Current Opinions in Open and Endovascular Treatment of Major Arterial Injuries in Pediatric Patient
Abstract
1. Introduction
2. Methods
3. Search Strategy
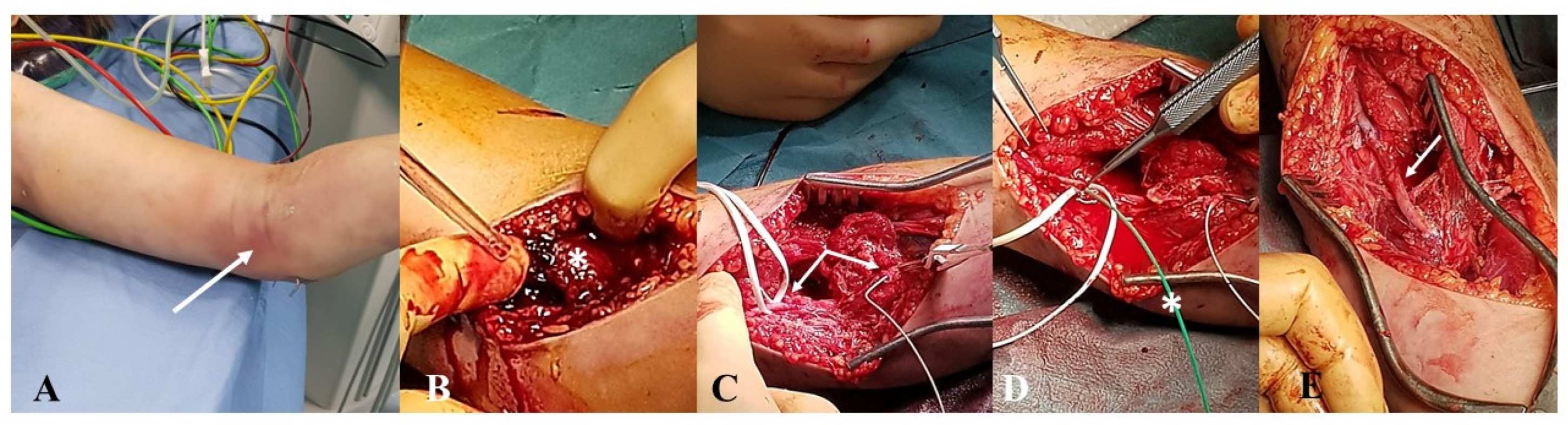
3.1. Institutional Case Report #1—Upper Extremity Injury and Open Surgery
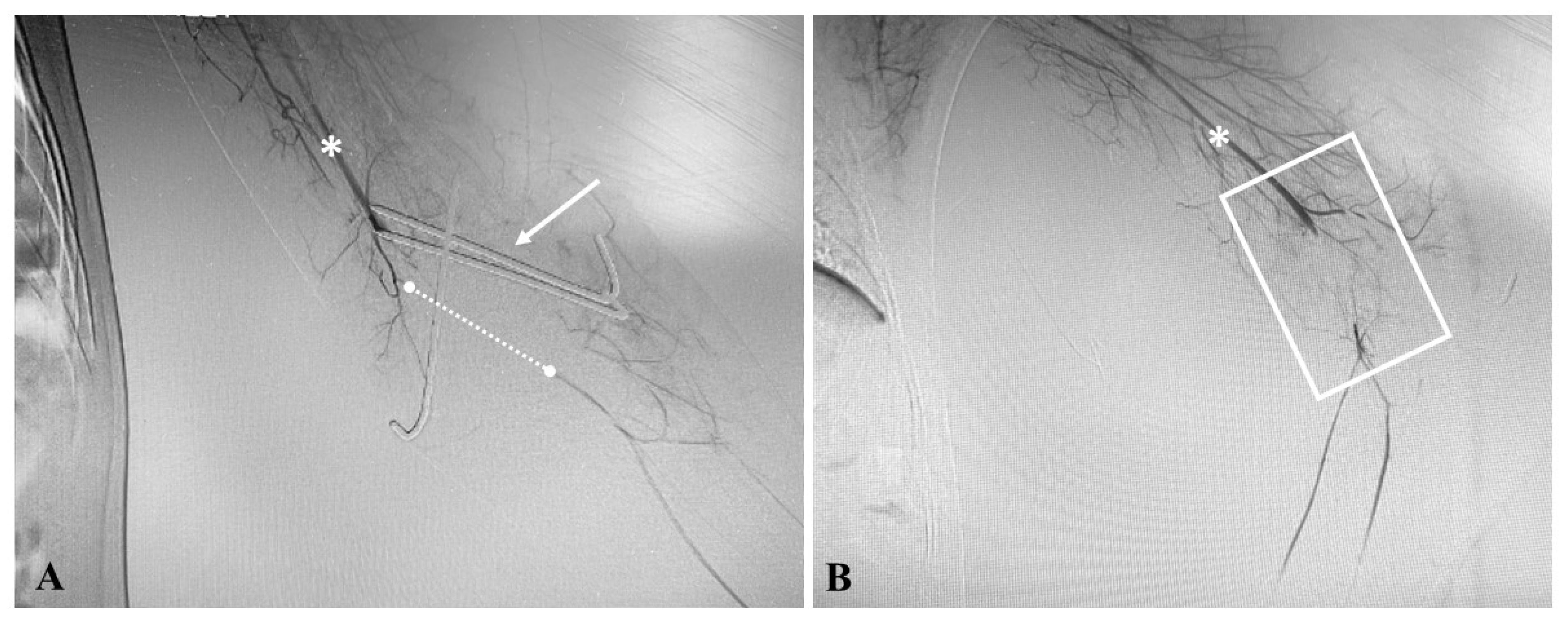
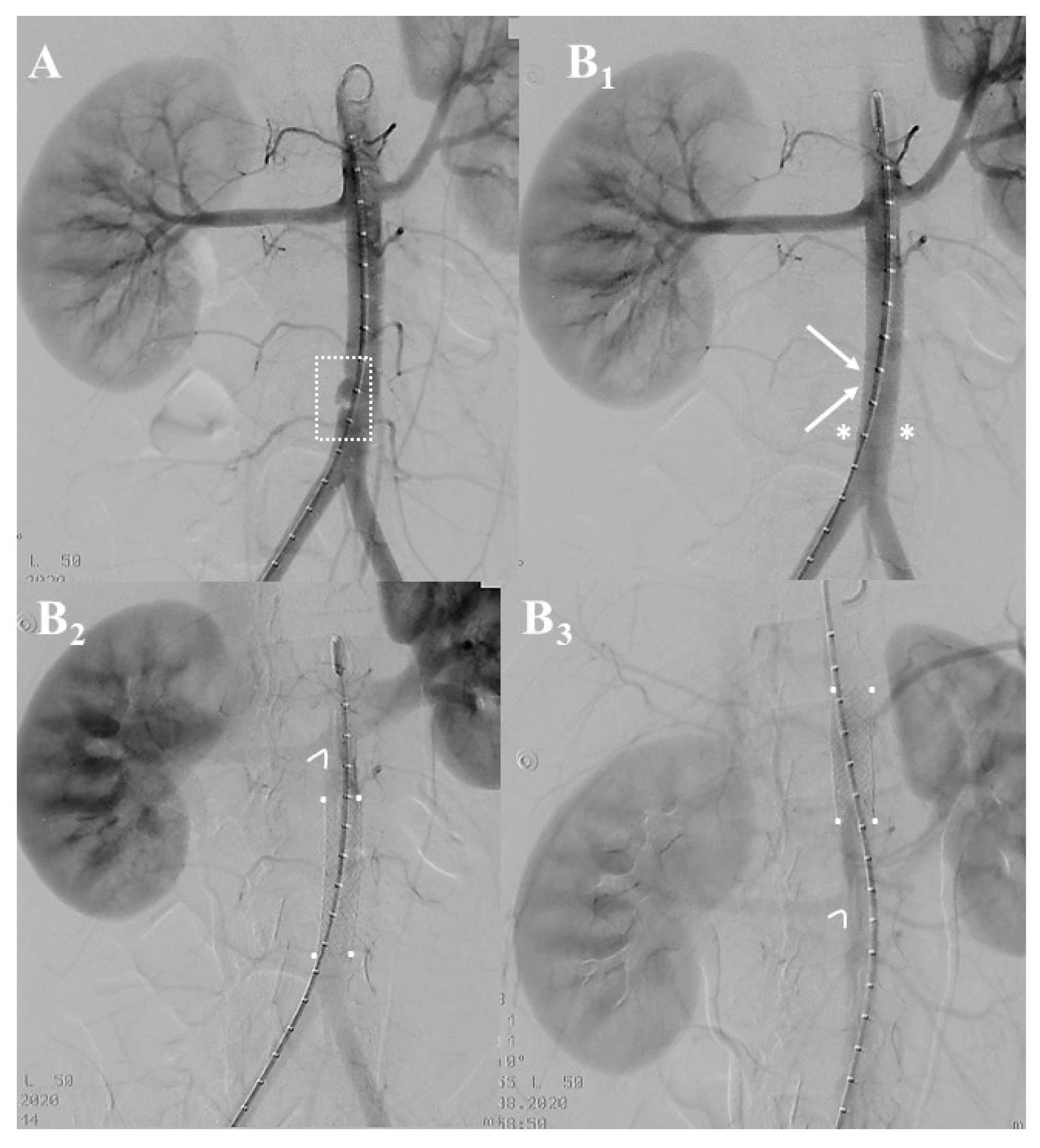
3.2. Institutional Case Report #2—Intra-Abdominal Injury and Endovascular Treatment
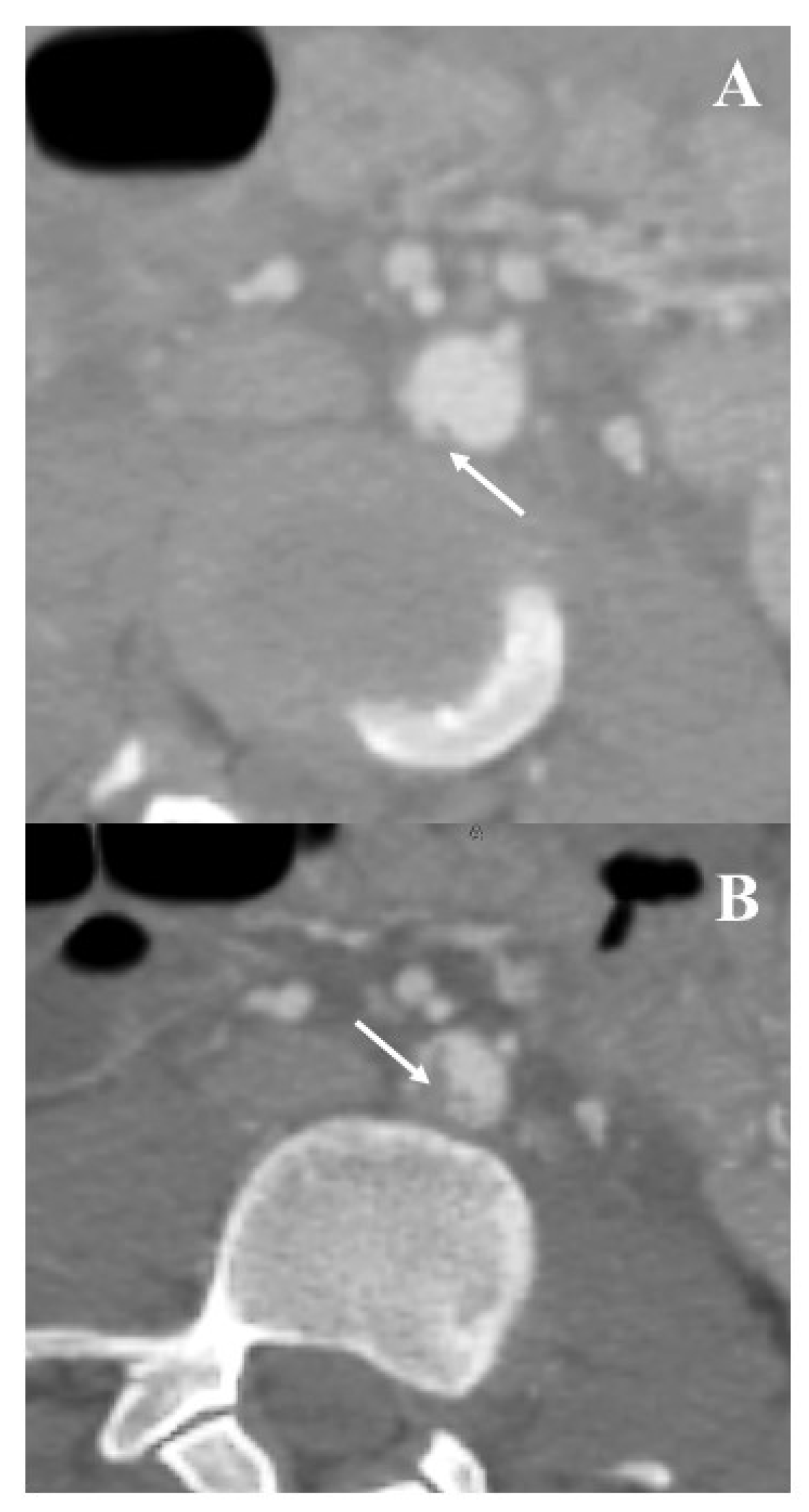
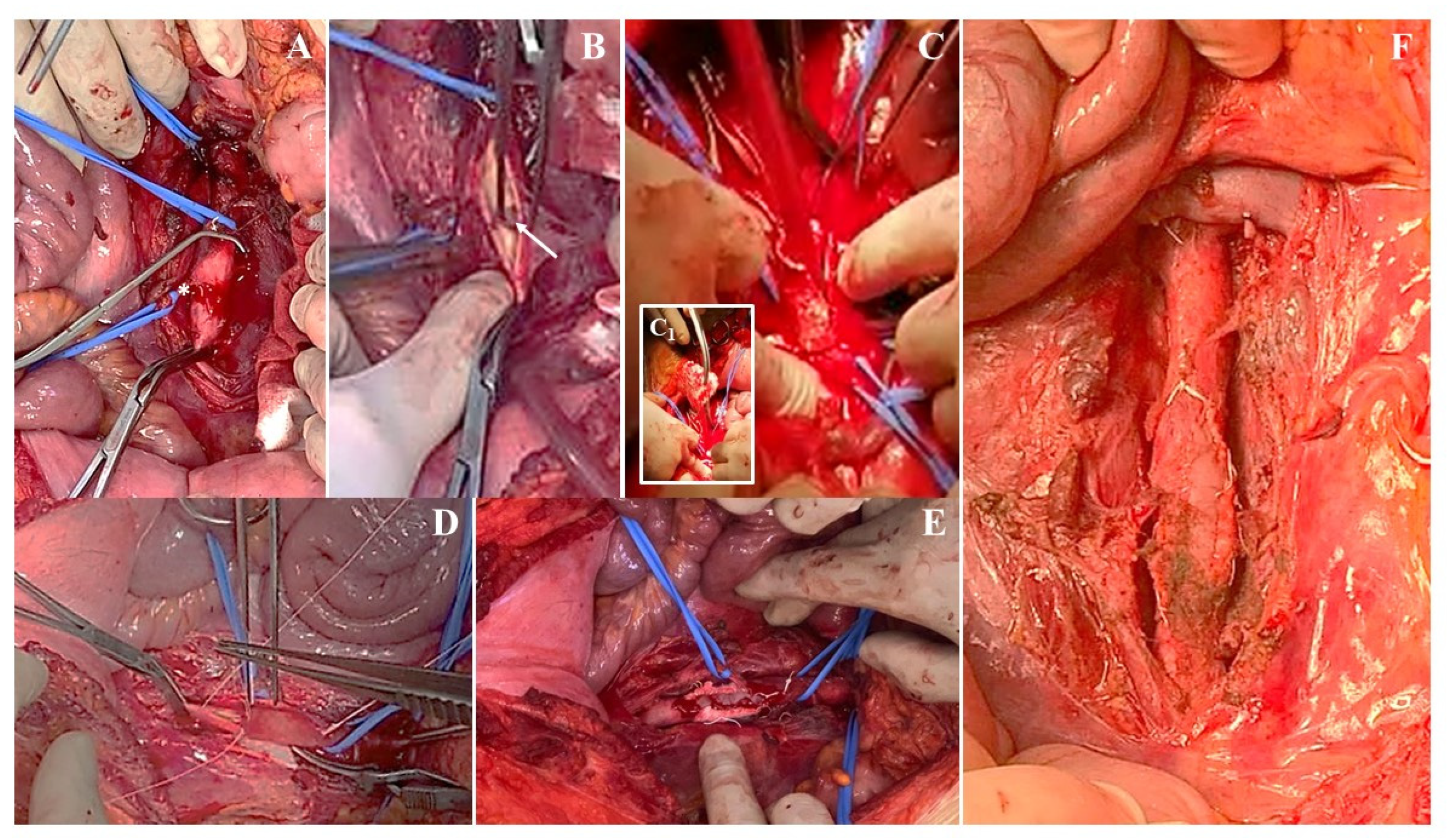
3.3. Institutional Case Report #3—Aortic Injury and Open Surgery for Failed Endovascular Treatment
4. Results
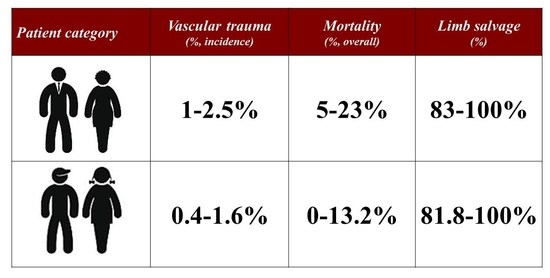
4.1. The Amount of Problem
4.2. Presenting Patterns and Type of Injuries
4.3. Vascular Diagnostic
4.4. Key Role of Pediatric Expertise
4.5. Essentials of Open Operative Repair
4.6. Endovascular Repair
4.7. Mortality and Morbidity
4.8. Postoperative Management
4.9. Follow-Up
4.10. Limitations
5. Conclusions
6. Future Directions
- Pediatric major arterial traumas, though infrequent, are still a matter of concern in the life of vascular and trauma surgeons. This means only a few cases per surgeon, and therefore, a possible solution to improve expertise in managing vascular lesions in pediatric patients could be better training in the management of vascular trauma during the education of pediatric (vascular) surgeons.
- Future research will be directed toward studying long-term outcomes. Long-term follow-up is needed in pediatric patients treated with endovascular repair for aortic traumas. Additionally, developing technologies such as readily available conduits for the reconstruction of extremity vessels that have the ability to grow with a child could eliminate the need for multiple reoperations and long-term morbidity. Lastly, further analysis is necessary to determine which pharmacological agents should be utilized, and the optimal length of treatment.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Barmparas, G.; Inaba, K.; Talving, P.; David, J.-S.; Lam, L.; Plurad, D.; Green, D.; Demetriades, D. Pediatric vs adult vascular trauma: A National Trauma Databank review. J. Pediatr. Surg. 2010, 45, 1404–1412. [Google Scholar] [CrossRef]
- Reichert, M.; Sartelli, M.; Askevold, I.H.; Braun, J.; Weigand, M.A.; Hecker, M.; Agnoletti, V.; Coccolini, F.; Catena, F.; Padberg, W.; et al. Pediatric trauma and emergency surgery: An international cross-sectional survey among WSES members. World J. Emerg. Surg. 2023, 18, 6. [Google Scholar] [CrossRef] [PubMed]
- Mommsen, P.; Zeckey, C.; Hildebrand, F.; Frink, M.; Khaladj, N.; Lange, N.; Krettek, C.; Probst, C. Traumatic extremity arterial injury in children: Epidemiology, diagnostics, treatment and prognostic value of Mangled Extremity Severity Score. J. Orthop. Surg. Res. 2010, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, J.D.; Macedo, F.I.B.; Chung, E.L.; Otero, C.A.; Pizano, L.R.; Namias, N. Management of lower extremity vascular injuries in pediatric trauma patients: A single Level I trauma center experience. J. Trauma Inj. Infect. Crit. Care 2014, 76, 1386–1389. [Google Scholar] [CrossRef]
- Kirkilas, M.; Notrica, D.M.; Langlais, C.S.; Muenzer, J.T.; Zoldos, J.; Graziano, K.; Kirkilas, M.; Notrica, D.M.; Langlais, C.S.; Muenzer, J.T.; et al. Outcomes of arterial vascular extremity trauma in pediatric patients. J. Pediatr. Surg. 2016, 51, 1885–1890. [Google Scholar] [CrossRef]
- Morão, S.; Ferreira, R.S.; Camacho, N.; Vital, V.P.; Pascoal, J.; Ferreira, M.E.; Capitão, L.M.; Gonçalves, F.B. Vascular Trauma in Children—Review from a Major Paediatric Center. Ann. Vasc. Surg. 2018, 49, 229–233. [Google Scholar] [CrossRef]
- Markovic, M.D.; Cvetkovic, S.D.; Koncar, I.B.; Dragas, M.V.; Markovic, D.M.; Kukic, B.P.; Kuzmanovic, I.B.; Dimic, A.D.; Sladojevic, M.M.; Davidovic, L.B. Treatment of pediatric vascular injuries: The experience of a single non-pediatric referral center. Int. Angiol. 2019, 38, 250–255. [Google Scholar] [CrossRef]
- Mousa, A.; Zakaria, O.M.; Hanbal, I.; Sultan, T.A.; El-Gibaly, A.M.; Zakaria, M.Y.; Nasr, M.A.; Bosat, B.E.; Sharabi, A.; Neinaa, M.; et al. Operative management of non-iatrogenic pediatric and adolescence peripheral arterial trauma: An experience from a resource challenged setting. Asian J. Surg. 2018, 42, 761–767. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Riaz, A.; Nazir, Z. Peripheral Arterial Injuries in Children: An Audit at a University Hospital in Developing Country. Ann. Vasc. Dis. 2020, 13, 158–162. [Google Scholar] [CrossRef]
- Kim, S.; Modrall, J.G.; Malekpour, F.; Siah, M.; Ramanan, B.; Tsai, S.; Timaran, C.; Kirkwood, M.L. A single-center experience on the management of pediatric blunt aortic injury. J. Vasc. Surg. 2022, 75, 1570–1576. [Google Scholar] [CrossRef]
- Baethge, C.; Goldbeck-Wood, S.; Mertens, S. SANRA—A scale for the quality assessment of narrative review articles. Res. Integr. Peer Rev. 2019, 4, 5. [Google Scholar] [CrossRef]
- Green, B.N.; Johnson, C.D.; Adams, A. Writing narrative literature reviews for peer-reviewed journals: Secrets of the trade. J. Chiropr. Med. 2006, 5, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Tashiro, J.; Hannay, W.M.; Naves, C.; Allen, C.J.; Perez, E.A.; Rey, J.; Sola, J.E. Mechanism and mortality of pediatric aortic injuries. J. Surg. Res. 2015, 198, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Klinkner, D.B.; Arca, M.J.; Lewis, B.D.; Oldham, K.T.; Sato, T.T. Pediatric vascular injuries: Patterns of injury, morbidity, and mortality. J. Pediatr. Surg. 2007, 42, 178–183. [Google Scholar] [CrossRef]
- Corneille, M.G.; Gallup, T.M.; Villa, C.; Richa, J.M.; Wolf, S.E.; Myers, J.G.; Dent, D.L.; Stewart, R.M. Pediatric Vascular Injuries: Acute Management and Early Outcomes. J. Trauma Inj. Infect. Crit. Care 2011, 70, 823–828. [Google Scholar] [CrossRef]
- Shah, S.R.; Wearden, P.D.; Gaines, B.A. Pediatric Peripheral Vascular Injuries: A Review of Our Experience. J. Surg. Res. 2009, 153, 162–166. [Google Scholar] [CrossRef]
- Wang, S.K.; Drucker, N.A.; Raymond, J.L.; Rouse, T.M.; Fajardo, A.; Lemmon, G.W.; Dalsing, M.C.; Gray, B.W. Long-term outcomes after pediatric peripheral revascularization secondary to trauma at an urban level I center. J. Vasc. Surg. 2018, 69, 857–862. [Google Scholar] [CrossRef]
- Myers, S.I.; Reed, M.K.; Black, C.T.; Burkhalter, K.J.; Lowry, P.A. Noniatrogenic pediatric vascular trauma. J. Vasc. Surg. 1989, 10, 0258–0265. [Google Scholar] [CrossRef]
- Kayssi, A.; Metias, M.; Langer, J.C.; Roche-Nagle, G.; Zani, A.; Forbes, T.L.; Wales, P.; King, S.K. The spectrum and management of noniatrogenic vascular trauma in the pediatric population. J. Pediatr. Surg. 2018, 53, 771–774. [Google Scholar] [CrossRef] [PubMed]
- Shahi, N.; Phillips, R.; Meier, M.; Nehler, M.; Jacobs, D.; Recicar, J.; Bensard, D.; Moulton, S. Anti-coagulation management in pediatric traumatic vascular injuries. J. Pediatr. Surg. 2020, 55, 324–330. [Google Scholar] [CrossRef]
- Brinkman, A.S.; Rogers, A.P.; Acher, C.W.; Wynn, M.M.; Nichol, P.F.; Ostlie, D.J.; Gosain, A. Evolution in management of adolescent blunt aortic injuries—A single institution 22-y experience. J. Surg. Res. 2015, 193, 523–527. [Google Scholar] [CrossRef] [PubMed]
- Soto, J.E.; Vásquez, D.M.; Rodríguez, G.; De La Cruz, L.A. Peripheral Vascular Trauma in Pediatrics: A Case Report and Literature Review. Cureus 2019, 11, e5744. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.A.; Matsumura, J.S.; Mitchell, R.S.; Farber, M.A.; Greenberg, R.K.; Azizzadeh, A.; Murad, M.H.; Fairman, R.M. Endovascular repair of traumatic thoracic aortic injury: Clinical practice guidelines of the Society for Vascular Surgery. J. Vasc. Surg. 2011, 53, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D. A Discourse on Pediatric Vascular Trauma. Endovasc Today 2021, 1, 62. [Google Scholar]
- McGovern, M.C.; Glasgow, J.F.T.; Stewart, M.C. Lesson of the week: Reye’s syndrome and aspirin: Lest we forget. BMJ 2001, 322, 1591–1592. [Google Scholar] [CrossRef]
- Cardneau, J.D.; Henke, P.K.; Upchurch, G.R.; Wakefield, T.W.; Graham, L.M.; Jacobs, L.A.; Greenfield, L.J.; Coran, A.G.; Stanley, J.C. Efficacy and durability of autogenous saphenous vein conduits for lower extremity arterial reconstructions in preadolescent children. J. Vasc. Surg. 2001, 34, 34–40. [Google Scholar] [CrossRef]
| Authors | References | Study | Series | Cases | Incidence | Upper Extremity | Lower Extremity | SAT | Torso | Endovascular | Mortality | Morbidity | Limb Salvage | Follow-Up | Reintervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n) | (Type) | (Years) | (n) | (%) | (%) | (%) | (%) | (%) | (%) | (In-Hospital) | (In-Hospital) | (%) | (Mean, Years) | (%) | |
| Barmparas, et al. | [1] | national registry | 2002–2006 | 1138 | 0.6 | 35.7 | 18.6 | 9.9 | 37.4 | 0 | 13.2 | n.r. | 91.8 | n.r. | n.r. |
| Mommsen, et al. | [3] | single-center | 1971–2006 | 44 | n.r. | 31.6 | 61.4 | not included | not included | 0 | n.r. | 18.2 | 81.8 | n.r. | n.r. |
| Sciarretta, et al. | [4] | single-center | 2006–2011 | 18 | 0.6 | not included | 100 | not included | not included | 0 | 5.5 | 22.2 | 94.5 | n.r. | n.r. |
| Kirkilas, et al. | [5] | single-center | 2008–2013 | 23 | n.r. | 73.9 | 26.1 | not included | not included | 0 | 0 | 22.0 | 87.0 | n.r. | n.r. |
| Morao, et al. | [6] | single-center | 2009–2015 | 21 | 0.5 | 75.0 | 25.0 | not included | not included | 0 | 0 | 0 | 100 | 4.3 | 0 |
| Markovic, et al. | [7] | single-center | 1993–2018 | 17 | n.r. | 70.6 | 29.4 | not included | not included | 0 | 0 | 11.8 | 94.1 | 2.3 | 6.2 |
| Mousa, et al. | [8] | multicenter | 2008–2015 | 149 | n.r. | 49.0 | 51.0 | not included | not included | 0 | 3 | 13.0 | 97.0 | n.r. | n.r. |
| Rehman, et al. | [9] | single-center | 2008–2018 | 75 | 4.4 | 56.0 | 44.0 | not included | not included | 0 | n.r. | n.r. | 92.6 | n.r. | n.r. |
| Kim, et al. | [10] | single-center | 2008–2019 | 16 | n.r. | not included | not included | not included | 100 | 6.2 | 0 | 0 | n.a. | 0.6 | 0 |
| Tashiro, et al. | [13] | multicenter | 1997–2009 | 468 | n.r. | not included | not included | not included | 100 | 4 | 35.5 | n.r. | n.r. | n.r. | n.r. |
| Klinkner, et al. | [14] | single-center | 1993–2005 | 103 | 1.1 | 22.3 | 23.3 | 19.4 | 13.5 | 1.0 | 9.7 | n.r. | 88.3 | n.r. | n.r. |
| Corneille, et al. | [15] | single-center | 1995–2008 | 116 | 1.4 | 38.7 | 24.1 | 6.9 | 25.9 | 0 | 12.1 | 12.9 | 97.3 | n.r. | n.r. |
| Shah, et al. | [16] | single-center | 2000–2006 | 42 | 0.4 | 78.4 | 21.6 | n.r. | n.r. | 0 | 0 | 7.1 | 100 | n.r | n.r |
| Wang, et al. | [17] | single-center | 2010–2017 | 23 | 1.6 | 60.9 | 30.4 | 8.7 | not included | 4.3 | 0 | 0 | 100 | 3.6 | 0 |
| Myers, et al. | [18] | single-center | 1985–1988 | 20 | 1.5 | 25.0 | 35.0 | 20.0 | 15.0 | 0 | 5.0 | 5.0 | 100 | 1.3 | 0 |
| Kayssi, et al. | [19] | single-center | 1994–2014 | 106 | 1.2 | 47.7 | 19.8 | 9.4 | 17.9 | 0.9 | 0.9 | 29.2 | 99.1 | n.r. | n.r. |
| Shahi, et al. | [20] | multicenter | 2008–2018 | 84 | n.r. | 39.3 | 28.6 | 7.1 | 25.0 | 3.6 | n.r. | 17.0 | 98.8 | n.r. | 17.9 |
| Brinkman, et al. | [21] | single-center | 1990–2013 | 17 | n.r. | not included | not included | not included | 100 | 41.1 | 0 | n.r. | 100 | 1.2 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franchin, M.; Righini, P.; D’Oria, M.; Mazzaccaro, D.; Nano, G.; Tozzi, M.; Selmo, G.; Piffaretti, G. Current Opinions in Open and Endovascular Treatment of Major Arterial Injuries in Pediatric Patient. J. Clin. Med. 2023, 12, 4906. https://doi.org/10.3390/jcm12154906
Franchin M, Righini P, D’Oria M, Mazzaccaro D, Nano G, Tozzi M, Selmo G, Piffaretti G. Current Opinions in Open and Endovascular Treatment of Major Arterial Injuries in Pediatric Patient. Journal of Clinical Medicine. 2023; 12(15):4906. https://doi.org/10.3390/jcm12154906
Chicago/Turabian StyleFranchin, Marco, Paolo Righini, Mario D’Oria, Daniela Mazzaccaro, Giovanni Nano, Matteo Tozzi, Gabriele Selmo, and Gabriele Piffaretti. 2023. "Current Opinions in Open and Endovascular Treatment of Major Arterial Injuries in Pediatric Patient" Journal of Clinical Medicine 12, no. 15: 4906. https://doi.org/10.3390/jcm12154906
APA StyleFranchin, M., Righini, P., D’Oria, M., Mazzaccaro, D., Nano, G., Tozzi, M., Selmo, G., & Piffaretti, G. (2023). Current Opinions in Open and Endovascular Treatment of Major Arterial Injuries in Pediatric Patient. Journal of Clinical Medicine, 12(15), 4906. https://doi.org/10.3390/jcm12154906