Abstract
The wearable cardioverter defibrillator (WCD) has been proven to be effective in preventing sudden cardiac death (SCD) in patients soon after acute myocardial infarction (AMI) and left ventricular ejection fraction (LVEF) ≤35%. The aim of this study was to assess whether a WCD may shorten the length of an initial hospital stay (total length, days in the intensive care unit (ICU) and in the acute cardiac care unit (ACCU)) among these patients. This was a single-centre, retrospective observational study of patients referred for the management of SCD risk post-AMI and LVEF ≤35%, in a tertiary care hospital. The clinical characteristics and length of index hospitalization of the group of patients discharged, with or without WCD, were compared. A propensity score analysis was performed, then weighted regression models were conducted. A total of 101 patients in the WCD group and 29 in the control group were enrolled in the analysis. In the weighted regression models, WCD significantly reduced the days spent in ACCU (p < 0.001). WCD patients had significantly fewer days spent in ACCU (5.5 ± 2.6 vs. 8.4 ± 12.8 days, p < 0.001) and shorter hospitalizations (10.2 ± 5.7 vs. 13.4 ± 17.6 days, p = 0.005), compared with the control group. It was concluded that the WCD appears to reduce the total length of hospitalization and lengths of stay in ACCU for patients post-AMI and with left ventricular dysfunction.
1. Introduction
The risk of sudden cardiac death (SCD) is increased after an acute myocardial infarction (AMI). A severe reduction of left ventricular ejection fraction (LVEF) is the strongest independent predictor of SCD soon thereafter [1]. Randomized clinical trials showed that implantable cardioverter-defibrillators (ICDs), in combination with optimal medical therapy (OMT), reduce the risk of SCD fourfold in patients who experience AMI with reduced LVEF [2,3]. However, randomized controlled trials did not provide empirical support for ICD implantation during the early-phase post-AMI period (particularly within 40 days) [4,5,6]. Furthermore, a significant proportion of patients improve their LVEF within the first three months. This is partly due to the slow up-titration of OMT in combination with cardiac remodeling during that time [7]. The wearable cardioverter defibrillator (WCD) can close the gap between hospital discharge and the best possible recovery obtained with medications.
The WCD (LifeVest®, ZOLL Medical Corporation, Pittsburgh, PA, USA) is a unique totally external medical device capable of detecting life-threatening arrhythmias and delivering the appropriate shocks for their termination [8,9].
Numerous prospective and retrospective studies have demonstrated the safety and effectiveness of the WCD [10,11,12]. The cost effectiveness of the WCD was analyzed in several publications in American and Italian settings [13,14,15,16]. Results of the studies indicated that the device was cost-effective, and one study found that the WCD was cost-saving, even when used after ICD explantation [17].
However, no studies to date have evaluated the direct impact of a WCD on the length of hospital stays among post-AMI patients.
Depending on the clinical presentation of patients with acute coronary syndrome (ACS), these patients can be managed in coronary intensive care units (ACCU) or an intensive care unit (ICU) [18,19]. Furthermore, in patients at high risk for SCD, the WCD is prescribed in the hospital prior to discharge.
The aim of our study was to assess whether a WCD could reduce total hospital stays, as well as the length of stay in ICU and ACCU, for post-AMI patients with severely reduced LVEF.
2. Materials and Methods
We conducted a single-centre, retrospective, observational study of consecutive post-AMI patients with LVEF ≤35%, over 18 years of age, admitted to the French University Hospital Arnaud de Villeneuve in Montpellier. Approval for the study was obtained from the institutional review board at Arnaud de Villeneuve Teaching Hospital in Montpellier, France (number IRB-MTP_2022_07_202201174).
Patients with the above-described inclusion criteria and additional WCD prescriptions, between June 2016 and March 2022, served as the study group. Patients discharged without a WCD (or ICD), from January to December 2022, formed the control group.
Patients who already had an ICD or cardiac resynchronization therapy defibrillator (CRT-D), were awaiting heart transplantation, had non-ischemic heart failure, LVEF >35% or were prescribed a WCD after ICD explantation, were excluded.
Components, functionality, and indications of the WCD are described in detail elsewhere [20,21].
For both study groups, the index event date began with the date of hospital admission for AMI. Baseline data included demographics, past medical history, and baseline medication. Electrocardiogram (ECG) and laboratory data were obtained from electronic medical records.
Echocardiographic parameters (such as left ventricular size and ejection fraction, right ventricular function, the presence of severe valvulopathy, as well as the presence of a left ventricular aneurysm or intraventricular thrombosis) were collected from the echocardiographic examination performed during the hospital stay and after coronary angiography (mean time of about five days post-AMI).
Data regarding the duration of hospitalization included total length of hospital stay, and days spent in ICU and ACCU.
For the WCD group, data on treatments were retrieved from the Zoll network database (LifeVest® Zoll Medical Corp., Pittsburgh, PA, USA).
Each patient was followed to gather information about re-hospitalizations, ICD implantations, or death.
Statistical Analysis
Continuous data were tested for normal distribution with the Kolmogorov–Smirnov test. Normally distributed continuous variables were presented as mean and standard deviations (SD). Continuous variables with skewed distributions were reported as medians and interquartile ranges (IQR). Categorical variables were summarized in terms of counts and percentages. Comparisons of continuous variables between groups were performed with Student’s t-test or the non-parametric Mann–Whitney test, as suitable. Categorical variables were compared with the Chi-square test.
To reduce the influences of potential selection bias, propensity score analyses were performed. In a first step, variables with an influence on WCD use were identified using a forward selection procedure. Variables with a potential influence were chosen a priori: age, gender, LVEF, ST-elevation ACS, multivessel disease, multivessel percutaneous coronary intervention, left ventricular aneurysm, left ventricular thrombosis and antiarrhythmic drugs use. Second, a logistic regression model adjusted for the selected variables was performed and inverse probability of treatment weighting was used to calculate weights for each patient. Finally, weighted regression models were conducted for total hospitalization, days in ICU and days in ACCU with WCD use as independent variables, respectively.
The following sensitivity analyses were performed: (1) weighted models were additionally adjusted for extra-corporeal membrane oxygenation (ECMO) use and heart transplantation (HTX); (2) variable selection, weighting and regression models were repeated excluding patients with ECMO or HTX; and (3) patients with longer hospital stays (more than 30 days) were excluded.
All p values < 0.05 were considered statistically significant. Data were analyzed using SAS (version 9.4).
3. Results
One hundred and thirty consecutive post-AMI patients with LVEF ≤35% were included in the study: 101 patients were discharged with a WCD while 29 were not (Figure 1).
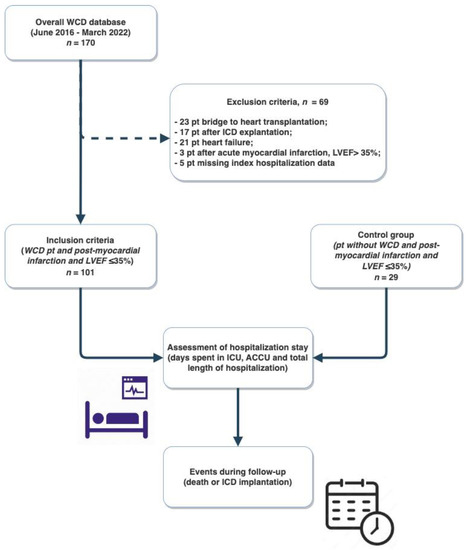
Figure 1.
Study flow-chart. WCD, wearable cardioverter defibrillator; pt, patients; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; ICU, intensive care unit; ACCU, acute cardiac care unit.
Mean age was 60.6 years (±10.9), 18.5% were female and 82.3% of patients had an ST-elevation ACS. All patients underwent coronary angiography after a mean time of 0 days (±1).
All patients were equipped with WCD during index hospitalization and previous to discharge, after a median time of 6.0 days (IQR 4.0–9.0) from the date of hospital admission. Baseline characteristics of the patients are depicted in Table 1. Clinical complications and the main resources used during the entire index hospitalization are shown in Table 2.

Table 1.
Baseline clinical characteristics of the patients.

Table 2.
Clinical complications and the main resources used during the entire index hospitalization.
Patients in the WCD group had significantly lower LVEF (28.2 ± 4.7 versus 32.1 ± 3.6; p < 0.001) and more cardiogenic shocks (23.8% versus 6.9%; p = 0.045).
ECMO was used with 11 patients (9 in the study group and 2 in the control group). In these patients, WCD was placed after weaning from ECMO (in seven patients) and earlier in two patients who subsequently had hemodynamic decompensation requiring circulatory support.
Three patients underwent HTX (two in the WCD group and one in the control group) in all cases after ECMO use.
The independent variables for WCD use were LVEF (p < 0.001) and left ventricular thrombosis (p = 0.034).
3.1. Hospitalization Times
In the context of management and treatment of ACS, 126 patients (96.9%) required ACCU care: 99 in the WCD group and 27 in the control group. Thirty-one patients (23.8%) required ICU care: 25 in the WCD group and 6 in the no-WCD group. Subsequently, all patients were transferred to the cardiology ward, from where they were discharged (except patients who died during hospitalization).
The different analyzes of hospitalization times are shown in Table 3 (a–c) and Supplementary Table S1.

Table 3.
(a)— Base propensity score model for hospitalization times. (b)— Propensity score model for hospitalization times without ECMO and HTX. (c)— Propensity score model for hospitalization times without patients with long (>30 days) hospital stay.
In the general descriptive analysis, the mean hospital stay was 13.5 days (±13.0); mean days spent in ICU were 1.8 days (±5.1); and mean days in ACCU were 5.8 days (±3.7). The maximum number of days spent in hospital was higher in the WCD group compared to the control group (total hospital stay = 74 vs. 56 days, p = 0.196; ICU stay = 33 vs. 19 days, p = 0.557; ACCU stay = 28 vs. 17 days, p = 0.100); however, the number was without statistical significance.
The use of ECMO and HTX emerged as independent variables for a higher total length of stay (p < 0.001) and for time spent in ICU (p < 0.001). HTX, but not ECMO use, emerged as an independent variable for days spent in ACCU (p < 0.001 and p = 0.739, respectively).
In the weighted regression model (adjusted for ECMO use and HTX), compared with the no-WCD group, WCD significantly reduced days spent in ACCU (5.8 ± 3.9 vs. 8.9 ± 13.5 days, p < 0.001), but not the total hospital stay length (13.6 ± 14.6 vs. 17.2 ± 36.3 days, p = 0.143) or days in ICU (1.7 ± 5.6 vs. 2.9 ± 15.4 days, p = 0.251).
After excluding patients from the analysis with ECMO/HTX, WCD use resulted in a borderline significance for fewer total lengths of hospitalization (10.4 ± 7.2 vs. 12.4 ± 15.2 days, p = 0.066), compared with the no-WCD group. Again, the WCD reduced the days spent in ACCU (5.7 ± 3.5 vs. 9.1 ± 14.6 days, p < 0.001).
Finally, excluding patients with hospital stays >30 days (nine patients in the study group and one patient in the control group), resulted in significantly fewer total lengths of hospitalization (10.2 ± 5.7 vs. 13.4 ± 17.6 days, p = 0.005), as well as ACCU stays (5.5 ± 2.6 vs. 8.4 ± 12.8 days, p < 0.001) for patients with a WCD. Days in ICU were not significantly reduced (0.7 ± 2.3 vs. 1.2 ± 8.2, p = 0.356). In this latest analysis, it was found that patients with a WCD spent an average of 3.2 days less in the hospital than those without a WCD, and an average of 3.0 days less in the ACCU.
No patients experienced WCD shock during index hospitalization. All patients were discharged, except three who died during index hospitalization: two in the WCD group for non-cardiovascular causes (hemorrhagic stroke and septic shock, respectively), and one in the no-WCD group (for cardiogenic shock).
3.2. Follow-Up Period
Median WCD wear time from the patients was 34 days (IQR 18–59 days), while the maximum wear time was 136 days. The main reasons for discontinuing the WCD were improvement of LVEF in 43 patients (44.8%), and ICD implantation in 45 patients (46.9%). Five patients discontinued WCD use for lack of tolerance, and three patients died (3.1%).
Four patients experienced WCD shock and were subsequently hospitalized: of these, two patients received seven appropriate shocks, while another two patients received one inappropriate treatment each. In no cases did the inappropriate shock from the WCD result in a patient’s death. All patients with appropriate WCD shocks underwent subsequent ICD implantation. No further adverse events were reported.
After a mean follow-up period of 37.3 months (±25.3), 46 (49.5%) patients underwent ICD implantation in the WCD group (after a mean time of 72.3 ± 44.5 days). In the control group, 11 patients (39.3%) underwent ICD implantation (after a mean time of 69.6 ± 69.5 days), without statistically significant difference (p = 0.344).
During the same time period, one patient died after hospitalization in the control group, and three patients died in the WCD group. All deaths occurred after WCD discontinuation.
4. Discussion
Our study was the first to show that WCD shortens the hospital stays of post-AMI patients with ventricular dysfunction, especially in terms of days spent in ACCU.
Compared with the general population, patients who experience an AMI are four to six times more likely to suffer from an SCD. Arrhythmic risk is at its maximum in the first month and decreases exponentially over the first six months [1]. ICDs for the primary prevention of SCDs in the early phase after an AMI (<40 days) failed to reduce overall mortality due to an increase in non-SCD and other adverse events, such as infection, inappropriate shock and heart failure. In addition, some patients regained full cardiac function after the early-phase post-AMI period [2,3,4,5,6,7]. Thus, the WCD is an interesting device that could effectively fill this time-gap.
In a randomized trial, WCD significantly reduced total mortality but not arrhythmic mortality in the intention-to-treat analysis [11]. Further per protocol and as-treated analyses showed that WCD significantly reduced total mortality, as well as arrhythmic and non-arrhythmic death [12,15,16,17,18,19,22]. Importantly, several large observational studies unanimously showed improved WCD compliance compared to the randomized trial, suggesting that the results of the per protocol and as-treated analyses are closer to real-life clinical practice [23]. For this reason, international guidelines suggest considering the use of WCDs, among other indications, in selected patients after the early phase of AMI [24,25].
Despite several cost-effectiveness analyses on the use of the WCD, no study until now has directly analysed the implications of WCD use on the total duration of hospitalization, and lengths of stay in ICU and ACCU.
In weighted regression models, the WCD significantly reduced the days spent in ACCU and this remained stable even after adjusting for a greater burden of cardiovascular disease (p < 0.001). After excluding outliers with long hospitalizations, WCD use resulted in significantly fewer days spent in ACCU (p < 0.001) and shorter total length of hospitalization (p = 0.005), compared with control group.
Patients with WCDs spent an average 3.2 days less in the hospital than those without WCDs, and an average of 3.0 days less in the ACCU.
In no weighted regression models, the WCD resulted in shorter ICU lengths of stay, although not statistically significant. This finding is in line with real-world data, in which the WCD is placed shortly before discharge from high-monitoring cardiology departments (e.g., ACCU), but does not impact the length of stay in ICUs.
In our study, patients in the WCD group had a higher disease burden than those in the control group, and notably had significantly lower LVEF and worse renal function (e.g., peak creatinine level). Additionally, patients in the WCD group experienced significantly more frequent cardiogenic shocks. The propensity score analyses were mandatory to adjust for diverging baseline variables of the groups, in order to provide more reliable comparison between groups. In this perspective, non-significant differences in mortality and ICD implantation rates may indicate better patient management and outcomes with a WCD.
It comes as no surprise that LVEF emerged as the most important independent variable for the use of a WCD, even after excluding patients from the analysis with long hospitalizations and those who required ECMO or HTX. LVEF is the most important SCD-risk marker, and therefore part of the WCD indication [1,3].
About 40% of patients in both groups presented with a late STE-ACS (>12 h). This finding is in line with real-world data. Patients who might benefit from WCD and/or ICD often have large myocardial infarction, and late presentation might be the cause, resulting in a poor chance of revascularization [26]. In this group of patients, WCD often finds a bridge indication to ICD because of the high arrhythmic risk of these patients.
Overall, post-AMI patients often undergo lengthy hospitalizations. This is linked to the intrinsic instability of the cardiological condition. In other cases, the clinician perceives the risk of SCD as too high for hospital discharge. The WCD allows for these patients to be safely discharged, so that they can return to their home environment under protected conditions, without increasing the risk of SCD. WCD allows the patient to enter a “virtual clinic” where there is 24/7 continuous monitoring. In contrast, for patients who still require hospitalization or cardiac rehabilitation, WCD allows patients to be transferred to wards with a lower degree of monitoring in complete safety because they are protected from an SCD. WCD acceptance by patients is high, enabling them to conduct daily life activities without major restrictions. Therefore, the WCD can help to prevent unnecessary ICD implantation (almost half of the patients improved their LVEF until the end of WCD use), and thereby contribute to the improvement of quality of life as well as reduce healthcare costs. This efficacy is not only expressed in a direct reduction of healthcare costs but indirectly allows for a reduction in the complications correlated with prolonged hospital stays or unnecessary ICD implantation [27,28].
The length of stay in our study tended to be longer than those reported in the literature [29] for the same patient population. The retrospective setting of our study does not allow us to arrive at firm conclusions; however, some assumptions can be made. In fact, 11 patients required ECMO. Of these, three required subsequent cardiac transplantation, which undoubtedly lengthened the overall hospitalization times. To this should be added that one-third of the patients in both groups underwent multivessel percutaneous coronary intervention, which was not always performed during the first session, and certainly lengthened the hospital stay.
The rate of appropriate WCD shocks in our study was 1.98%, and the same was reported for inappropriate shocks. This indicates that use of the WCD helped to prevent two serious cardiovascular events. The results are in line with those published in the literature [10,30]. Therefore, the WCD is a useful and feasible device for the prevention of SCD in post-AMI patients with severe left ventricular dysfunction. The WCD has an excellent cost-effectiveness and is able to reduce the duration of hospitalizations, especially stays in ACCU.
Limitations
Our study has several limitations that warrant consideration. First, this was a single-centre analysis with a retrospective design performed in a non-randomized manner. This poses some inherent limitations, including selection bias and unknown confounding factors that could not be controlled. Second, there was a limited number of patients included with some important differences between groups. Thus, we tried to reduce the impact of selection bias by constructing different sensitivity analysis models assessing the effects of various measurable and unmeasurable confounders. Third, our results may not be generalizable to hospitals with different organizational levels, especially for patients with lower disease complexity. Fourth, our study did not include analyses of some laboratory and echocardiographic parameters over time. In addition, an analysis of hemodynamic parameters or cognitive function, which might have indirectly influenced the choice to place WCD, was not included. Subsequent analyses may focus specifically on the parameters listed above. Finally, it is possible to imagine a direct reduction in healthcare costs with the use of WCDs; however, our analysis is currently unable to answer this question. A sub-analysis of the costs of WCDs versus hospitalization is still ongoing.
5. Conclusions
The WCD was effective for the outpatient management of patients at a high risk of an SCD following a myocardial infarction. Our data support that WCD may allow for earlier discharges, resulting in fewer days spent in ACCU and lower hospital lengths of stay. This is important to consider within the context of modern hospital organizations, and in view of the better allocation of economic resources. Confirmation by a prospective randomized clinical trial is as necessary as ever.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12154884/s1, Table S1: Hospitalization times, descriptive analysis.
Author Contributions
Conceptualization, F.R.; Methodology, F.R.; Investigation, Q.D., F.M., M.G., G.C., G.B., V.D., J.-C.M., F.L., J.-L.P. and F.R.; Resources, F.R.; Data curation, L.S.C.; Writing—original draft, L.S.C.; Writing—review & editing, L.S.C., J.-L.P., G.C. and F.R.; Visualization, L.S.C. and F.R.; Supervision, F.R.; Project administration, F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Approval for the study was obtained from the institutional review board at Arnaud de Villeneuve Teaching Hospital in Montpellier, France (number IRB-MTP_2022_07_202201174).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to restrictions eg privacy or ethical.
Conflicts of Interest
FR has received honoraria for lectures from Astra Zeneca, Servier, Boehringer, Astra Zeneca, Vifor, Bayer, Pfizer, Novartis, Servier, Novonordisk, Air liquid, Abbott and more specifically regarding this manuscript from Zoll for other lectures and consulting.
Abbreviations
| ACCU | Acute cardiac care unit |
| ACS | Acute coronary syndrome |
| AMI | Acute myocardial infarction |
| CRT-D | Cardiac resynchronization therapy defibrillator |
| ECG | Electrocardiogram |
| ECMO | Extra-corporeal membrane oxygenation |
| HTX | Heart transplantation |
| ICD | Implantable cardioverter-defibrillator |
| ICU | Intensive care unit |
| IQR | Interquartile ranges |
| LVEF | Left ventricular ejection fraction |
| OMT | Optimal medical therapy |
| SCD | Sudden cardiac death |
| SD | Standard deviation |
| WCD | Wearable cardioverter defibrillator |
References
- Solomon, S.D.; Zelenkofske, S.; McMurray, J.J.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden Death in Patients with Myocardial Infarction and Left Ventricular Dysfunction, Heart Failure, or Both. N. Engl. J. Med. 2005, 352, 2581–2588. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.E.; Lee, K.L.; Fisher, J.D.; Josephson, M.E.; Prystowsky, E.N.; Hafley, G. A Randomized Study of the Prevention of Sudden Death in Patients with Coronary Artery Disease. N. Engl. J. Med. 1999, 341, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Elayi, C.S.; Charnigo, R.J.; Heron, P.M.; Lee, B.K.; Olgin, J.E. Primary Prevention of Sudden Cardiac Death Early Post-Myocardial Infarction. Circ. Arrhythmia Electrophysiol. 2017, 10, 194. [Google Scholar] [CrossRef] [PubMed]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgärtner, F.; et al. Defibrillator Implantation Early after Myocardial Infarction. N. Engl. J. Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Kuck, K.H.; Dorian, P.; Roberts, R.S.; Hampton, J.R.; Hatala, R.; Fain, E.; Gent, M.; Connolly, S.J. Prophylactic Use of an Implantable Cardioverter–Defibrillator after Acute Myocardial Infarction. N. Engl. J. Med. 2004, 351, 2481–2488. [Google Scholar] [CrossRef]
- Solomon, S.D.; Glynn, R.J.; Greaves, S.; Ajani, U.; Rouleau, J.-L.; Menapace, F.; Arnold, J.M.O.; Hennekens, C.; Pfeffer, M.A. Recovery of ventricular function after myocardial infarction in the reperfusion era: The healing and early afterload reducing therapy study. Ann. Intern. Med. 2001, 134, 451–458. [Google Scholar] [CrossRef]
- Chieng, D.; Paul, V.; Denman, R. Current Device Therapies for Sudden Cardiac Death Prevention—The ICD, Subcutaneous ICD and Wearable ICD. Heart Lung Circ. 2018, 28, 65–75. [Google Scholar] [CrossRef]
- Piccini, J.P.; Allen, L.A.; Kudenchuk, P.J.; Page, R.L.; Patel, M.R.; Turakhia, M.P. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death. Circulation 2016, 133, 1715–1727. [Google Scholar] [CrossRef]
- Feldman, A.M.; Klein, H.; Tchou, P.; Murali, S.; Hall, W.J.; Mancini, D.; Boehmer, J.; Harvey, M.; Heilman, M.S.; Szymkiewicz, S.J.; et al. Use of a Wearable Defibrillator in Terminating Tachyarrhythmias in Patients at High Risk for Sudden Death: Results of WEARIT/BIROAD. Pacing Clin. Electrophysiol. 2004, 27, 4–9. [Google Scholar] [CrossRef]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter–Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Olgin, J.E.; Lee, B.K.; Vittinghoff, E.; Morin, D.P.; Zweibel, S.; Rashba, E.; Chung, E.H.; Borggrefe, M.; Hulley, S.; Lin, F.; et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020, 31, 1009–1018. [Google Scholar] [CrossRef]
- Healy, C.A.; Carrillo, R.G. Wearable cardioverter-defibrillator for prevention of sudden cardiac death after infected implantable cardioverter-defibrillator removal: A cost-effectiveness evaluation. Heart Rhythm. 2015, 12, 1565–1573. [Google Scholar] [CrossRef]
- Sanders, G.D.; Owens, D.K.; Hlatky, M.A. Potential Cost-effectiveness of Wearable Cardioverter-Defibrillator Early Post Myocardial Infarction. J. Innov. Card. Rhythm. Manag. 2015, 6, 1929–1940. [Google Scholar] [CrossRef]
- Botto, G.L.; Mantovani, L.G.; Cortesi, P.A.; De Ponti, R.; D’Onofrio, A.; Biffi, M.; Capucci, A.; Casu, G.; Notarstefano, P.; Scaglione, M.; et al. The value of wearable cardioverter defibrillator in adult patients with recent myocardial infarction: Economic and clinical implications from a health technology assessment perspective. Int. J. Cardiol. 2022, 356, 12–18. [Google Scholar] [CrossRef] [PubMed]
- Clark, M.A.; Szymkiewicz, S.; Volosin, K.; Kabulski, G.M.; Northup, A.; Wiggins, B.S. Mortality and Costs Associated with Wearable Cardioverter-defibrillators after Acute Myocardial Infarction: A Retrospective Cohort Analysis of Medicare Claims Data. J. Innov. Card. Rhythm Manag. 2019, 10, 3866–3873. [Google Scholar] [CrossRef] [PubMed]
- Boriani, G.; Mantovani, L.G.; Cortesi, P.A.; De Ponti, R.; D’Onofrio, A.; Arena, G.; Curnis, A.; Forleo, G.; Guerra, F.; Porcu, M.; et al. Cost-minimization analysis of a wearable cardioverter defibrillator in adult patients undergoing ICD explant procedures: Clinical and economic implications. Clin. Cardiol. 2021, 44, 1497–1505. [Google Scholar] [CrossRef] [PubMed]
- Gage, A.; Higgins, A.; Lee, R. Cardiac Critical Care: The Evolution of a Novel Subspecialty. Methodist DeBakey Cardiovasc. J. 2022, 18, 24–29. [Google Scholar] [CrossRef]
- Chen, R.; Strait, K.M.; Dharmarajan, K.; Li, S.-X.; Ranasinghe, I.; Martin, J.; Fazel, R.; Masoudi, F.A.; Cooke, C.R.; Nallamothu, B.K.; et al. Hospital variation in admission to intensive care units for patients with acute myocardial infarction. Am. Heart J. 2015, 170, 1161–1169. [Google Scholar] [CrossRef]
- Reek, S.; Burri, H.; Roberts, P.R.; Perings, C.; Epstein, A.E.; Klein, H.U.; Lip, G.; Gorenek, B.; Sticherling, C.; Fauchier, L.; et al. The wearable cardioverter-defibrillator: Current technology and evolving indications. Europace 2016, 19, 335–345. [Google Scholar] [CrossRef]
- Klein, H.U.; Goldenberg, I.; Moss, A.J. Risk stratification for implantable cardioverter defibrillator therapy: The role of the wearable cardioverter-defibrillator. Eur. Heart J. 2013, 34, 2230–2242. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, E.; Weeda, E.; Kohn, C.; D’Souza, B.; Russo, A.; Noreika, S.; Coleman, C. Wearable Cardioverter-defibrillators for the Prevention of Sudden Cardiac Death: A Meta-analysis. J. Innov. Card. Rhythm. Manag. 2018, 9, 3151–3162. [Google Scholar] [CrossRef] [PubMed]
- Wäßnig, N.K.; Günther, M.; Quick, S.; Pfluecke, C.; Rottstädt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience with the Wearable Cardioverter-Defibrillator in Patients at High Risk for Sudden Cardiac Death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef] [PubMed]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, A.N.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef]
- Bodin, A.; Bisson, A.; Fauchier, L. When is a wearable defibrillator indicated? Expert Rev. Med Devices 2021, 18, 51–56. [Google Scholar] [CrossRef]
- Cho, K.H.; Han, X.; Ahn, J.H.; Hyun, D.Y.; Kim, M.C.; Sim, D.S.; Hong, Y.J.; Kim, J.H.; Ahn, Y.; Hwang, J.Y.; et al. Long-Term Outcomes of Patients with Late Presentation of ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2021, 77, 1859–1870. [Google Scholar] [CrossRef]
- Sud, M.; Yu, B.; Wijeysundera, H.C.; Austin, P.C.; Ko, D.T.; Braga, J.; Cram, P.; Spertus, J.A.; Domanski, M.; Lee, D.S. Associations Between Short or Long Length of Stay and 30-Day Readmission and Mortality in Hospitalized Patients With Heart Failure. JACC Heart Fail. 2017, 5, 578–588. [Google Scholar] [CrossRef]
- Hoogervorst-Schilp, J.; Langelaan, M.; Spreeuwenberg, P.; De Bruijne, M.C.; Wagner, C. Excess length of stay and economic consequences of adverse events in Dutch hospital patients. BMC Health Serv. Res. 2015, 15, 531. [Google Scholar] [CrossRef]
- Toušek, P.; Bauer, D.; Neuberg, M.; Nováčková, M.; Mašek, P.; Tu˚ma, P.; Kočka, V.; Moťovská, Z.; Widimský, P. Patient characteristics, treatment strategy, outcomes, and hospital costs of acute coronary syndrome: 3 years of data from a large high-volume centre in Central Europe. Eur. Heart J. Suppl. 2022, 24, B3–B9. [Google Scholar] [CrossRef]
- Chung, M.K.; Szymkiewicz, S.J.; Shao, M.; Zishiri, E.; Niebauer, M.J.; Lindsay, B.D.; Tchou, P.J. Aggregate National Experience with the Wearable Cardioverter-Defibrillator: Event Rates, Compliance, and Survival. J. Am. Coll. Cardiol. 2010, 56, 194–203. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).