Nitrofuran Derivatives Cross-Resistance Evidence—Uropathogenic Escherichia coli Nitrofurantoin and Furazidin In Vitro Susceptibility Testing
Abstract
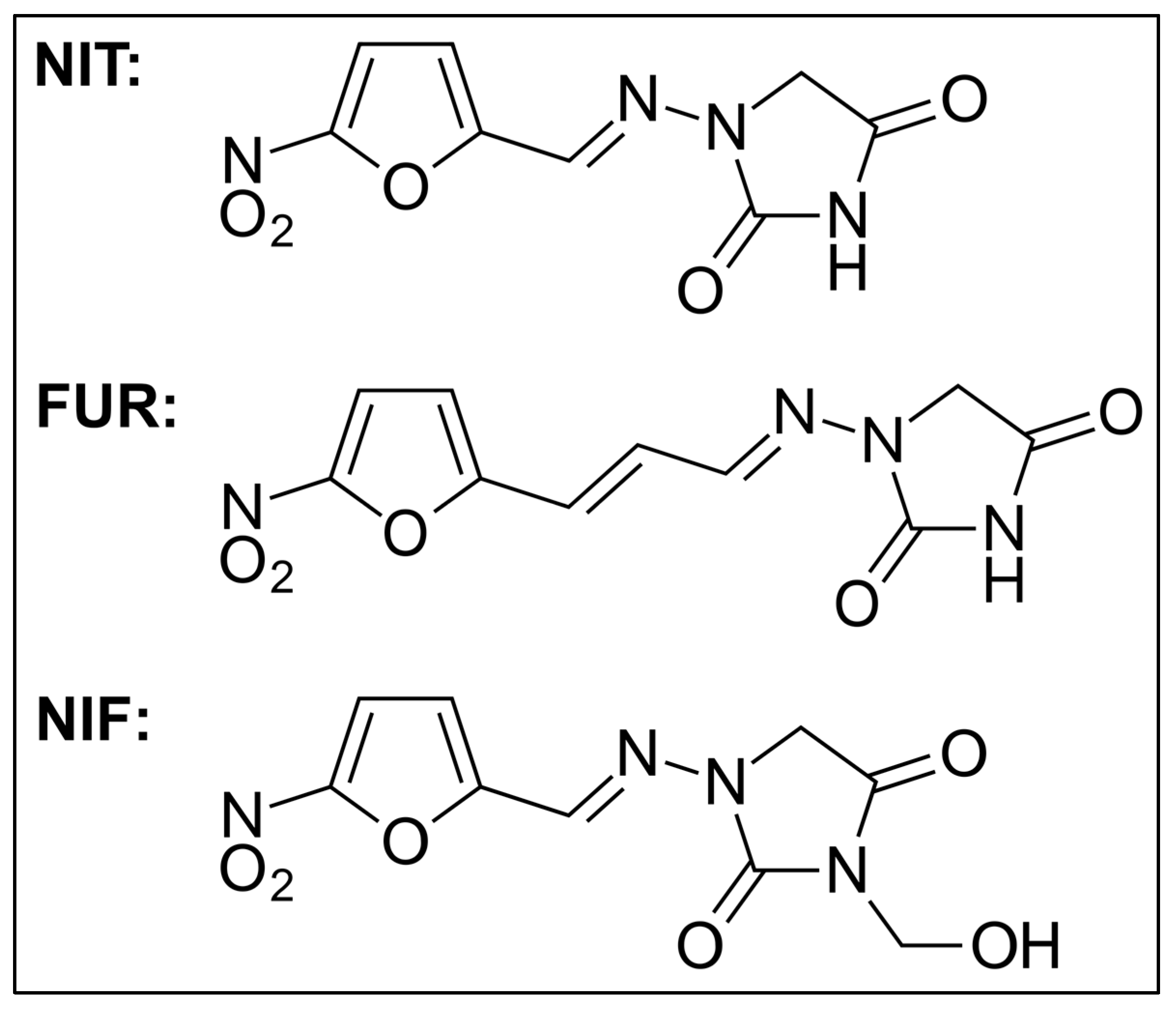
1. Introduction
2. Materials and Methods
Ethical Issues
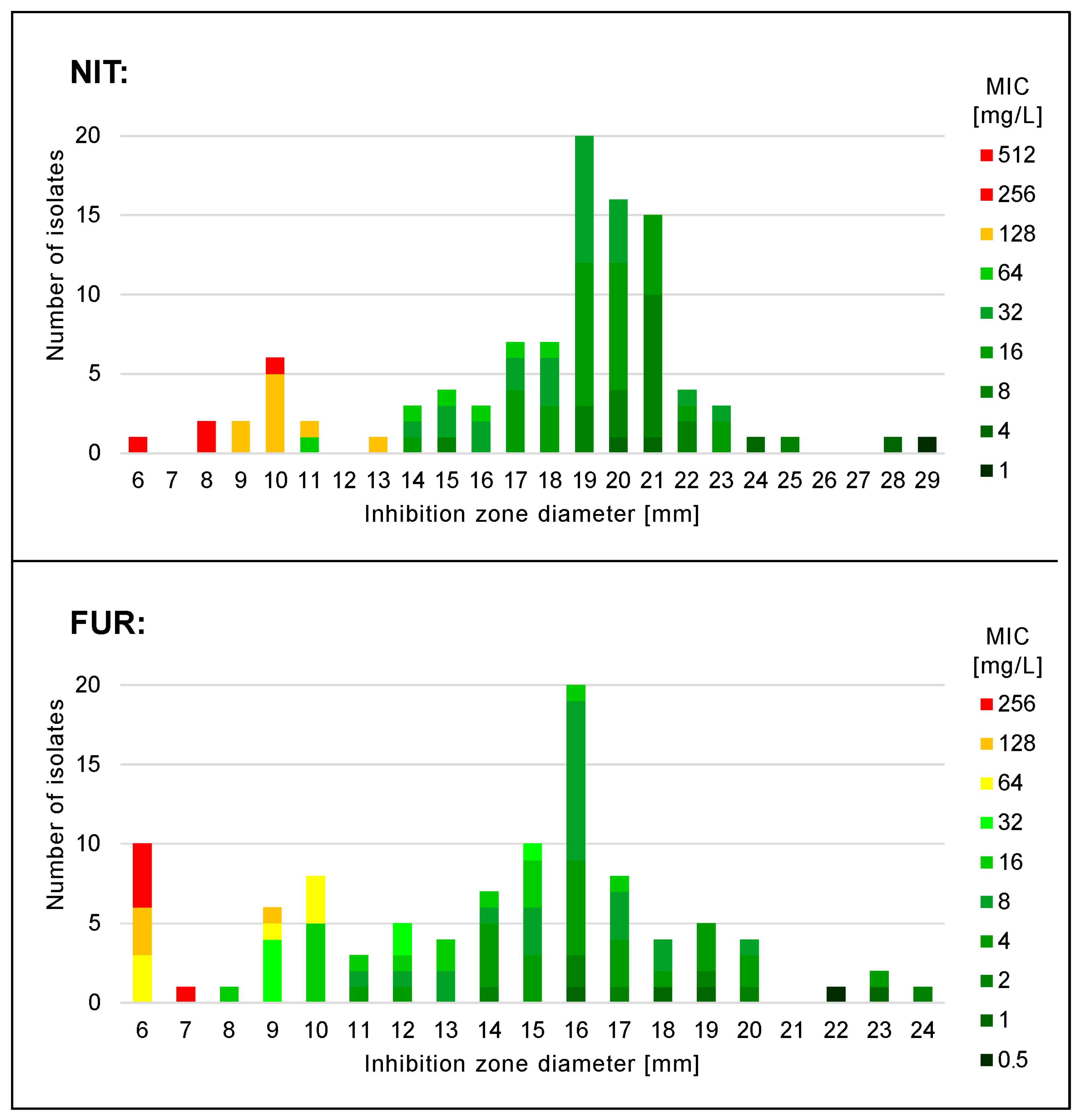
3. Results
4. Discussion
Study Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trześniewska-Ofiara, Z.; Mendrycka, M.; Cudo, A.; Szmulik, M.; Woźniak-Kosek, A. Hospital Urinary Tract Infections in Healthcare Units on the Example of Mazovian Specialist Hospital Ltd. Front. Cell. Infect. Microbiol. 2022, 12, 891796. [Google Scholar] [CrossRef] [PubMed]
- Kolman, K.B. Cystitis and Pyelonephritis: Diagnosis, Treatment, and Prevention. Prim. Care 2019, 46, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Deltourbe, L.; Lacerda Mariano, L.; Hreha, T.N.; Hunstad, D.A.; Ingersoll, M.A. The impact of biological sex on diseases of the urinary tract. Mucosal Immunol. 2022, 15, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Daniel, M.; Szymanik-Grzelak, H.; Sierdziński, J.; Podsiadły, E.; Kowalewska-Młot, M.; Pańczyk-Tomaszewska, M. Epidemiology and Risk Factors of UTIs in Children-A Single-Center Observation. J. Pers. Med. 2023, 13, 138. [Google Scholar] [CrossRef] [PubMed]
- Mody, L.; Juthani-Mehta, M. Urinary tract infections in older women: A clinical review. JAMA 2014, 311, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Jermakow, K.; Pajączkowska, M.; Krzyżanowska, B.; Nowicka, J.; Dworniczek, E. The growing importance of Enterococcus and Streptococcus Agalactiae in uncomplicated urinary tract infections. Fam. Med. Prim. Care Rev. 2016, 18, 250–252. [Google Scholar] [CrossRef]
- Holm, A.; Cordoba, G.; Sørensen, T.M.; Jessen, L.R.; Frimodt-Møller, N.; Siersma, V.; Bjerrum, L. Clinical accuracy of point-of-care urine culture in general practice. Scand. J. Prim. Health Care 2017, 35, 170–177. [Google Scholar] [CrossRef]
- Stefaniuk, E.; Suchocka, U.; Bosacka, K.; Hryniewicz, W. Etiology and antibiotic susceptibility of bacterial pathogens responsible for community-acquired urinary tract infections in Poland. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1363–1369. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Moghadami, M.; Shirazi, B.; Tabari, P.; Moosavi, M. What Left for Us for Urinary Tract Infection Treatment? An Experience from the South of Iran. Adv. Biomed. Res. 2021, 10, 52. [Google Scholar]
- Watts, V.; Brown, B.; Ahmed, M.; Charlett, A.; Chew-Graham, C.; Cleary, P.; Decraene, V.; Dodgson, K.; George, R.; Hopkins, S.; et al. Routine laboratory surveillance of antimicrobial resistance in community-acquired urinary tract infections adequately informs prescribing policy in England. JAC Antimicrob. Resist. 2020, 2, dlaa022. [Google Scholar] [CrossRef]
- Wagenlehner, F.M.E.; Bjerklund Johansen, T.E.; Cai, T.; Koves, B.; Kranz, J.; Pilatz, A.; Tandogdu, Z. Epidemiology, definition and treatment of complicated urinary tract infections. Nat. Rev. Urol. 2020, 17, 586–600. [Google Scholar] [CrossRef]
- Medina-Polo, J.; Naber, K.G.; Bjerklund Johansen, T.E. Healthcare-associated urinary tract infections in urology. GMS Infect. Dis. 2021, 9, Doc05. [Google Scholar] [PubMed]
- Bitew, A.; Zena, N.; Abdeta, A. Bacterial and Fungal Profile, Antibiotic Susceptibility Patterns of Bacterial Pathogens and Associated Risk Factors of Urinary Tract Infection Among Symptomatic Pediatrics Patients Attending St. Paul’s Hospital Millennium Medical College: A Cross-Sectional Study. Infect. Drug Resist. 2022, 15, 1613–1624. [Google Scholar] [PubMed]
- Meers, P.D.; Chow, C.K. Bacteriostatic and bactericidal actions of boric acid against bacteria and fungi commonly found in urine. J. Clin. Pathol. 1990, 43, 484–487. [Google Scholar] [CrossRef]
- Nzalie, R.N.; Gonsu, H.K.; Koulla-Shiro, S. Bacterial Etiology and Antibiotic Resistance Profile of Community-Acquired Urinary Tract Infections in a Cameroonian City. Int. J. Microbiol. 2016, 2016, 3240268. [Google Scholar] [CrossRef]
- Erdem, I.; Kara Ali, R.; Ardic, E.; Elbasan Omar, S.; Mutlu, R.; Topkaya, A.E. Community-acquired Lower Urinary Tract Infections: Etiology, Antimicrobial Resistance, and Treatment Results in Female Patients. J. Glob. Infect. Dis. 2018, 10, 129–132. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, G.V.; Babiker, A.; Master, R.N.; Luu, T.; Mathur, A.; Bordon, J. Antibiotic Resistance among Urinary Isolates from Female Outpatients in the United States in 2003 and 2012. Antimicrob. Agents Chemother. 2016, 60, 2680–2683. [Google Scholar] [CrossRef]
- NkontCho, F.; Sainte-Rose, V.; Abboud, P.; Portecop, P.; Pujo, J.M.; Cook, F.; Walter, G.; Mounier, R.; Resiere, D.; Houcke, S.; et al. Antimicrobial Susceptibility of Community-Acquired Urine Bacterial Isolates in French Amazonia. Am. J. Trop. Med. Hyg. 2023, 108, 927–935. [Google Scholar] [CrossRef]
- Bitew, A.; Molalign, T.; Chanie, M. Species distribution and antibiotic susceptibility profile of bacterial uropathogens among patients complaining urinary tract infections. BMC Infect. Dis. 2017, 17, 654. [Google Scholar] [CrossRef]
- Kot, B.; Grużewska, A.; Szweda, P.; Wicha, J.; Parulska, U. Antibiotic Resistance of Uropathogens Isolated from Patients Hospitalized in District Hospital in Central Poland in 2020. Antibiotics 2021, 10, 447. [Google Scholar] [CrossRef]
- Dejonckheere, Y.; Desmet, S.; Knops, N. A study of the 20-year evolution of antimicrobial resistance patterns of pediatric urinary tract infections in a single center. Eur. J. Pediatr. 2022, 181, 3271–3281. [Google Scholar] [CrossRef] [PubMed]
- Bonkat, G.; Bartoletti, R.; Bruyère, F.; Cai, T.; Geerlings, S.E.; Köves, B.; Schubert, S.; Wagelehner, F. EAU Guidelines on Urological Infections; European Association of Urology Guidelines Office: Arnhem, The Netherlands, 2021. [Google Scholar]
- Hryniewicz, W.; Holecki, M. Rekomendacje Diagnostyki, Terapii i Profilaktyki Zakażeń Układu Moczowego u Dorosłych [Recommendations for Diagnostics, Therapy and Prevention of Urinary Tract Infections in Adults]; Narodowy Program Ochrony Antybiotyków: Warsaw, Poland, 2015. [Google Scholar]
- Kranz, J.; Schmidt, S.; Lebert, C.; Schneidewind, L.; Mandraka, F.; Kunze, M.; Helbig, S.; Vahlensieck, W.; Naber, K.; Schmiemann, G.; et al. The 2017 Update of the German Clinical Guideline on Epidemiology, Diagnostics, Therapy, Prevention, and Management of Uncomplicated Urinary Tract Infections in Adult Patients. Part II: Therapy and Prevention. Urol. Int. 2018, 100, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Conklin, J.D. The pharmacokinetics of nitrofurantoin and its related bioavailability. Antibiot. Chemother. 1978, 25, 233–252. [Google Scholar] [PubMed]
- Männistö, P.; Karttunen, P. Pharmacokinetics of furagin, a new nitrofurantoin congener, on human volunteers. Int. J. Clin. Pharmacol. Biopharm. 1979, 17, 264–270. [Google Scholar]
- Mancuso, G.; Midiri, A.; Gerace, E.; Marra, M.; Zummo, S.; Biondo, C. Urinary Tract Infections: The Current Scenario and Future Prospects. Pathogens 2023, 12, 623. [Google Scholar] [CrossRef]
- Willems, C.S.; van den Broek D’Obrenan, J.; Numans, M.E.; Verheij, T.J.; van der Velden, A.W. Cystitis: Antibiotic prescribing, consultation, attitudes and opinions. Fam. Pract. 2014, 31, 149–155. [Google Scholar] [CrossRef]
- Leydon, G.M.; Turner, S.; Smith, H.; Little, P.; UTIS team. The journey from self-care to GP care: A qualitative interview study of women presenting with symptoms of urinary tract infection. Br. J. Gen. Pract. 2009, 59, e219–e225. [Google Scholar] [CrossRef]
- Kahlmeter, G.; Poulsen, H.O. Antimicrobial susceptibility of Escherichia coli from community-acquired urinary tract infections in Europe: The ECO·SENS study revisited. Int. J. Antimicrob. Agents 2012, 39, 45–51. [Google Scholar] [CrossRef]
- Naber, K.G.; Schitob, G.; Bottoc, H.; Palou, J.; Mazzei, T. Surveillance Study in Europe and Brazil on Clinical Aspects and AntimicrobialResistance Epidemiology in Females with Cystitis (ARESC): Implications for Empiric Therapy. Eur. Urol. 2008, 54, 1164–1178. [Google Scholar] [CrossRef]
- Adugna, B.; Sharew, B.; Jemal, M. Bacterial Profile, Antimicrobial Susceptibility Pattern, and Associated Factors of Community- and Hospital-Acquired Urinary Tract Infection at Dessie Referral Hospital, Dessie, Northeast Ethiopia. Int. J. Microbiol. 2021, 2021, 5553356. [Google Scholar] [CrossRef]
- Bangash, K.; Mumtaz, H.; Mehmood, M.; Hingoro, M.A.; Khan, Z.Z.; Sohail, A.; Ullah, S.; Maqbool, D.; Umm-E-Farwa; Jamal, N.; et al. Twelve-year trend of Escherichia coli antibiotic resistance in the Islamabad population. Ann. Med. Surg. 2022, 78, 103855. [Google Scholar] [CrossRef] [PubMed]
- Koningstein, M.; van der Bij, A.K.; de Kraker, M.E.; Monen, J.C.; Muilwijk, J.; de Greeff, S.C.; Geerlings, S.E.; Leverstein-van Hall, M.A.; ISIS-AR Study Group. Recommendations for the empirical treatment of complicated urinary tract infections using surveillance data on antimicrobial resistance in the Netherlands. PLoS ONE 2014, 9, e86634. [Google Scholar] [CrossRef] [PubMed]
- Gautam, G.; Gogoi, S.; Saxena, S.; Kaur, R.; Dhakad, M.S. Nitrofurantoin Susceptibility Pattern in Gram-Negative Urinary Isolates: In Need of Increased Vigilance. J. Lab. Physicians 2021, 13, 252–256. [Google Scholar] [CrossRef] [PubMed]
- Stoltidis-Claus, C.; Rosenberger, K.D.; Mandraka, F.; Quante, X.; Gielen, J.; Hoffmann, D.; Wisplinghoff, H.; Jazmati, N. Antimicrobial resistance of clinical Enterobacterales isolates from urine samples, Germany, 2016 to 2021. Eurosurveillance 2023, 28, 2200568. [Google Scholar] [CrossRef]
- Firissa, Y.B.; Shelton, D.; Azazh, A.; Engida, H.; Kifle, F.; Debebe, F. Prevalence and Antimicrobial Sensitivity Patterns of Uropathogens, in Tikur Anbessa Specialized Hospital Emergency Medicine Department Addis Ababa, Ethiopia. Infect. Drug Resist. 2023, 16, 1649–1656. [Google Scholar] [CrossRef]
- Patel, H.B.; Soni, S.T.; Bhagyalaxmi, A.; Patel, N.M. Causative agents of urinary tract infections and their antimicrobial susceptibility patterns at a referral center in Western India: An audit to help clinicians prevent antibiotic misuse. J. Fam. Med. Prim. Care 2019, 8, 154–159. [Google Scholar] [CrossRef]
- Sanchez, G.V.; Master, R.N.; Karlowsky, J.A.; Bordon, J.M. In vitro antimicrobial resistance of urinary Escherichia coli isolates among U.S. outpatients from 2000 to 2010. Antimicrob. Agents Chemother. 2012, 56, 2181–2183. [Google Scholar] [CrossRef]
- Salles, M.J.; Zurita, J.; Mejía, C.; Villegas, M.V.; Latin America Working Group on Bacterial Resistance. Resistant gram-negative infections in the outpatient setting in Latin America. Epidemiol. Infect. 2013, 141, 2459–2472. [Google Scholar] [CrossRef]
- Fasugba, O.; Mitchell, B.G.; Mnatzaganian, G.; Das, A.; Collignon, P.; Gardner, A. Five-Year Antimicrobial Resistance Patterns of Urinary Escherichia coli at an Australian Tertiary Hospital: Time Series Analyses of Prevalence Data. PLoS ONE 2016, 11, e0164306. [Google Scholar] [CrossRef]
- Cuningham, W.; Perera, S.; Coulter, S.; Nimmo, G.R.; Yarwood, T.; Tong, S.Y.C.; Wozniak, T.M. Antibiotic resistance in uropathogens across northern Australia 2007–20 and impact on treatment guidelines. JAC Antimicrob. Resist. 2021, 3, dlab127. [Google Scholar] [CrossRef]
- Gofron, Z.F.; Aptekorz, M.; Gibas, K.W.; Kabała, M.; Martirosian, G. Retrospective Study of the Etiology, Laboratory Findings, and Management of Patients with Urinary Tract Infections and Urosepsis from a Urology Center in Silesia, Southern Poland Between 2017 and 2020. Med. Sci. Monit. 2022, 28, e935478. [Google Scholar] [CrossRef]
- Klesiewicz, K.; Karczewska, E.; Nowak, P.; Mrowiec, P.; Skiba-Kurek, I.; Białecka, J.; Majka, Z.; Berdzik-Kalarus, S.; Budak, A.; Zajdel, P. Comparative in vitro studies of furazidin and nitrofurantoin activities against common uropathogens including multidrug-resistant strains of E. coli and S. aureus. Acta Pol. Pharm. 2018, 75, 803. [Google Scholar]
- ISO 20776-1:2019; Susceptibility Testing of Infectious Agents and Evaluation of Performance of Antimicrobial Susceptibility Test Devices—Part 1: Broth Micro-Dilution Reference Method for Testing the In Vitro Activity of Antimicrobial Agents against Rapidly Growing Aerobic Bacteria Involved in Infectious Diseases. ISO: Geneva, Switzerland, 2019.
- EUCAST. Reading Guide for Broth Microdilution; Ver. 4.0; EUCAST: Växjö, Sweden, 2022; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Reading_guide_BMD_v_4.0_2022.pdf (accessed on 30 June 2023).
- EUCAST. Antimicrobial Susceptibility Testing: Disk Diffusion Method; Ver. 10.0; EUCAST: Växjö, Sweden, 2022; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Manual_v_10.0_EUCAST_Disk_Test_2022.pdf (accessed on 30 June 2023).
- EUCAST. Reading Guide: Disk Diffusion Method for Antimicrobial Susceptibility Testing; Ver. 9.0; EUCAST: Växjö, Sweden, 2022; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Disk_test_documents/2022_manuals/Reading_guide_v_9.0_EUCAST_Disk_Test_2022.pdf (accessed on 30 June 2023).
- Bielec, F.; Brauncajs, M.; Pastuszak-Lewandoska, D. Comparison of Substance Sources in Experimental Antimicrobial Susceptibility Testing. Sci. Pharm. 2023, 91, 10. [Google Scholar] [CrossRef]
- EUCAST. Breakpoint Tables for Interpretation of MICs and Zone Diameters; Ver. 13.0; EUCAST: Växjö, Sweden, 2023; Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_13.0_Breakpoint_Tables.pdf (accessed on 30 June 2023).
- Paul, H.E.; Harrington, C.M.; Bender, R.C.; Briggs, W.P. Resistance and cross resistance of bacteria to nitrofurans. Proc. Soc. Exp. Biol. Med. 1952, 79, 199–204. [Google Scholar] [CrossRef]
- Borkowska-Opacka, B.; Truszczyński, M. Krzyzowa oporność na zwiazki nitrofuranowe szczepów Escherichia coli izolowanych od świń [Cross resistance of E. coli strains isolated from swine to nitrofuran compounds]. Med. Dosw. Mikrobiol. 1972, 24, 1–7. [Google Scholar]
- Mattelaer, J.J. A new urinary antiseptic with prolonged action, urfadyn PL, in the treatment of urinary tract infections. Acta Urol. Belg. 1982, 50, 270–275. [Google Scholar] [PubMed]
- CLSI. M100: Performance Standards for Antimicrobial Susceptibility Testing, 33rd ed.; CLSI: Wayne, PA, USA, 2023; Available online: https://em100.edaptivedocs.net/GetDoc.aspx?doc=CLSI%20M100%20ED33:2023 (accessed on 30 July 2023).
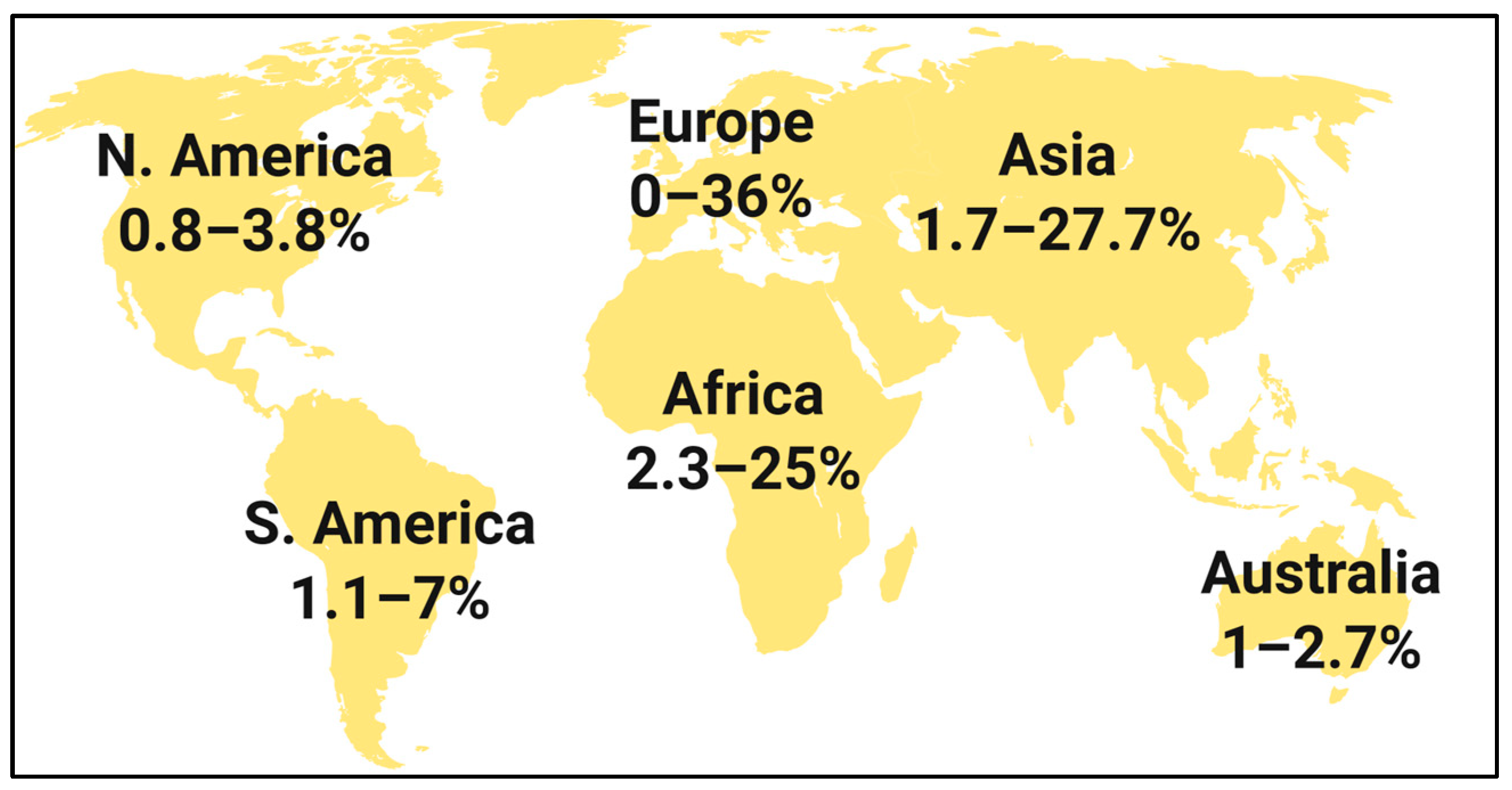
| Bacteria | CA-UTI | HA-UTI | |
|---|---|---|---|
| Gram-negative | Escherichia coli | 51–84% | 25–45% |
| Klebsiella pneumoniae | 4–17% | 10–38% | |
| CESP group | 2–9% | 4–11% | |
| Non-fermenters | 0–7% | 8–19% | |
| Gram-positive | Enterococcus spp. | 2–16% | 4–42% |
| Staphylococcus spp. | 1–8% | 3–6% | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bielec, F.; Brauncajs, M.; Pastuszak-Lewandoska, D. Nitrofuran Derivatives Cross-Resistance Evidence—Uropathogenic Escherichia coli Nitrofurantoin and Furazidin In Vitro Susceptibility Testing. J. Clin. Med. 2023, 12, 5166. https://doi.org/10.3390/jcm12165166
Bielec F, Brauncajs M, Pastuszak-Lewandoska D. Nitrofuran Derivatives Cross-Resistance Evidence—Uropathogenic Escherichia coli Nitrofurantoin and Furazidin In Vitro Susceptibility Testing. Journal of Clinical Medicine. 2023; 12(16):5166. https://doi.org/10.3390/jcm12165166
Chicago/Turabian StyleBielec, Filip, Małgorzata Brauncajs, and Dorota Pastuszak-Lewandoska. 2023. "Nitrofuran Derivatives Cross-Resistance Evidence—Uropathogenic Escherichia coli Nitrofurantoin and Furazidin In Vitro Susceptibility Testing" Journal of Clinical Medicine 12, no. 16: 5166. https://doi.org/10.3390/jcm12165166
APA StyleBielec, F., Brauncajs, M., & Pastuszak-Lewandoska, D. (2023). Nitrofuran Derivatives Cross-Resistance Evidence—Uropathogenic Escherichia coli Nitrofurantoin and Furazidin In Vitro Susceptibility Testing. Journal of Clinical Medicine, 12(16), 5166. https://doi.org/10.3390/jcm12165166








