Near-Infrared Spectroscopy-Guided, Individualized Arterial Blood Pressure Management for Carotid Endarterectomy under General Anesthesia: A Randomized, Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
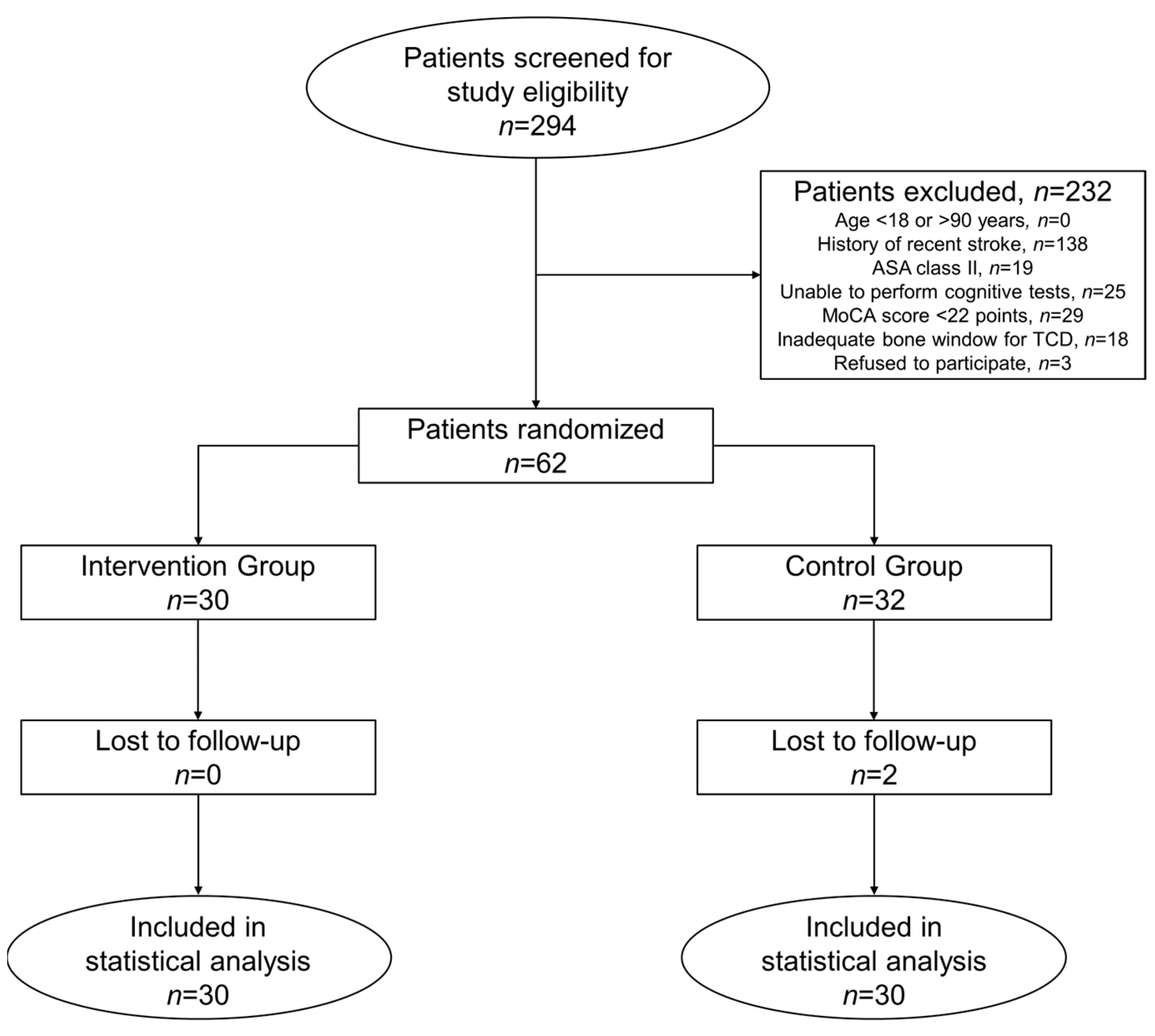
2.1. Study Patients
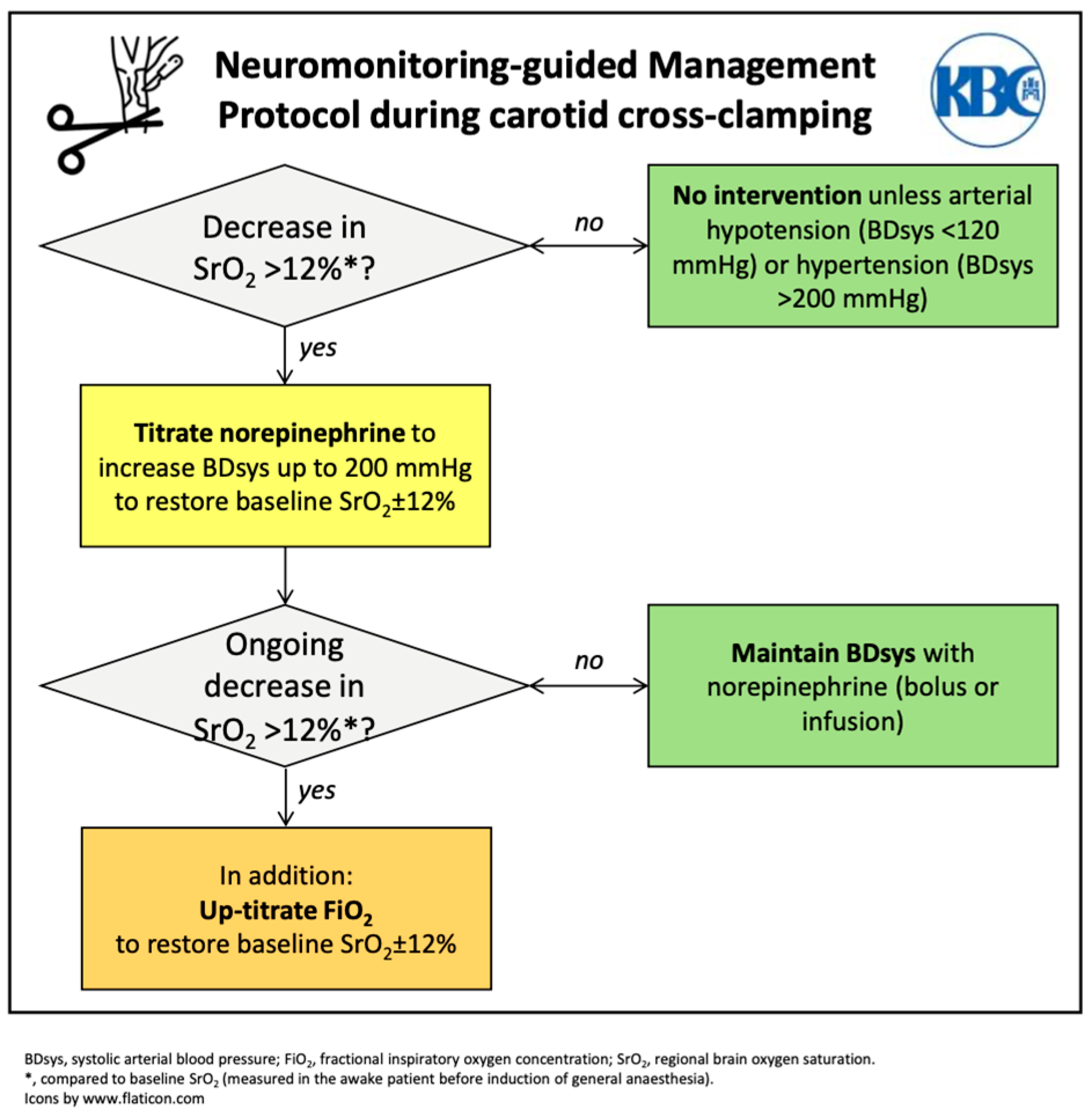
2.2. Interventions
2.3. Data Collection
2.4. Outcomes
2.5. Statistical Analysis
3. Results
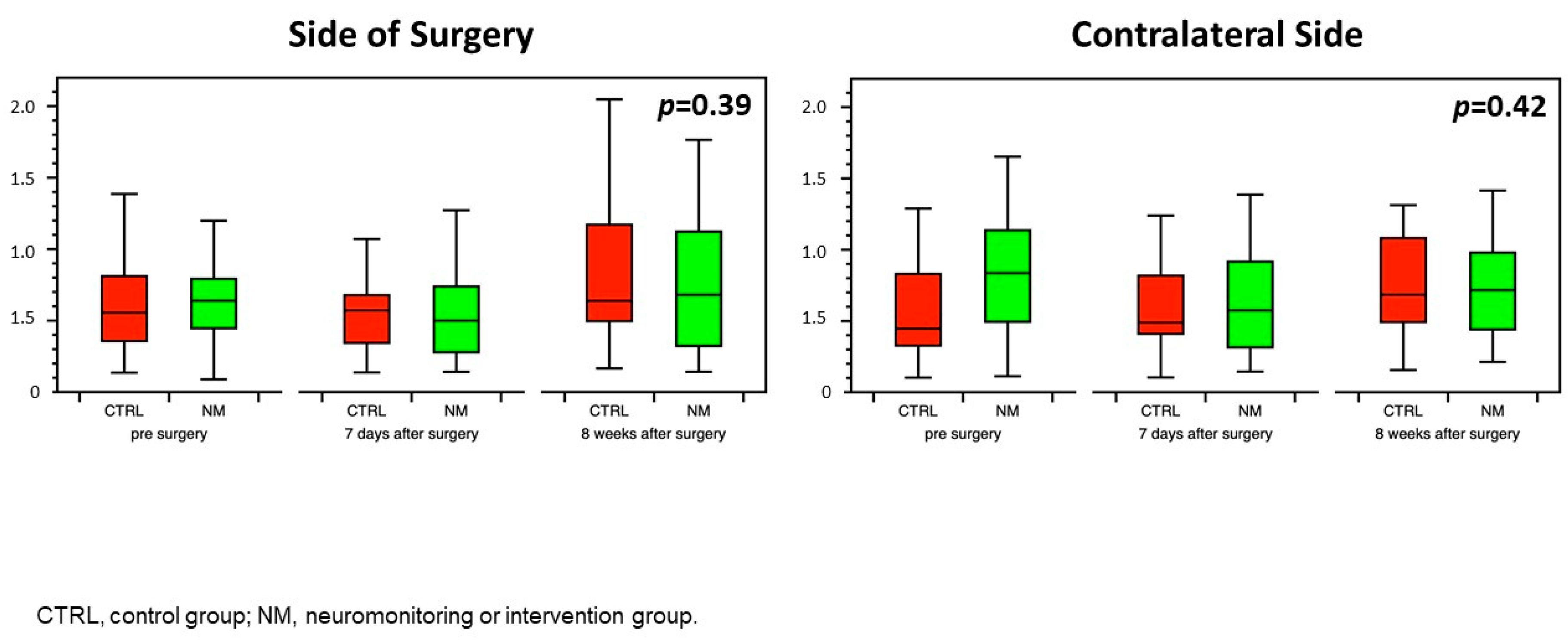
3.1. Neurocognitive Function
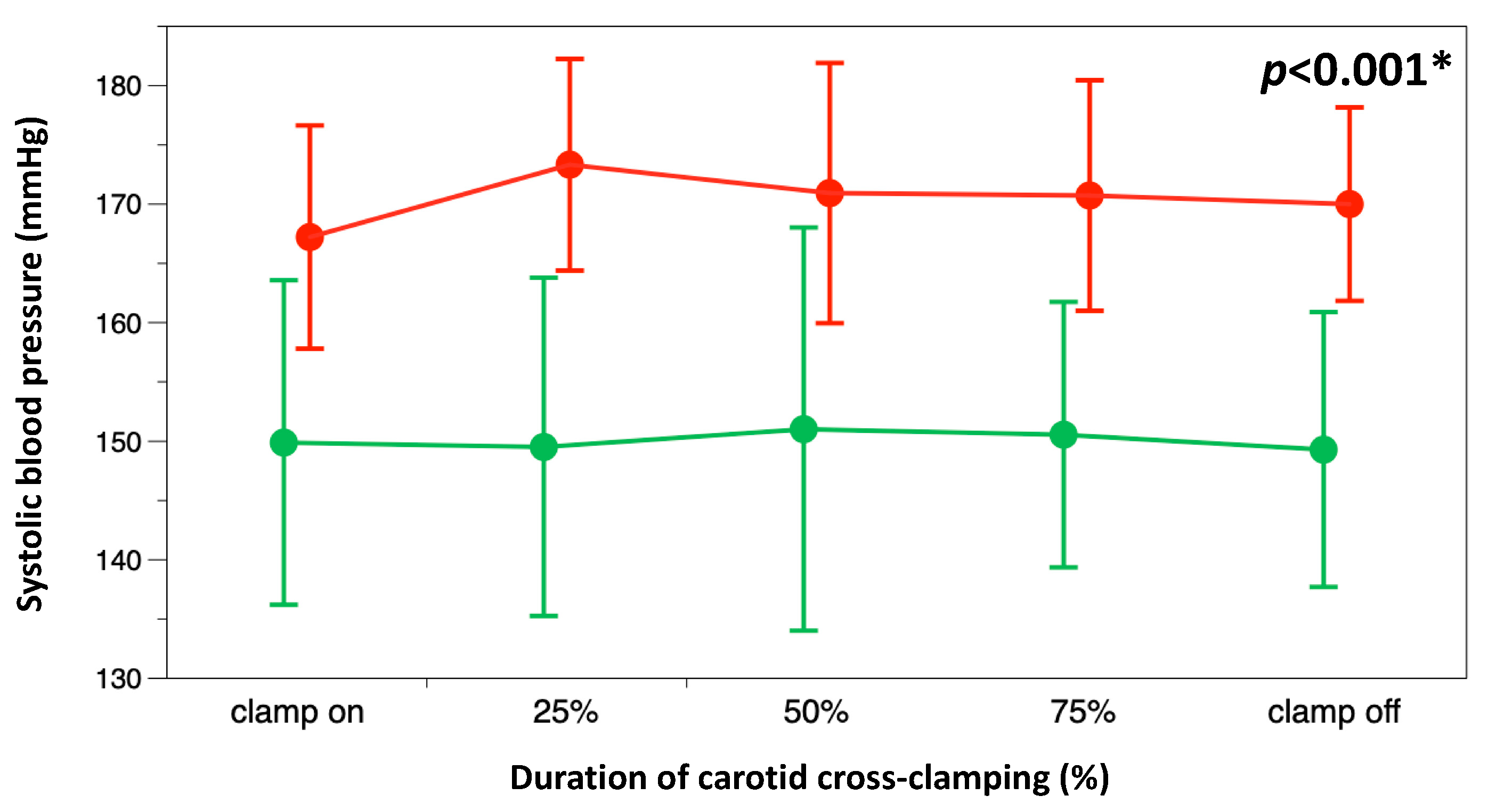
3.2. Secondary Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Oh, E.C.; Sridharan, N.D.; Avgerinos, E.D. Cognitive Function after Carotid Endarterectomy in Asymptomatic Patients. J. Cardiovasc. Surg. 2023, 64, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Piegza, M.; Więckiewicz, G.; Wierzba, D.; Piegza, J. Cognitive Functions in Patients after Carotid Artery Revascularization—A Narrative Review. Brain Sci. 2021, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, N.D.; Asaadi, S.; Thirumala, P.D.; Avgerinos, E.D. A Systematic Review of Cognitive Function after Carotid Endarterectomy in Asymptomatic Patients. J. Vasc. Surg. 2022, 75, 2074–2085. [Google Scholar] [CrossRef]
- Lattanzi, S.; Carbonari, L.; Pagliariccio, G.; Bartolini, M.; Cagnetti, C.; Viticchi, G.; Buratti, L.; Provinciali, L.; Silvestrini, M. Neurocognitive Functioning and Cerebrovascular Reactivity after Carotid Endarterectomy. Neurology 2018, 90, e307–e315. [Google Scholar] [CrossRef]
- Stoneham, M.D.; Thompson, J.P. Arterial Pressure Management and Carotid Endarterectomy. Br. J. Anaesth. 2009, 102, 442–452. [Google Scholar] [CrossRef]
- Kaya, K.; Zavriyev, A.I.; Orihuela-Espina, F.; Simon, M.V.; LaMuraglia, G.M.; Pierce, E.T.; Franceschini, M.A.; Sunwoo, J. Intraoperative Cerebral Hemodynamic Monitoring during Carotid Endarterectomy via Diffuse Correlation Spectroscopy and Near-Infrared Spectroscopy. Brain Sci. 2022, 12, 1025. [Google Scholar] [CrossRef]
- Stoneham, M.D.; Warner, O. Blood Pressure Manipulation during Awake Carotid Surgery to Reverse Neurological Deficit after Carotid Cross-clamping. Br. J. Anaesth. 2001, 87, 641–644. [Google Scholar] [CrossRef][Green Version]
- LeSar, C.J.; Sprouse, R.L.; Harris, W.B. Permissive Hypertension during Awake Eversion Carotid Endarterectomy: A Physiologic Approach for Cerebral Protection. J. Am. Coll. Surg. 2014, 218, 760–766. [Google Scholar] [CrossRef]
- Howell, S.J. Carotid Endarterectomy. Br. J. Anaesth. 2007, 99, 119–131. [Google Scholar] [CrossRef]
- Vanpeteghem, C.; Moerman, A.; De Hert, S. Perioperative Hemodynamic Management of Carotid Artery Surgery. J. Cardiothorac. Vasc. Anesth. 2016, 30, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Heyer, E.J.; Mergeche, J.L.; Anastasian, Z.H.; Kim, M.; Mallon, K.A.; Connolly, E.S. Arterial Blood Pressure Management During Carotid Endarterectomy and Early Cognitive Dysfunction. Neurosurgery 2014, 74, 245–253. [Google Scholar] [CrossRef]
- Gassner, M.; Bauman, Z.; Parish, S.; Koenig, C.; Martin, J.; Hans, S. Hemodynamic Changes in Patients Undergoing Carotid Endarterectomy under Cervical Block and General Anesthesia. Ann. Vasc. Surg. 2014, 28, 1680–1685. [Google Scholar] [CrossRef]
- Schmittinger, C.A.; Torgersen, C.; Luckner, G.; Schröder, D.C.H.; Lorenz, I.; Dünser, M.W. Adverse Cardiac Events during Catecholamine Vasopressor Therapy: A Prospective Observational Study. Intensive Care Med. 2012, 38, 950–958. [Google Scholar] [CrossRef]
- Moritz, S.; Kasprzak, P.; Arlt, M.; Taeger, K.; Metz, C. Accuracy of Cerebral Monitoring in Detecting Cerebral Ischemia during Carotid Endarterectomy: A Comparison of Transcranial Doppler Sonography, near-Infrared Spectroscopy, Stump Pressure, and Somatosensory Evoked Potentials. Anesthesiology 2007, 107, 563–569. [Google Scholar] [CrossRef]
- Pedrini, L.; Magnoni, F.; Sensi, L.; Pisano, E.; Ballestrazzi, M.S.; Cirelli, M.R.; Pilato, A. Is Near-Infrared Spectroscopy a Reliable Method to Evaluate Clamping Ischemia during Carotid Surgery? Stroke Res. Treat. 2012, 2012, 156975. [Google Scholar] [CrossRef]
- Kondov, S.; Bothe, D.; Beyersdorf, F.; Czerny, M.; Harloff, A.; Pooth, J.-S.; Kaier, K.; Schöllhorn, J.; Kreibich, M.; Siepe, M.; et al. Routine versus Selective Near-Infrared Spectroscopy-Guided Shunting during Carotid Eversion Endarterectomy. Interdiscip. CardioVascular Thorac. Surg. 2023, 36, ivad005. [Google Scholar] [CrossRef] [PubMed]
- Yücel, C.; Ketenciler, S.; Gürsoy, M.; Türkmen, S.; Kayalar, N. The Effect of Hemodynamic Parameters on Cerebral Oxygenization During Carotid Endarterectomy. Braz. J. Cardiovasc. Surg. 2022, 37, 80–87. [Google Scholar] [CrossRef]
- Mille, T.; Tachimiri, M.E.; Klersy, C.; Ticozzelli, G.; Bellinzona, G.; Blangetti, I.; Pirrelli, S.; Lovotti, M.; Odero, A. Near Infrared Spectroscopy Monitoring During Carotid Endarterectomy: Which Threshold Value Is Critical? Eur. J. Vasc. Endovasc. Surg. 2004, 27, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, M.; Zhou, Y.; Ji, J.; Raithel, D.; Qiao, T. Effects of Carotid Endarterectomy on Cerebral Reperfusion and Cognitive Function in Patients with High Grade Carotid Stenosis: A Perfusion Weighted Magnetic Resonance Imaging Study. Eur. J. Vasc. Endovasc. Surg. 2015, 50, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Inčiūra, D.; Antuševas, A.; Aladaitis, A.; Gimžauskaitė, A.; Velička, L.; Kavaliauskienė, Ž. Near-Infrared Spectroscopy as a Predictor of Cerebral Ischaemia during Carotid Endarterectomy in Awake Patients. Vascular 2020, 28, 301–308. [Google Scholar] [CrossRef]
- Butcher, N.J.; Monsour, A.; Mew, E.J.; Chan, A.-W.; Moher, D.; Mayo-Wilson, E.; Terwee, C.B.; Chee-A-Tow, A.; Baba, A.; Gavin, F.; et al. Guidelines for Reporting Outcomes in Trial Reports: The CONSORT-Outcomes 2022 Extension. JAMA 2022, 328, 2252. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, G.G.; Eliasziw, M.; Barr, H.W.; Clagett, G.P.; Barnes, R.W.; Wallace, M.C.; Taylor, D.W.; Haynes, R.B.; Finan, J.W.; Hachinski, V.C.; et al. The North American Symptomatic Carotid Endarterectomy Trial: Surgical Results in 1415 Patients. Stroke 1999, 30, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Bowie, C.R.; Harvey, P.D. Administration and Interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Meagher, J.; Leonard, M.; Donoghue, L.; O’Regan, N.; Timmons, S.; Exton, C.; Cullen, W.; Dunne, C.; Adamis, D.; Maclullich, A.J.; et al. Months Backward Test: A Review of Its Use in Clinical Studies. WJP 2015, 5, 305. [Google Scholar] [CrossRef]
- Markus, H.S.; Harrison, M.J. Estimation of Cerebrovascular Reactivity Using Transcranial Doppler, Including the Use of Breath-Holding as the Vasodilatory Stimulus. Stroke 1992, 23, 668–673. [Google Scholar] [CrossRef]
- Müller, M.; Voges, M.; Piepgras, U.; Schimrigk, K. Assessment of Cerebral Vasomotor Reactivity by Transcranial Doppler Ultrasound and Breath-Holding: A Comparison With Acetazolamide as Vasodilatory Stimulus. Stroke 1995, 26, 96–100. [Google Scholar] [CrossRef]
- Dünser, M.W.; Ruokonen, E.; Pettilä, V.; Ulmer, H.; Torgersen, C.; Schmittinger, C.A.; Jakob, S.; Takala, J. Association of Arterial Blood Pressure and Vasopressor Load with Septic Shock Mortality: A Post Hoc Analysis of a Multicenter Trial. Crit. Care 2009, 13, R181. [Google Scholar] [CrossRef]
- Jian, L.; Ahmed, S.; Fuhai, J.; Lingzhong, M. Monitoring Cerebral Ischemia during Carotid Endarterectomy and Stenting. J. Biomed. Res. 2017, 31, 11. [Google Scholar] [CrossRef]
- Estruch-Pérez, M.J.; Barberá-Alacreu, M.; Ausina-Aguilar, A.; Soliveres-Ripoll, J.; Solaz-Roldán, C.; Morales-Suárez-Varela, M.M. Bispectral Index Variations in Patients with Neurological Deficits during Awake Carotid Endarterectomy. Eur. J. Anaesthesiol. 2010, 27, 359–363. [Google Scholar] [CrossRef]
- Jonsson, M.; Lindström, D.; Wanhainen, A.; Djavani Gidlund, K.; Gillgren, P. Near Infrared Spectroscopy as a Predictor for Shunt Requirement During Carotid Endarterectomy. Eur. J. Vasc. Endovasc. Surg. 2017, 53, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.M.; McInnis, C.L.; Ross-White, A.; Day, A.G.; Norman, P.A.; Boyd, J.G. Overview and Diagnostic Accuracy of Near Infrared Spectroscopy in Carotid Endarterectomy: A Systematic Review and Meta-Analysis. Eur. J. Vasc. Endovasc. Surg. 2021, 62, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Editorial Expression of Concern. Neurosurgery 2023, 93, 489. [CrossRef]
- Milne, B.; Gilbey, T.; Gautel, L.; Kunst, G. Neuromonitoring and Neurocognitive Outcomes in Cardiac Surgery: A Narrative Review. J. Cardiothorac. Vasc. Anesth. 2022, 36, 2098–2113. [Google Scholar] [CrossRef] [PubMed]
- Trafidło, T.; Gaszyński, T.; Gaszyński, W.; Nowakowska-Domagała, K. Intraoperative Monitoring of Cerebral NIRS Oximetry Leads to Better Postoperative Cognitive Performance: A Pilot Study. Int. J. Surg. 2015, 16, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Murniece, S.; Soehle, M.; Vanags, I.; Mamaja, B. Near Infrared Spectroscopy Based Clinical Algorithm Applicability During Spinal Neurosurgery and Postoperative Cognitive Disturbances. Medicina 2019, 55, 179. [Google Scholar] [CrossRef] [PubMed]
- Thanaboriboon, C.; Vanichvithya, P.; Jinaworn, P. What Is the Risk of Intraoperative Cerebral Oxygen Desaturation in Patients Undergoing Shoulder Surgery in the Beach Chair Position? Clin. Orthop. Relat. Res. 2021, 479, 2677–2687. [Google Scholar] [CrossRef]
- Usman, R.; Jamil, M.; Haq, I.U.; Memon, A.A. Neurocognitive Improvement in Patients Undergoing Carotid Endarterectomy for Atherosclerotic Occlusive Carotid Artery Disease. Ann. Vasc. Dis. 2016, 9, 307–311. [Google Scholar] [CrossRef]


| Intervention Group | Control Group | p-Value | ||
|---|---|---|---|---|
| n | 30 | 30 | ||
| Age | years | 70 (63–73) | 64 (67–72) | 0.76 |
| Female sex | n (%) | 5 (17) | 9 (30) | 0.36 |
| Body Mass Index | kg/m² | 27 (26–29) | 27 (25–28) | 0.6 |
| Right-handedness | n (%) | 30 (100) | 28 (93.3) | 0.49 |
| School education > 12 years | n (%) | 19 (63.3) | 9 (30) | 0.02 * |
| Comorbid conditions | ||||
| chronic arterial hypertension | n (%) | 27 (90) | 27 (90) | 1 |
| congestive heart failure | n (%) | 8 (26.7) | 9 (30) | 1 |
| diabetes mellitus II | n (%) | 7 (23.3) | 10 (33.3) | 0.57 |
| chronic kidney disease | n (%) | 1 (3.3) | 2 (6.7) | 1 |
| chronic obstructive pulmonary disease | n (%) | 1 (3.3) | 1 (3.3) | 1 |
| Antihypertensive drugs | ||||
| ACE-inhibitor/sartan | n (%) | 17 (56.7) | 23 (76.7) | 0.17 |
| calcium channel blocker | n (%) | 16 (53.3) | 15 (50) | 1 |
| central antihypertensive | n (%) | 10 (33.3) | 7 (23.3) | 0.57 |
| diuretic | n (%) | 9 (30) | 16 (53.3) | 0.12 |
| beta-blocker | n (%) | 9 (30) | 14 (46.7) | 0.29 |
| alpha-blocker | n (%) | 2 (6.7) | 1 (3.3) | 1 |
| ASA physical status | 3 (3–3) | 3 (3–3) | 0.33 | |
| III | n (%) | 30 (100) | 29 (96.7) | 1 |
| IV | n (%) | 0 (0) | 1 (3.3) | |
| NASCET stenosis grade | ||||
| side of surgery | % | 80 (71–90) | 85 (80–90) | 0.35 |
| contralateral side | % | 30 (0–50) | 45 (3–54) | 0.32 |
| Symptomatic stenosis | n (%) | 11 (37) | 16 (53.3) | 0.3 |
| Montreal Cognitive Assessment test | points | 25 (24–27) | 25 (24–27) | 0.82 |
| Trail marking test A | s | 50 (37–69) | 61 (48–80) | 0.6 |
| Trail marking test B | S | 110 (81–186) | 147 (120–192) | 0.42 |
| Month backward test | s | 32 (20–50) | 36 (24–75) | 0.23 |
| Breath Holding Index | ||||
| side of surgery | 80 (71–90) | 85 (80–90) | 0.52 | |
| contralateral side | 29 (0–50) | 45 (3–54) | 0.14 |
| Intervention Group | Control Group | ||||||
|---|---|---|---|---|---|---|---|
| n = 30 | n = 30 | ||||||
| Type of Complication | n (%) | Postop Day | Management | Type of Complication | n (%) | Postop Day | Management |
| Arterial hypertension * | 1 (3.3%) | Rebleeding | 3 (10%) | ||||
| Patient 1 | 2 | Antihypertensive | Patient 1 | 1 | surgical revision | ||
| therapy | |||||||
| Patient 2 | 1 | surgical revision | |||||
| Patient 3 | 1 | surgical revision | |||||
| Arterial hypertension * | 3 (10%) | ||||||
| Patient 1 | 2 | antihypertensive therapy | |||||
| Patient 2 | 3 | antihypertensive therapy | |||||
| Patient 3 | 2 | antihypertensive therapy | |||||
| Unstable angina | 1 (3.3%) | ||||||
| Patient 1 | 2 | anti-ischemic, antihypertensive therapy | |||||
| New neurological deficit | 1 (3.3%) | ||||||
| Patient 1 | 0 | symptomatic care as per stroke protocol | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomić Mahečić, T.; Malojčić, B.; Tonković, D.; Mažar, M.; Baronica, R.; Juren Meaški, S.; Crkvenac Gregorek, A.; Meier, J.; Dünser, M.W. Near-Infrared Spectroscopy-Guided, Individualized Arterial Blood Pressure Management for Carotid Endarterectomy under General Anesthesia: A Randomized, Controlled Trial. J. Clin. Med. 2023, 12, 4885. https://doi.org/10.3390/jcm12154885
Tomić Mahečić T, Malojčić B, Tonković D, Mažar M, Baronica R, Juren Meaški S, Crkvenac Gregorek A, Meier J, Dünser MW. Near-Infrared Spectroscopy-Guided, Individualized Arterial Blood Pressure Management for Carotid Endarterectomy under General Anesthesia: A Randomized, Controlled Trial. Journal of Clinical Medicine. 2023; 12(15):4885. https://doi.org/10.3390/jcm12154885
Chicago/Turabian StyleTomić Mahečić, Tina, Branko Malojčić, Dinko Tonković, Mirabel Mažar, Robert Baronica, Snježana Juren Meaški, Andrea Crkvenac Gregorek, Jens Meier, and Martin W. Dünser. 2023. "Near-Infrared Spectroscopy-Guided, Individualized Arterial Blood Pressure Management for Carotid Endarterectomy under General Anesthesia: A Randomized, Controlled Trial" Journal of Clinical Medicine 12, no. 15: 4885. https://doi.org/10.3390/jcm12154885
APA StyleTomić Mahečić, T., Malojčić, B., Tonković, D., Mažar, M., Baronica, R., Juren Meaški, S., Crkvenac Gregorek, A., Meier, J., & Dünser, M. W. (2023). Near-Infrared Spectroscopy-Guided, Individualized Arterial Blood Pressure Management for Carotid Endarterectomy under General Anesthesia: A Randomized, Controlled Trial. Journal of Clinical Medicine, 12(15), 4885. https://doi.org/10.3390/jcm12154885






