Impact of Osteoporosis Pharmacotherapy on Functional Outcomes after Ischemic Stroke
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Data Collection and Definition of Parameters
2.3. Bone Mineral Density Measurements
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, S.B.; Cho, A.H.; Butcher, K.S.; Kim, T.W.; Ryu, S.Y.; Kim, Y.I. Low bone mineral density is associated with poor clinical outcome in acute ischemic stroke. Int. J. Stroke 2013, 8, 68–72. [Google Scholar] [CrossRef]
- Jorgensen, L.; Jacobsen, B.K. Functional status of the paretic arm affects the loss of bone mineral in the proximal humerus after stroke: A 1-year prospective study. Calcif. Tissue Int. 2001, 68, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ramnemark, A.; Nilsson, M.; Borssen, B.; Gustafson, Y. Stroke, a major and increasing risk factor for femoral neck fracture. Stroke 2000, 31, 1572–1577. [Google Scholar] [CrossRef]
- Dennis, M.S.; Lo, K.M.; McDowall, M.; West, T. Fractures after stroke: Frequency, types, and associations. Stroke 2002, 33, 728–734. [Google Scholar] [CrossRef]
- Nordstrom, A.; Eriksson, M.; Stegmayr, B.; Gustafson, Y.; Nordstrom, P. Low bone mineral density is an independent risk factor for stroke and death. Cerebrovasc. Dis. 2010, 29, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Carda, S.; Cisari, C.; Invernizzi, M.; Bevilacqua, M. Osteoporosis after stroke: A review of the causes and potential treatments. Cerebrovasc. Dis. 2009, 28, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, E.; Austin, P.C.; Alibhai, S.M.H.; Cheung, A.M.; Cram, P.; Casaubon, L.K.; Fang, J.; Porter, J.; Smith, E.E.; Prager, M.; et al. Screening and Treatment for Osteoporosis After Stroke. Stroke 2019, 50, 1564–1566. [Google Scholar] [CrossRef]
- Winstein, C.J.; Stein, J.; Arena, R.; Bates, B.; Cherney, L.R.; Cramer, S.C.; Deruyter, F.; Eng, J.J.; Fisher, B.; Harvey, R.L.; et al. Guidelines for Adult Stroke Rehabilitation and Recovery: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2016, 47, e98–e169. [Google Scholar] [CrossRef]
- Poole, K.E.; Reeve, J.; Warburton, E.A. Falls, fractures, and osteoporosis after stroke: Time to think about protection? Stroke 2002, 33, 1432–1436. [Google Scholar] [CrossRef]
- Sallehuddin, H.; Ong, T.; Md Said, S.; Ahmad Tarmizi, N.A.; Loh, S.P.; Lim, W.C.; Nadarajah, R.; Lim, H.T.; Mohd Zambri, N.H.; Ho, Y.Y.; et al. Non-pharmacological interventions for bone health after stroke: A systematic review. PLoS ONE 2022, 17, e0263935. [Google Scholar] [CrossRef]
- Sulter, G.; Steen, C.; De Keyser, J. Use of the Barthel index and modified Rankin scale in acute stroke trials. Stroke 1999, 30, 1538–1541. [Google Scholar] [CrossRef]
- Ganesh, A.; Luengo-Fernandez, R.; Rothwell, P.M. Late functional improvement and 5-year poststroke outcomes: A population-based cohort study. J. Neurol. Neurosurg. Psychiatry 2020, 91, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, A.; Luengo-Fernandez, R.; Wharton, R.M.; Gutnikov, S.A.; Silver, L.E.; Mehta, Z.; Rothwell, P.M.; Oxford Vascular, S. Time Course of Evolution of Disability and Cause-Specific Mortality After Ischemic Stroke: Implications for Trial Design. J. Am. Heart Assoc. 2017, 6, e005788. [Google Scholar] [CrossRef]
- Genant, H.K.; Cooper, C.; Poor, G.; Reid, I.; Ehrlich, G.; Kanis, J.; Nordin, B.E.; Barrett-Connor, E.; Black, D.; Bonjour, J.P.; et al. Interim report and recommendations of the World Health Organization Task-Force for Osteoporosis. Osteoporos. Int. 1999, 10, 259–264. [Google Scholar] [CrossRef]
- Poole, K.E.; Loveridge, N.; Rose, C.M.; Warburton, E.A.; Reeve, J. A single infusion of zoledronate prevents bone loss after stroke. Stroke 2007, 38, 1519–1525. [Google Scholar] [CrossRef]
- Ikai, T.; Uematsu, M.; Eun, S.S.; Kimura, C.; Hasegawa, C.; Miyano, S. Prevention of secondary osteoporosis postmenopause in hemiplegia. Am. J. Phys. Med. Rehabil. 2001, 80, 169–174. [Google Scholar] [CrossRef]
- Dehghani, F.; Conrad, A.; Kohl, A.; Korf, H.W.; Hailer, N.P. Clodronate inhibits the secretion of proinflammatory cytokines and NO by isolated microglial cells and reduces the number of proliferating glial cells in excitotoxically injured organotypic hippocampal slice cultures. Exp. Neurol. 2004, 189, 241–251. [Google Scholar] [CrossRef]
- Li, W.; Cheong, Y.K.; Wang, H.; Ren, G.; Yang, Z. Neuroprotective Effects of Etidronate and 2,3,3-Trisphosphonate Against Glutamate-Induced Toxicity in PC12 Cells. Neurochem. Res. 2016, 41, 844–854. [Google Scholar] [CrossRef]
- Inoue, R.; Matsuki, N.A.; Jing, G.; Kanematsu, T.; Abe, K.; Hirata, M. The inhibitory effect of alendronate, a nitrogen-containing bisphosphonate on the PI3K-Akt-NFkappaB pathway in osteosarcoma cells. Br. J. Pharmacol. 2005, 146, 633–641. [Google Scholar] [CrossRef]
- Giuliani, N.; Pedrazzoni, M.; Passeri, G.; Girasole, G. Bisphosphonates inhibit IL-6 production by human osteoblast-like cells. Scand. J. Rheumatol. 1998, 27, 38–41. [Google Scholar]
- Cantatore, F.P.; Acquista, C.A.; Pipitone, V. Evaluation of bone turnover and osteoclastic cytokines in early rheumatoid arthritis treated with alendronate. J. Rheumatol. 1999, 26, 2318–2323. [Google Scholar]
- Zameer, S.; Najmi, A.K.; Vohora, D.; Akhtar, M. Bisphosphonates: Future perspective for neurological disorders. Pharmacol. Rep. 2018, 70, 900–907. [Google Scholar] [CrossRef]
- Gamboa, A.; Duaso, E.; Marimon, P.; Sandiumenge, M.; Escalante, E.; Lumbreras, C.; Tarrida, A. Oral bisphosphonate prescription and non-adherence at 12 months in patients with hip fractures treated in an acute geriatric unit. Osteoporos. Int. 2018, 29, 2309–2314. [Google Scholar] [CrossRef]
- Marsden, J.; Gibson, L.M.; Lightbody, C.E.; Sharma, A.K.; Siddiqi, M.; Watkins, C. Can early onset bone loss be effectively managed in post-stroke patients? An integrative review of the evidence. Age Ageing 2008, 37, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.Y.; Sung, S.F.; Huang, H.K. Drug treatment strategies for osteoporosis in stroke patients. Expert Opin. Pharmacother. 2020, 21, 811–821. [Google Scholar] [CrossRef]
- Chen, L.R.; Ko, N.Y.; Chen, K.H. Medical Treatment for Osteoporosis: From Molecular to Clinical Opinions. Int. J. Mol. Sci. 2019, 20, 2213. [Google Scholar] [CrossRef]
- Black, D.M.; Delmas, P.D.; Eastell, R.; Reid, I.R.; Boonen, S.; Cauley, J.A.; Cosman, F.; Lakatos, P.; Leung, P.C.; Man, Z.; et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N. Engl. J. Med. 2007, 356, 1809–1822. [Google Scholar] [CrossRef]
- Peter, R.; Mishra, V.; Fraser, W.D. Severe hypocalcaemia after being given intravenous bisphosphonate. BMJ 2004, 328, 335–336. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.J.; Brown, S. Severe hypocalcemia after intravenous bisphosphonate therapy in occult vitamin D deficiency. N. Engl. J. Med. 2003, 348, 1503–1504. [Google Scholar] [CrossRef]
- An, T.; Hao, J.; Sun, S.; Li, R.; Yang, M.; Cheng, G.; Zou, M. Efficacy of statins for osteoporosis: A systematic review and meta-analysis. Osteoporos. Int. 2017, 28, 47–57. [Google Scholar] [CrossRef]
- Fiordellisi, W.; White, K.; Schweizer, M. A Systematic Review and Meta-analysis of the Association Between Vitamin K Antagonist Use and Fracture. J. Gen. Intern. Med. 2019, 34, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.M.; Yang, S.H.; Liang, C.C.; Huang, H.K. Proton pump inhibitor use and the risk of osteoporosis and fracture in stroke patients: A population-based cohort study. Osteoporos. Int. 2018, 29, 153–162. [Google Scholar] [CrossRef] [PubMed]

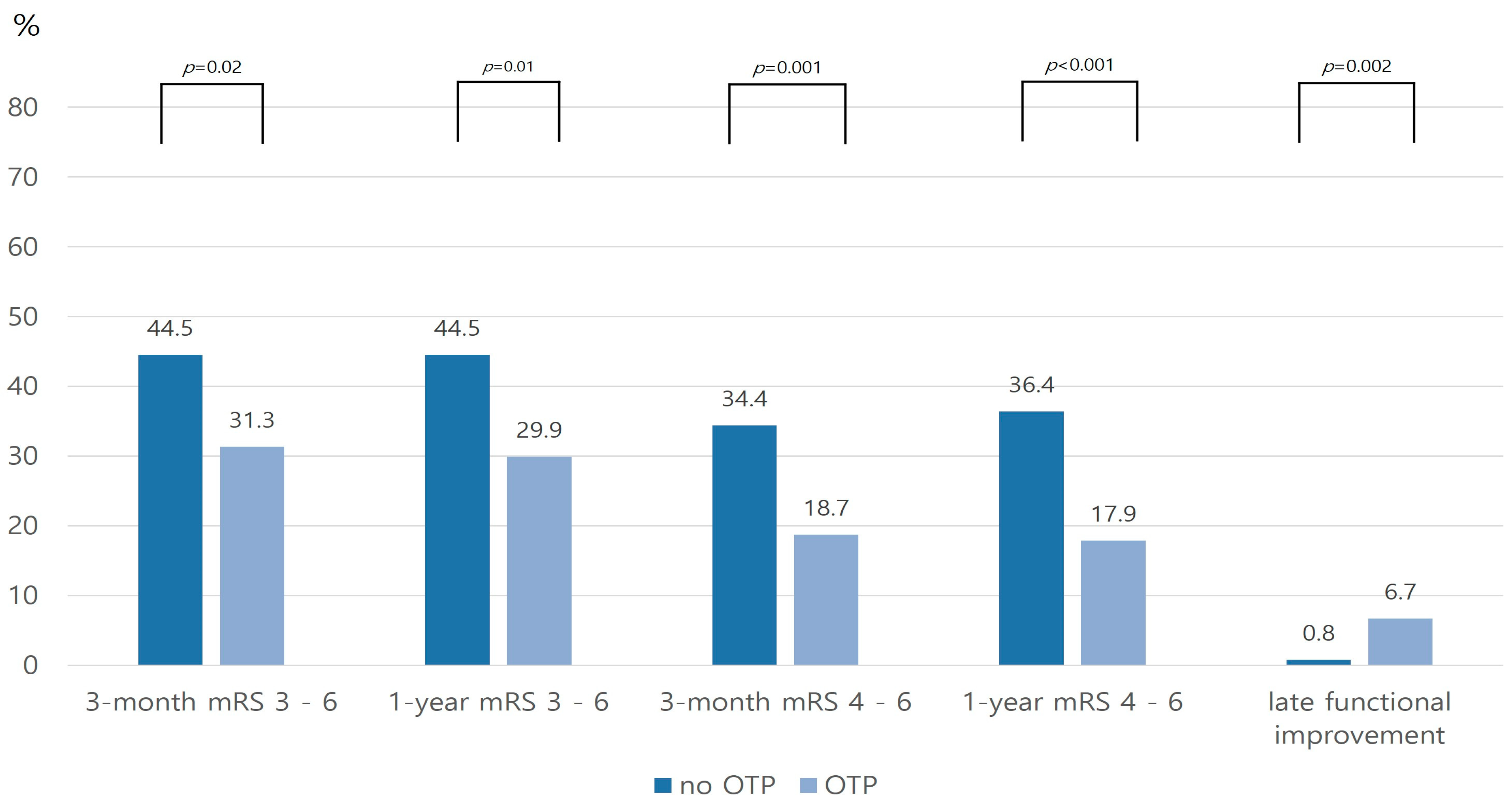

| Total Cohort | PSM Cohort | |||||
|---|---|---|---|---|---|---|
| No OPT (n = 247) | OPT (n = 134) | p-Value | No OPT (n = 134) | OPT (n = 134) | p-Value | |
| Age, years (SD) | 75.5 (11.0) | 75.8 (10.9) | 0.73 † | 76.0 (10.2) | 75.8 (10.9) | 0.89 |
| Female, n (%) | 171 (69.2) | 106 (79.1) | 0.04 * | 106 (79.1) | 106 (79.1) | 1.000 |
| BMI, kg/m2 (IQR) | 23.0 (21–25) | 22.2 (20.0–25.0) | 0.03 ‡ | 22.6 (20.8–24.0) | 22.2 (20.0–25.0) | 0.91 |
| Initial NIHSS score (IQR) | 4 (1–8) | 3 (1–5) | 0.01 ‡ | 2 (1–5) | 3 (1–5) | 0.53 |
| Stroke subtypes, n (%) | 0.32 * | 0.75 | ||||
| SVO | 72 (29.1) | 42 (31.3) | 48 (35.8) | 42 (31.3) | ||
| LAA | 72 (29.1) | 47 (35.1) | 39 (29.1) | 47 (35.1) | ||
| CE | 55 (22.3) | 20 (14.9) | 20 (14.9) | 20 (14.9) | ||
| Others | 48 (19.4) | 25 (18.7) | 27 (20.1) | 25 (18.7) | ||
| Prior stroke, n (%) | 58 (23.5) | 38 (28.4) | 0.32 * | 36 (26.9) | 38 (28.4) | 0.89 |
| Hypertension, n (%) | 156 (63.2) | 88 (65.7) | 0.66 * | 94 (70.1) | 88 (65.7) | 0.51 |
| Diabetes mellitus, n (%) | 82 (33.2) | 36 (26.9) | 0.21 * | 45 (33.6) | 36 (26.9) | 0.29 |
| Hyperlipidemia, n (%) | 32 (13.0) | 16 (11.9) | 0.87 * | 5 (11.2) | 16 (11.9) | 1.00 |
| Current smoking, n (%) | 16 (6.5) | 6 (4.5) | 0.50 * | 6 (4.5) | 6 (4.5) | 1.00 |
| Prior antiplatelet, n (%) | 69 (27.9) | 47 (35.1) | 0.16 * | 48 (35.8) | 47 (35.1) | 1.00 |
| Prior anticoagulation, n (%) | 23 (9.3) | 7 (5.2) | 0.17 * | 6 (4.5) | 7 (5.2) | 1.00 |
| Prior statin, n (%) | 25 (10.1) | 20 (14.9) | 0.19 * | 18 (13.4) | 20 (14.9) | 0.86 |
| Reperfusion therapy, n (%) | 0.27 * | 0.37 | ||||
| No | 209 (84.6) | 120 (89.6) | 125 (93.3) | 120 (89.6) | ||
| IVT | 15 (6.1) | 9 (6.7) | 8 (6.0) | 9 (6.7) | ||
| EVT | 10 (4.0) | 2 (1.5) | 0 (0.0) | 2 (1.5) | ||
| IVT+EVT | 13 (5.3) | 3 (2.2) | 1 (0.7) | 3 (2.2) | ||
| Lesions, n (%) | 0.71 * | 0.95 | ||||
| Anterior | 198 (80.2) | 103 (76.9) | 101 (75.4) | 103 (76.9) | ||
| Posterior | 35 (14.6) | 24 (17.9) | 26 (19.4) | 24 (17.9) | ||
| Multiple | 13 (5.3) | 7 (5.2) | 7 (5.2) | 7 (5.2) | ||
| Rehabilitation, n (%) | 57 (23.1) | 35 (26.1) | 0.53 * | 36 (26.9) | 35 (26.1) | 1.00 |
| Vertebral fracture, n (%) | 12 (4.9) | 11 (8.2) | 0.26 * | 9 (6.7) | 11 (8.2) | 0.82 |
| Femur fracture, n (%) | 6 (2.4) | 3 (2.2) | 0.91 * | 4 (3.0) | 3 (2.2) | 1.00 |
| mRS at discharge > 2, n (%) | 92 (38.0) | 42 (30.2) | 0.15 * | 42 (31.3) | 42 (31.3) | 1.00 |
| Laboratory findings | ||||||
| Osteocalcin, ng/mL (SD) | 13.4 (16.9) | 14.2 (12.5) | 0.59 † | 13.4 (16.6) | (14.2 ± 12.5) | 0.64 |
| CTx, ng/mL (SD) | 0.5 (1.2) | 0.5 (0.5) | 0.94 † | 0.6 (1.7) | 0.5 (0.5) | 0.60 |
| 25 (OH) Vitamin D, ng/mL (SD) | 16.1 (8.6) | 16.6 (10.4) | 0.63 † | 16.2 (8.3) | 16.6 (10.4) | 0.78 |
| Calcium, mg/dL (SD) | 9.1 (0.4) | 9.1 (0.5) | 0.31 † | 9.1 (0.4) | 9.1 (0.5) | 0.51 |
| Phosphorus, mg/dL (SD) | 3.3 (0.5) | 3.4 (0.7) | 0.11 † | 3.3 (0.5) | 3.4 (0.7) | 0.17 |
| Creatinine, mg/dL (SD) | 1.0 (0.9) | 1.0 (0.9) | 0.93 † | 1.0 (0.9) | 1.0 (0.9) | 0.81 |
| Hemoglobin, mg/dL (SD) | 13.0 (2.2) | 12.9 (1.9) | 0.52 † | 12.9 (2.0) | 12.9 (1.9) | 0.87 |
| Platelet count, uL/103 (SD) | 223.6 (66.9) | 228.3 (58.8) | 0.50 † | 230.4 (58.0) | 228.3 (58.8) | 0.77 |
| LDL, mg/dL (SD) | 97.8 (38.3) | 99.2 (33.3) | 0.72 † | 98.9 (38.5) | 99.2 (33.3) | 0.96 |
| HbA1c, % (SD) | 6.0 (1.3) | 5.9 (0.9) | 0.15 † | 5.9 (1.2) | 5.9 (0.9) | 0.60 |
| CRP, mg/dL (SD) | 11.7 (29.7) | 8.6 (30.1) | 0.34 † | 8.9 (19.9) | 8.6 (30.1) | 0.93 |
| Initial glucose, mg/dL (SD) | 135.4 (56.5) | 131.9 (39.5) | 0.49 † | 136.8 (62.9) | 131.9 (39.5) | 0.45 |
| SBP, mmHg (SD) | 149.1 (28.1) | 149.0 (26.7) | 0.97 † | 146.8 (28.9) | 149.0 (26.7) | 0.51 |
| T3, μU/mL (SD) | 73.2 (23.4) | 74.6 (21.5) | 0.57 † | 75.6 (22.1) | 74.6 (21.5) | 0.73 |
| fT4, μU/mL (SD) | 1.0 (0.18) | 1.0 (0.2) | 0.49 † | 1.0 (0.2) | 1.0 (0.2) | 0.91 |
| TSH, μU/mL (SD) | 1.6 (1.2) | 1.6 (1.2) | 0.91 † | 1.5 (1.0) | 1.6 (1.2) | 0.60 |
| 1-Year Dependency (mRS 3–6) | 1-Year Poor Functional Outcome (mRS 4–6) | Late Improvement Functional Outcome | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| OPT | 0.52 | 0.27–0.996 | 0.24 | 0.10–0.57 | 6.16 | 1.12–33.79 |
| Age | 1.05 | 1.01–1.09 | 1.05 | 1.001–1.09 | 1.08 | 0.99–1.17 |
| Female | 0.996 | 0.42–2.37 | 0.62 | 0.22–1.72 | 0.90 | 0.17–4.95 |
| BMI | 0.92 | 0.82–1.03 | 0.97 | 0.85–1.11 | 0.91 | 0.73–1.14 |
| Initial NIHSS | 1.32 | 1.19–1.45 | 1.42 | 1.26–1.59 | 0.99 | 0.82–1.18 |
| Stroke subtypes | ||||||
| SVO | ref | ref | ref | |||
| LAA | 1.39 | 0.63–3.08 | 1.21 | 0.44–3.29 | 0.90 | 0.17–4.63 |
| CE | 1.17 | 0.40–3.38 | 1.02 | 0.28–3.68 | 0.73 | 0.09–6.08 |
| Others | 0.88 | 0.33–2.31 | 1.04 | 0.33–3.30 | 0.21 | 0.02–3.00 |
| Rehab | 1.35 | 0.68–2.70 | 0.29 | 0.11–0.77 | 0.75 | 0.16–3.43 |
| Fracture | 10.57 | 3.47–32.21 | 15.95 | 4.87–52.26 | 0.27 | 0.02–3.68 |
| Vitamin D | 0.98 | 0.94–1.01 | 0.97 | 0.93–1.02 | 0.93 | 0.85–1.02 |
| Calcium | 1.43 | 0.71–2.92 | 1.62 | 0.68–3.89 | 0.30 | 0.08–1.11 |
| Phosphorus | 1.17 | 0.72–1.91 | 1.30 | 0.67–3.89 | 0.92 | 0.32–2.68 |
| Osteocalcin | 1.02 | 0.99–1.04 | 1.01 | 0.97–1.05 | 0.95 | 0.88–1.03 |
| CTx | 0.91 | 0.63–1.39 | 0.50 | 0.10–2.51 | 1.03 | 0.50–2.15 |
| 3-Month Poor Functional Outcome in PSM Cohort (mRS 4–6) | 3-Month Poor Functional Outcome in Total Cohort (mRS 4–6) | |||
|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |
| OPT | 0.30 | 0.13–0.72 | 0.49 | 0.25–0.94 |
| Age | 1.05 | 1.01–1.10 | 1.03 | 1.00–1.06 |
| Female | 0.45 | 0.16–1.27 | 0.96 | 0.48–1.92 |
| BMI | 0.95 | 0.83–1.09 | 0.95 | 0.48–1.92 |
| Initial NIHSS | 1.43 | 1.26–1.61 | 1.29 | 1.20–1.39 |
| Stroke subtypes | ||||
| SVO | ref | ref | ||
| LAA | 1.11 | 0.39–3.17 | 1.13 | 0.50–2.58 |
| CE | 1.79 | 0.51–6.29 | 1.83 | 0.71–4.69 |
| Others | 1.22 | 0.37–4.00 | 1.82 | 0.75–4.45 |
| Rehab | 0.16 | 0.05–0.49 | 0.09 | 0.03–0.24 |
| Vitamin D | 0.96 | 0.91–1.01 | 0.97 | 0.94–1.01 |
| Calcium | 1.24 | 0.51–3.03 | 0.67 | 0.32–1.39 |
| Phosphorus | 1.41 | 0.77–2.59 | 1.11 | 0.63–1.94 |
| Osteocalcin | 1.01 | 0.97–1.05 | 0.997 | 0.97–1.02 |
| CTx | 0.44 | 0.09–2.21 | 0.77 | 0.25–2.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sohn, J.-H.; Kim, C.; Kim, Y.; Park, S.Y.; Lee, S.-H. Impact of Osteoporosis Pharmacotherapy on Functional Outcomes after Ischemic Stroke. J. Clin. Med. 2023, 12, 4905. https://doi.org/10.3390/jcm12154905
Sohn J-H, Kim C, Kim Y, Park SY, Lee S-H. Impact of Osteoporosis Pharmacotherapy on Functional Outcomes after Ischemic Stroke. Journal of Clinical Medicine. 2023; 12(15):4905. https://doi.org/10.3390/jcm12154905
Chicago/Turabian StyleSohn, Jong-Hee, Chulho Kim, Yerim Kim, So Young Park, and Sang-Hwa Lee. 2023. "Impact of Osteoporosis Pharmacotherapy on Functional Outcomes after Ischemic Stroke" Journal of Clinical Medicine 12, no. 15: 4905. https://doi.org/10.3390/jcm12154905
APA StyleSohn, J.-H., Kim, C., Kim, Y., Park, S. Y., & Lee, S.-H. (2023). Impact of Osteoporosis Pharmacotherapy on Functional Outcomes after Ischemic Stroke. Journal of Clinical Medicine, 12(15), 4905. https://doi.org/10.3390/jcm12154905





