---|---|---|---|
| 95% CI | ||||
| B | Lower | Upper | Sig. | |
| Male sex | 0.152 | 0.022 | 0.283 | 0.022 * |
| Baseline age (yr) | 0.022 | −0.001 | 0.045 | 0.059 |
| Atropine QON use | 0.048 | −0.128 | 0.225 | 0.591 |
| Total follow-up period (m) † | 0.001 | −0.005 | 0.007 | 0.779 |
| Baseline AL (mm) | −0.019 | −0.088 | 0.050 | 0.594 |
| Baseline SEq (D) | 0.011 | −0.038 | 0.060 | 0.654 |
| Parameter | The Current Study | Chia et al. (ATOM2) | Yam et al. (LAMP2) | |
|---|---|---|---|---|
| QON | HS | 0th–2nd Year | 0th–2nd Year | |
| Atropine concentration (%) | 0.125 | 0.125 | 0.1 | 0.05 |
| Compliance | >80% | >80% | >80% | >80% |
| Washout period | 6 months | 6 months | 2 weeks | None |
| Baseline age (yr) | 10.6 ± 2.4 | 10.2 ± 2.6 | 9.7 ± 1.6 | 8.32 ± 1.71 |
| Male sex (%) | 52.6 | 47.7 | 53.5 | 53.8 |
| Baseline AL (mm) | 23.81 ± 0.96 | 23.97 ± 1.02 | 25.1 ± 0.8 | 24.88 ± 0.91 |
| Annual AL changes (mm/yr) | 0.16 ± 0.10 | 0.18 ± 0.12 | 0.14 ± 0.14 | 0.195 ± 0.175 |
| Baseline SEq (D) | −1.56 ± 1.65 | −1.43 ± 1.41 | −4.5 ± 1.4 | −3.93 ± 1.63 |
| Annual SEq changes (D/yr) | −0.29 ± 0.44 | −0.34 ± 0.36 | −0.19 ± 0.30 | −0.275 ± 0.43 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-R.; Chen, S.-C.; Wan, T.-Y.; Chuang, L.-H.; Chen, H.-C.; Yeh, L.-K.; Kuo, Y.-K.; Wu, P.-C.; Chen, Y.-W.; Lai, I.-C.; et al. Treatment of Myopia with Atropine 0.125% Once Every Night Compared with Atropine 0.125% Every Other Night: A Pilot Study. J. Clin. Med. 2023, 12, 5220. https://doi.org/10.3390/jcm12165220
Chen Z-R, Chen S-C, Wan T-Y, Chuang L-H, Chen H-C, Yeh L-K, Kuo Y-K, Wu P-C, Chen Y-W, Lai I-C, et al. Treatment of Myopia with Atropine 0.125% Once Every Night Compared with Atropine 0.125% Every Other Night: A Pilot Study. Journal of Clinical Medicine. 2023; 12(16):5220. https://doi.org/10.3390/jcm12165220
Chicago/Turabian StyleChen, Zi-Rong, Shin-Chieh Chen, Tsung-Yao Wan, Lan-Hsin Chuang, Hung-Chi Chen, Lung-Kun Yeh, Yu-Kai Kuo, Pei-Chang Wu, Yun-Wen Chen, Ing-Chou Lai, and et al. 2023. "Treatment of Myopia with Atropine 0.125% Once Every Night Compared with Atropine 0.125% Every Other Night: A Pilot Study" Journal of Clinical Medicine 12, no. 16: 5220. https://doi.org/10.3390/jcm12165220
APA StyleChen, Z.-R., Chen, S.-C., Wan, T.-Y., Chuang, L.-H., Chen, H.-C., Yeh, L.-K., Kuo, Y.-K., Wu, P.-C., Chen, Y.-W., Lai, I.-C., Hwang, Y.-S., & Liu, C.-F. (2023). Treatment of Myopia with Atropine 0.125% Once Every Night Compared with Atropine 0.125% Every Other Night: A Pilot Study. Journal of Clinical Medicine, 12(16), 5220. https://doi.org/10.3390/jcm12165220







