Intraoperative Neurophysiological Monitoring in Syringomyelia Surgery: A Multimodal Approach
Abstract
1. Introduction
2. Methods
2.1. Pre- and Postoperative Neurophysiological Evaluations
2.1.1. Electromyography
2.1.2. Somatosensory Evoked Potentials
2.1.3. Transcranial Magnetic Stimulation
2.2. Surgical Procedure
2.3. Anesthesia
2.4. Intraoperative Neurophysiological Monitoring
2.4.1. Somatosensory Evoked Potentials
2.4.2. Motor Evoked Potentials
2.4.3. D-Wave
2.4.4. Free-Running Electromyography
2.4.5. Dorsal Column Mapping
2.4.6. Root Mapping
2.4.7. Other Techniques
3. Results
Illustrative Cases
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vandertop, W.P. Syringomyelia. Neuropediatrics 2014, 45, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Ji, S.; Niu, P.; Zhang, B.; Shao, D.; Li, Y.; Xie, S.; Jiang, Z. Knowledge Mapping of Syringomyelia from 2003 to 2022: A Bibliometric Analysis. J. Clin. Neurosci. 2023, 110, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, N.; Cacciola, F. Adult Syringomielia. Classification, Pathogenesis and Therapeutic Approaches. J. Neurosurg. Sci. 2005, 49, 65–72. [Google Scholar]
- Cacciola, F.; Capozza, M.; Perrini, P.; Benedetto, N.; Di Lorenzo, N. Syringopleural Shunt as a Rescue Procedure in Patients with Syringomyelia Refractory to Restoration of Cerebrospinal Fluid Flow. Neurosurgery 2009, 65, 471–476; discussion 476. [Google Scholar] [CrossRef]
- Sala, F.; Skrap, B.; Kothbauer, K.F.; Deletis, V. Intraoperative Neurophysiology in Intramedullary Spinal Cord Tumor Surgery. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2022; Volume 186, pp. 229–244. [Google Scholar] [CrossRef]
- Verla, T.; Fridley, J.S.; Khan, A.B.; Mayer, R.R.; Omeis, I. Neuromonitoring for Intramedullary Spinal Cord Tumor Surgery. World Neurosurg. 2016, 95, 108–116. [Google Scholar] [CrossRef]
- Sala, F.; Palandri, G.; Basso, E.; Lanteri, P.; Deletis, V.; Faccioli, F.; Bricolo, A. Motor Evoked Potential Monitoring Improves Outcome after Surgery for Intramedullary Spinal Cord Tumors: A Historical Control Study. Neurosurgery 2006, 58, 1129–1143. [Google Scholar] [CrossRef] [PubMed]
- Nuwer, M.R.; Emerson, R.G.; Galloway, G.; Legatt, A.D.; Lopez, J.; Minahan, R.; Yamada, T.; Goodin, D.S.; Armon, C.; Chaudhry, V.; et al. Evidence-Based Guideline Update: Intraoperative Spinal Monitoring with Somatosensory and Transcranial Electrical Motor Evoked Potentials. J. Clin. Neurophysiol. 2012, 29, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Simon, M.V.; Chiappa, K.H.; Borges, L.F. Phase Reversal of Somatosensory Evoked Potentials Triggered by Gracilis Tract Stimulation: Case Report of a New Technique for Neurophysiologic Dorsal Column Mapping. Neurosurgery 2012, 70, E783–E788. [Google Scholar] [CrossRef]
- Nair, D.; Kumaraswamy, V.M.; Braver, D.; Kilbride, R.D.; Borges, L.F.; Simon, M.V. Dorsal Column Mapping via Phase Reversal Method: The Refined Technique and Clinical Applications. Neurosurgery 2014, 74, 437–446; discussion 446. [Google Scholar] [CrossRef]
- Mehta, A.I.; Mohrhaus, C.A.; Husain, A.M.; Karikari, I.O.; Hughes, B.; Hodges, T.; Gottfried, O.; Bagley, C.A. Dorsal Column Mapping for Intramedullary Spinal Cord Tumor Resection Decreases Dorsal Column Dysfunction. J. Spinal Disord. Tech. 2012, 25, 205–209. [Google Scholar] [CrossRef]
- Deletis, V.; Seidel, K.; Sala, F.; Raabe, A.; Chudy, D.; Beck, J.; Kothbauer, K.F. Intraoperative Identification of the Corticospinal Tract and Dorsal Column of the Spinal Cord by Electrical Stimulation. J. Neurol. Neurosurg. Psychiatry 2018, 89, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Scibilia, A.; Terranova, C.; Rizzo, V.; Raffa, G.; Morelli, A.; Esposito, F.; Mallamace, R.; Buda, G.; Conti, A.; Quartarone, A.; et al. Intraoperative Neurophysiological Mapping and Monitoring in Spinal Tumor Surgery: Sirens or Indispensable Tools? Neurosurg. Focus 2016, 41, E18. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, O.; Lidar, Z.; Constantini, S.; Salame, K.; Bitan-Talmor, Y.; Korn, A. Continuous Mapping of the Corticospinal Tracts in Intramedullary Spinal Cord Tumor Surgery Using an Electrified Ultrasonic Aspirator. J. Neurosurg. Spine 2017, 27, 161–168. [Google Scholar] [CrossRef]
- Olmsted, Z.T.; Ryu, B.; Phayal, G.; Green, R.; Lo, S.-F.L.; Sciubba, D.M.; Silverstein, J.W.; D’Amico, R.S. Direct Wave Intraoperative Neuromonitoring for Spinal Tumor Resection: A Focused Review. World Neurosurg. X 2023, 17, 100139. [Google Scholar] [CrossRef] [PubMed]
- Deletis, V.; Sala, F. Intraoperative Neurophysiological Monitoring of the Spinal Cord during Spinal Cord and Spine Surgery: A Review Focus on the Corticospinal Tracts. Clin. Neurophysiol. 2008, 119, 248–264. [Google Scholar] [CrossRef] [PubMed]
- Yanni, D.S.; Ulkatan, S.; Deletis, V.; Barrenechea, I.J.; Sen, C.; Perin, N.I. Utility of Neurophysiological Monitoring Using Dorsal Column Mapping in Intramedullary Spinal Cord Surgery. J. Neurosurg. Spine 2010, 12, 623–628. [Google Scholar] [CrossRef]
- Moncho, D.; Poca, M.A.; Minoves, T.; Ferré, A.; Sahuquillo, J. Interside Latency Differences in Brainstem Auditory and Somatosensory Evoked Potentials. Defining Upper Limits to Determine Asymmetry. J. Clin. Neurophysiol. 2015, 32, 424–427. [Google Scholar] [CrossRef]
- Sindou, M.; Mertens, P.; Wael, M. Microsurgical DREZotomy for Pain Due to Spinal Cord and/or Cauda Equina Injuries: Long-Term Results in a Series of 44 Patients. Pain 2001, 92, 159–171. [Google Scholar] [CrossRef]
- MacDonald, D.B.; Dong, C.; Quatrale, R.; Sala, F.; Skinner, S.; Soto, F.; Szelényi, A. Recommendations of the International Society of Intraoperative Neurophysiology for Intraoperative Somatosensory Evoked Potentials. Clin. Neurophysiol. 2019, 130, 161–179. [Google Scholar] [CrossRef]
- Macdonald, D.B.; Skinner, S.; Shils, J.; Yingling, C. American Society of Neurophysiological Monitoring Intraoperative Motor Evoked Potential Monitoring—A Position Statement by the American Society of Neurophysiological Monitoring. Clin. Neurophysiol. 2013, 124, 2291–2316. [Google Scholar] [CrossRef]
- Nuwer, M.R. New Alert Criteria for Intraoperative Somatosensory Evoked Potential Monitoring. Clin. Neurophysiol. 2019, 130, 155–156. [Google Scholar] [CrossRef]
- MacDonald, D.B. Motor Evoked Potential Warning Criteria. J. Clin. Neurophysiol. 2017, 34, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Skinner, S.A.; Transfeldt, E.E.; Mehbod, A.A.; Mullan, J.C.; Perra, J.H. Electromyography Detects Mechanically-Induced Suprasegmental Spinal Motor Tract Injury: Review of Decompression at Spinal Cord Level. Clin. Neurophysiol. 2009, 120, 754–764. [Google Scholar] [CrossRef]
- Sahuquillo, J.; Rubio, E.; Poca, M.A.; Rovira, A.; Rodriguez-Baeza, A.; Cervera, C. Posterior Fossa Reconstruction: A Surgical Technique for the Treatment of Chiari I Malformation and Chiari I/Syringomyelia Complex—Preliminary Results and Magnetic Resonance Imaging Quantitative Assessment of Hindbrain Migration. Neurosurgery 1994, 35, 874–884; discussion 884–885. [Google Scholar] [CrossRef] [PubMed]
- Batzdorf, U.; Klekamp, J.; Johnson, J.P. A Critical Appraisal of Syrinx Cavity Shunting Procedures. J. Neurosurg. 1998, 89, 382–388. [Google Scholar] [CrossRef]
- Pencovich, N.; Korn, A.; Constantini, S. Intraoperative Neurophysiologic Monitoring during Syringomyelia Surgery: Lessons from a Series of 13 Patients. Acta Neurochir. 2013, 155, 785–791; discussion 791. [Google Scholar] [CrossRef]
- Barzilai, O.; Roth, J.; Korn, A.; Constantini, S. The Value of Multimodality Intraoperative Neurophysiological Monitoring in Treating Pediatric Chiari Malformation Type I. Acta Neurochir. 2016, 158, 335–340. [Google Scholar] [CrossRef]
- Anderson, R.C.; Emerson, R.G.; Dowling, K.C.; Feldstein, N.A. Attenuation of Somatosensory Evoked Potentials during Positioning in a Patient Undergoing Suboccipital Craniectomy for Chiari I Malformation with Syringomyelia. J. Child Neurol. 2001, 16, 936–939. [Google Scholar] [CrossRef] [PubMed]
- Sala, F.; Squintani, G.; Tramontano, V.; Coppola, A.; Gerosa, M. Intraoperative Neurophysiological Monitoring during Surgery for Chiari Malformations. Neurol. Sci. 2011, 32 (Suppl. S3), S317–S319. [Google Scholar] [CrossRef]
- Bose, B.; Sestokas, A.K.; Schwartz, D.M. Neurophysiological Monitoring of Spinal Cord Function during Instrumented Anterior Cervical Fusion. Spine J. 2004, 4, 202–207. [Google Scholar] [CrossRef]
- Ando, M.; Tamaki, T.; Yoshida, M.; Kawakami, M.; Kubota, S.; Nakagawa, Y.; Iwasaki, H.; Tsutsui, S.; Yamada, H. Intraoperative Spinal Cord Monitoring Using Combined Motor and Sensory Evoked Potentials Recorded from the Spinal Cord during Surgery for Intramedullary Spinal Cord Tumor. Clin. Neurol. Neurosurg. 2015, 133, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Sutter, M.; Eggspuehler, A.; Grob, D.; Jeszenszky, D.; Benini, A.; Porchet, F.; Mueller, A.; Dvorak, J. The Diagnostic Value of Multimodal Intraoperative Monitoring (MIOM) during Spine Surgery: A Prospective Study of 1,017 Patients. Eur. Spine J. 2007, 16 (Suppl. S2), S162–S170. [Google Scholar] [CrossRef] [PubMed]
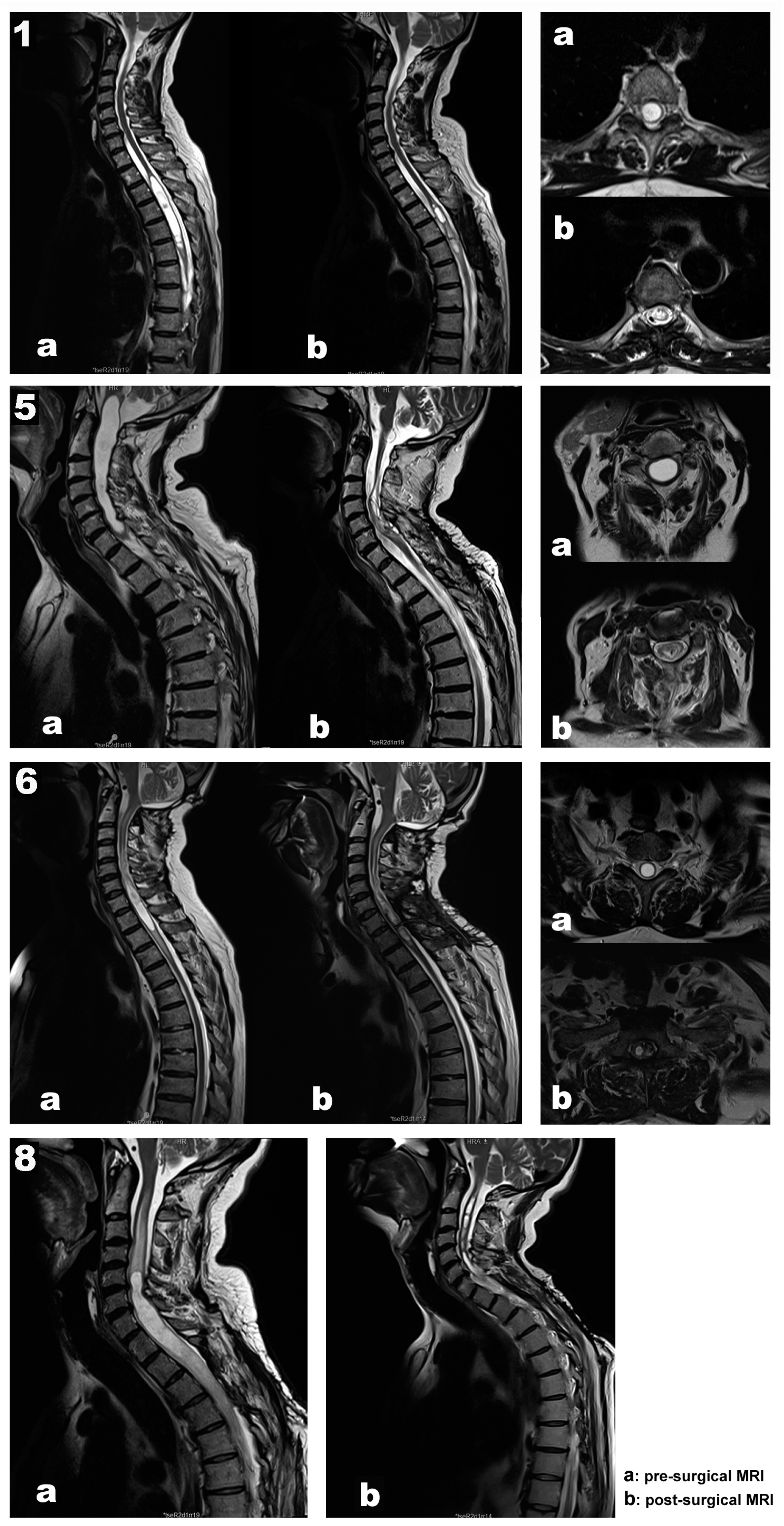
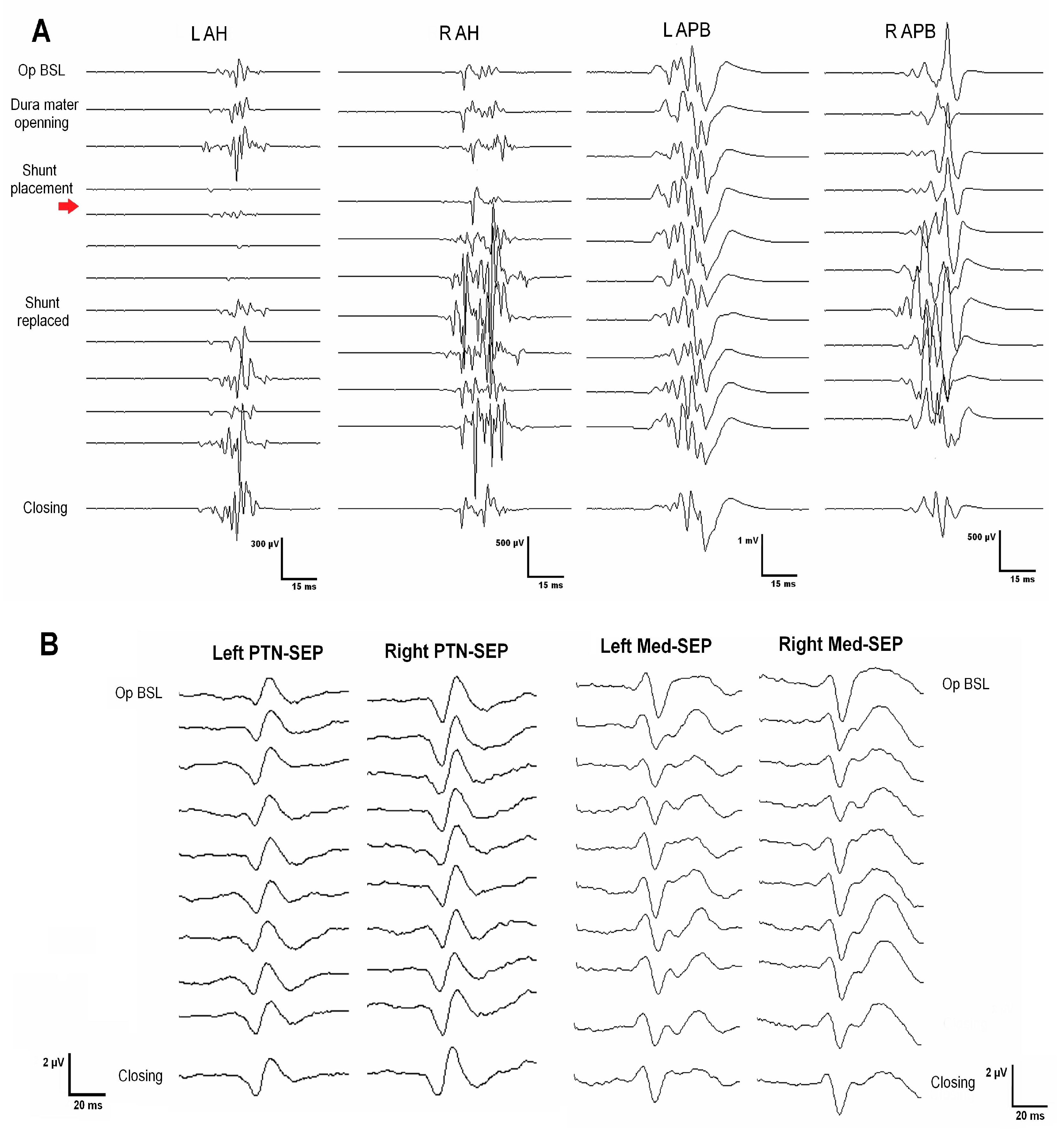


| Case No. | Age | Gender | Type | Syrinx Location | Surgery | Pre-Operative Clinical Examination | IOMN Modalities | IOMN Finding and Events | Clinical Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 67 | Female | Post-traumatic | Th2–Th6 | Th4–Th5 laminectomy Left DREZ myelotomy Syringoperitoneal shunt | Loss of sensation of the left LL associated with left LL weakness | MEPs SEPs Free-EMG Root mapping | Transitory loss of left AHB MEP recovered after shunt repositioning SEPs remained stable throughout surgery Root identification | No new deficits Subjective clinical improvement |
| 2 | 44 | Male | Post-traumatic | Holocord | C7–Th2 laminectomy Right DREZ myelotomy Syringopleural shunt | Gait disturbances Muscle weakness | MEPs SEPs Free-EMG Root mapping | Root identification Significant decrement of LLs MEP (R > L) after shunt placement, not recovered SSPEs remained stable throughout surgery | Worsening Temporary paraplegia Worsening of distal strength in right LL |
| 3 | 48 | Male | Post-traumatic | Holocord | Reintervention (2 previous surgery without IONM) C7–Th1 laminectomy Left DREZ myelotomy Syringoperitoneal shunt | Sensory disturbances on the left hemithorax, hemiabdomen, and left UL LLs pain | MEPs SEPs Free-EMG Root mapping | Root identification SEPs and MEPs remained stable throughout surgery | Pain improvement Transitory left UL improvement lasting some weeks Some months later, strength worsening |
| 4 | 31 | Male | Post-traumatic | C5–Th1 | C5–C7 laminectomy Left DREZ myelotomy Subarachnoid space reconstruction | Right UL weakness Subjective sensory disturbances on ULs | MEPs SEPs Free-EMG Root mapping | LL SEPs absent baselines Root identification SEPs and MEPs remained stable throughout surgery | No new deficits Improvement of the sensory disturbances on ULs Stable right UL weakness |
| 5 | 70 | Male | Hemangioblastoma related | Medulla–C7 | C5–Th1 laminectomy Midline myelotomy Subarachnoid space reconstruction | Paresthesias in ULs Ataxia Mild muscle weakness in LLs | MEPs SEPs Free-EMG D-wave DCM | Transitory MEP decrement after prone position, recovered after neck reposition DCM for median raphe identification Transitory EMG discharges upon shunt placement Stable MEPs, SEPs, and D-wave | No new deficits Proximal UL and LL strength improvement, which persists one year later |
| 6 | 61 | Male | Chiari malformation | C6–Th2 | C7-Th1 laminectomy Left DREZ myelotomy Syringoperitoneal shunt | Progressive paraparesis Gait disturbances | MEPs SEPs Free-EMG DCM Root mapping | Prepositional baselines remains stable DCM for left DC and safe zone entry identification at DREZ Root identification SEPs and MEPs stable throughout surgery | No new deficits Distal left UL strength improvement One year later, progressive strength and sensory worsening |
| 7 | 74 | Male | Idiopathic | C2–Th8 | Th1–Th3 laminectomy Left DREZ myelotomy Syringopleural shunt | Ataxia, progressive left LL spasticity, and LL weakness Right UL pain | MEPs SEPs Free EMG D-wave Root mapping | Root mapping MEP, SEP, and D-wave stable throughout surgery | No new deficits LL strength improvement Reduction of spasticity and pain |
| 8 | 48 | Male | Post-traumatic | Holocord | Reintervention (3 previous surgeries) C5–Th1 laminectomy Left DREZ myelotomy Syringoperitoneal shunt | Muscle weakness No more data is available | MEPs SEPs Free-EMG Root mapping | Prepositional baselines stable Root C7 and C8 mapping Sudden loss of left MEPs (ADM, APB, TA, and recto femoris) after shunt placement, not recovered by the end of the surgery | Worsening Hemiparesis (LLs > ULs) |
| 9 | 76 | Female | Spinal arachnoid cyst | Th7–conus | Th8–Th9 laminectomy Midline myelotomy Syringoperitoneal shunt | LLs weakness Ataxia | MEPs SEPs Free-EMG DCM | Prepositional baselines stable DCM (no responses) LL SEP absent baselines MEPs stable throughout surgery | Slight worsening of the ataxia Initially, neuropathic pain worsens; after a few months, similar neuropathic pain to the pre-operative condition |
| 10 | 57 | Male | Post-meningitis | Medulla–holocord | Th6–Th8 laminectomy Midline myelotomy Syringopleural shunt | Cervico-dorsal myelitis Slight weakness in the left UL LLs paraparesis | MEPs SEPs Free-EMG DCM | LL SEP absent baselines DCM (identification of left DC and medial sulcus. Not response at right DC) SEPs and MEPs stable throughout surgery | No new deficits Left UL strength improvement |
| Case | Preoperative | Postoperative | Electrodiagnostic Outcome | |
|---|---|---|---|---|
| 1 | EMG | Upper limbs not explored. Normal LL | Upper limbs not explored | Unknown |
| SEP | Mild impairment of dorsal columns in both LL (normal UL) | Mild impairment of dorsal columns in both LL (UL not explored) | Stable | |
| TMS | Normal pyramidal tract conduction in both LL (UL not explored) | Uninjured pyramidal tract in both LL (UL not explored) | Stable | |
| 2 | EMG | Right UL: complete denervation at C8/D1 level and severe at C5–C7 Left UL: almost complete denervation C5–C8/D1 (brachial plexopathy) | Not explored | Unknown |
| SEP | Severe impairment of dorsal columns in left UL (absent cortical potential) and moderate in both LL (normal right UL) | Moderate impairment of dorsal columns in both LL (UL not explored) | Stable for LL. Unknown for UL | |
| TMS | Normal pyramidal tract conduction in both LL (UL not explored) | Mild impairment of pyramidal tract in both LL (UL not explored) | Deterioration of LL CST. Unknown for UL | |
| 3 | EMG | Mild acute denervation at C5–C7 left levels. Severe acute denervation at C8 and L3–S1 left levels. Mild chronic denervation at C7–C8 and L5 right side levels. | Not explored | Deterioration of left roots/anterior horn |
| SEP | Moderate impairment of dorsal columns in left LL and mild in right LL. Normal for both ULs | Stable for right LL, moderate impairment for left LL (amplitude decrement of cortical potential). Mild impairment for both ULs at the cervico-medullary level, with normal cortical conduction. | Stable, mild changes for left LL and cervico-medullary level. | |
| TMS | Uninjured pyramidal tract in right UL and LL. Severe impairment on the left side (absence of responses for left LL) | Stable for right limbs. Persistent abolition of motor cortical response for UL and LL from the left side | Stable | |
| 4 | EMG | Bilateral moderate–severe chronic denervation at C7–C8/D1 levels | Bilateral moderate–severe chronic denervation at C7–C8/D1 levels | Stable |
| SEP | Severe impairment of dorsal columns for both LL (absent cortical potential). UL not explored | Mild impairment of dorsal columns for right UL. Left UL and both LL not explored | Unknown | |
| TMS | Severe impairment of pyramidal tract for both LLs. ULs not explored | Not explored | Unknown | |
| 5 | EMG | Moderate–severe acute denervation at right C8/D1 level and mild left C8/D1 | Mild chronic denervation at bilateral C8/D1 level | Improved degree of denervation at right C8/D1. Stable for the left side |
| SEP | Mild impairment of dorsal columns in both upper limbs and right lower limb (normal left LL) | Mild impairment of dorsal columns in both upper limbs and right lower limb (normal left LL) | Stable | |
| TMS | Mild impairment of pyramidal tract in left LL (normal right LL and both UL) | Mild impairment of pyramidal tract in left LL (normal right LL and both UL) | Stable | |
| 6 | EMG | Severe chronic denervation at left C5–C8 levels and mild–moderate at right C6–C7 | Severe chronic denervation at left C7 level and moderate left C5–C6 (right UL not explored) | Improved degree of denervation at left C5–C6. Unknown evolution for right UL. |
| SEP | Mild impairment of dorsal columns in right UL and left LL; moderate impairment in left UL and right LL | Severe impairment in left UL, moderate in right LL, and mild in right UL and left LL | Moderate deterioration of left UL dorsal column | |
| TMS | Mild impairment of pyramidal tract in right UL and LL, moderate in left UL (normal left LL) | Mild impairment of pyramidal tract in right UL and LL and moderate in left UL and LL | Global mild deterioration for CST | |
| 7 | EMG | Not explored | Not explored | Unknown |
| SEP | Moderate impairment of dorsal columns for both LL (left > right) at the cervical–lumbar segment. Mild impairment for both ULs | Moderate impairment of dorsal columns for LLs at the cervical–lumbar segment. Mild–moderate impairment for both ULs | Global mild deterioration of dorsal columns conduction | |
| TMS | Severe impairment of pyramidal tract for left LL (absence of potential). Mild–moderate impairment for right LL. Normal for both ULs | Not explored | Unknown | |
| 8 | EMG | Not explored | Not explored | Unknown |
| SEP | Moderate impairment of dorsal columns in left LL, mild in right LL. Mild impairment for both ULs at the cervico-medullary level | Severe impairment of dorsal columns in left LL (absence of cortical potential) Mild in right LL. Mild–moderate impairment for both ULs at the cervico-medullary level (right > left) | Deterioration of left dorsal column conduction | |
| TMS | Moderate impairment of pyramidal tract in left UL. Severe impairment for left LL (absence of potential). Normal for right UL and LL | Severe impairment (absence of potential) of the pyramidal tract in left UL. Mild impairment for right UL and left LL. Uninjured pyramidal tract in right LL | Deterioration for right UL CST. Improvement for left LL CST. | |
| 9 | EMG | Not explored | Not explored | Unknown |
| SEP | Severe impairment of dorsal columns for both LL (absent cortical potential). ULs not explored | Severe impairment of dorsal columns for both LL (absent cortical potential). Normal function for both ULs | Stable | |
| TMS | Mild impairment of pyramidal tract for both LLs (left > right) ULs not explored | Not explored | Unknown | |
| 10 | EMG | Moderate–severe acute denervation at left C5–C8/D1 levels Mild–moderate on the right side. Bilateral moderate–severe acute denervation at L3-S1 levels | Not explored | Unknown |
| SEP | Mild impairment of dorsal columns for left UL, normal for right UL. Severe impairment of dorsal columns for both LL (absent cortical potential) | Not explored | Unknown | |
| TMS | Mild impairment of pyramidal tract for left UL, moderate for right UL and severe for both LLs | Not explored | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez Roldán, M.Á.; Moncho, D.; Rahnama, K.; Santa-Cruz, D.; Lainez, E.; Baiget, D.; Chocrón, I.; Gándara, D.; Bescós, A.; Sahuquillo, J.; et al. Intraoperative Neurophysiological Monitoring in Syringomyelia Surgery: A Multimodal Approach. J. Clin. Med. 2023, 12, 5200. https://doi.org/10.3390/jcm12165200
Sánchez Roldán MÁ, Moncho D, Rahnama K, Santa-Cruz D, Lainez E, Baiget D, Chocrón I, Gándara D, Bescós A, Sahuquillo J, et al. Intraoperative Neurophysiological Monitoring in Syringomyelia Surgery: A Multimodal Approach. Journal of Clinical Medicine. 2023; 12(16):5200. https://doi.org/10.3390/jcm12165200
Chicago/Turabian StyleSánchez Roldán, M. Ángeles, Dulce Moncho, Kimia Rahnama, Daniela Santa-Cruz, Elena Lainez, Daniel Baiget, Ivette Chocrón, Darío Gándara, Agustín Bescós, Juan Sahuquillo, and et al. 2023. "Intraoperative Neurophysiological Monitoring in Syringomyelia Surgery: A Multimodal Approach" Journal of Clinical Medicine 12, no. 16: 5200. https://doi.org/10.3390/jcm12165200
APA StyleSánchez Roldán, M. Á., Moncho, D., Rahnama, K., Santa-Cruz, D., Lainez, E., Baiget, D., Chocrón, I., Gándara, D., Bescós, A., Sahuquillo, J., & Poca, M. A. (2023). Intraoperative Neurophysiological Monitoring in Syringomyelia Surgery: A Multimodal Approach. Journal of Clinical Medicine, 12(16), 5200. https://doi.org/10.3390/jcm12165200









