Prevention of Early Sudden Cardiac Death after Myocardial Infarction Using the Wearable Cardioverter Defibrillator—Results from a Real-World Cohort
Abstract
1. Introduction
2. Materials and Methods
2.1. Austrian WCD Registry
2.2. Austrian “VEST” Cohort
2.3. WCD Diagnostics and Treatments
2.4. Automatically Triggered Alarms
2.5. Manually Recorded Alarms
Appropriate and Inappropriate Treatments
2.6. Follow-Up
2.7. Data Analysis
3. Results
3.1. Patient Characteristics
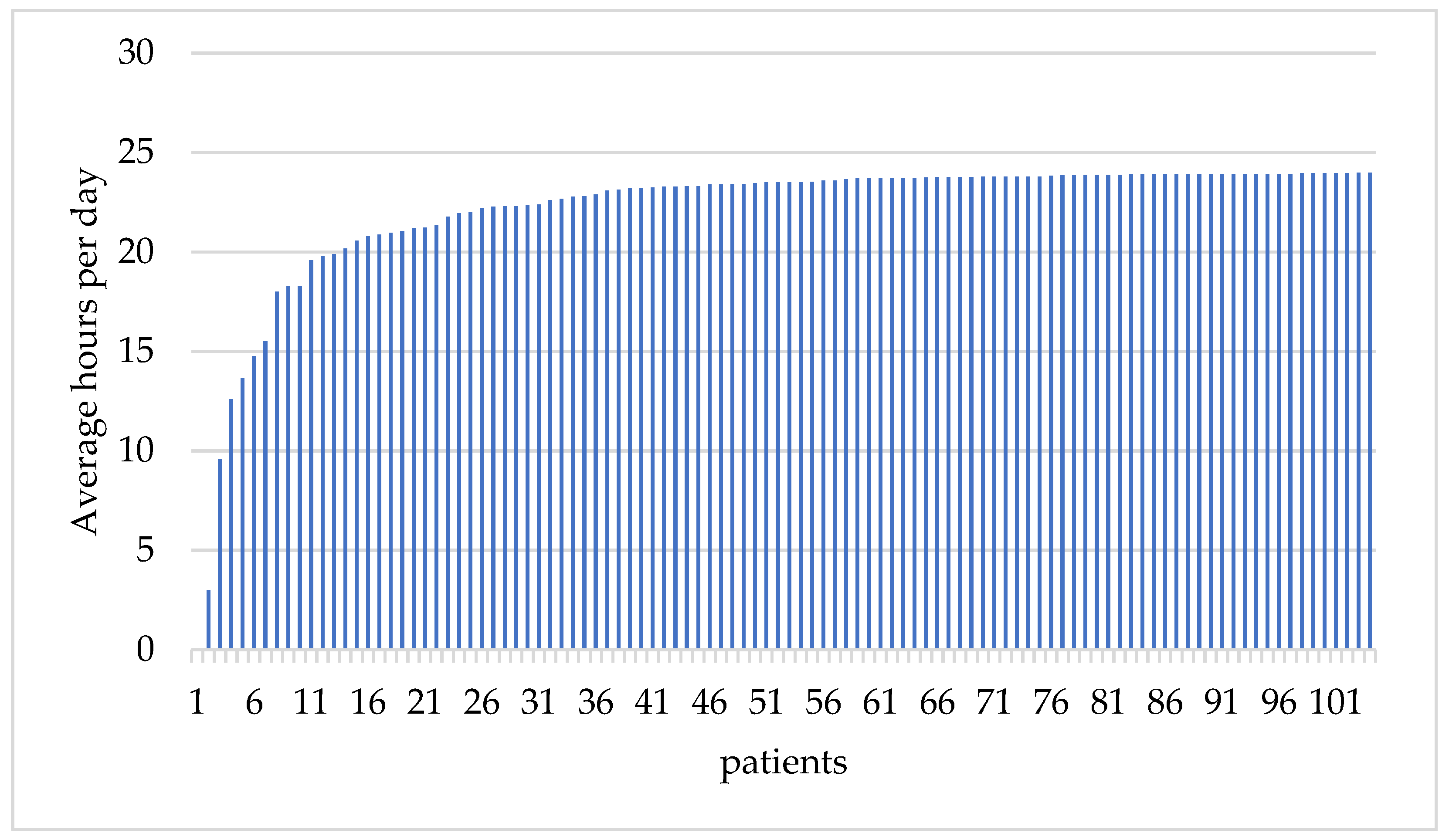
3.2. WCD Prescription and Compliance
3.3. WCD Treatments
3.4. Total Mortality
3.5. Non-Arrhythmic Mortality
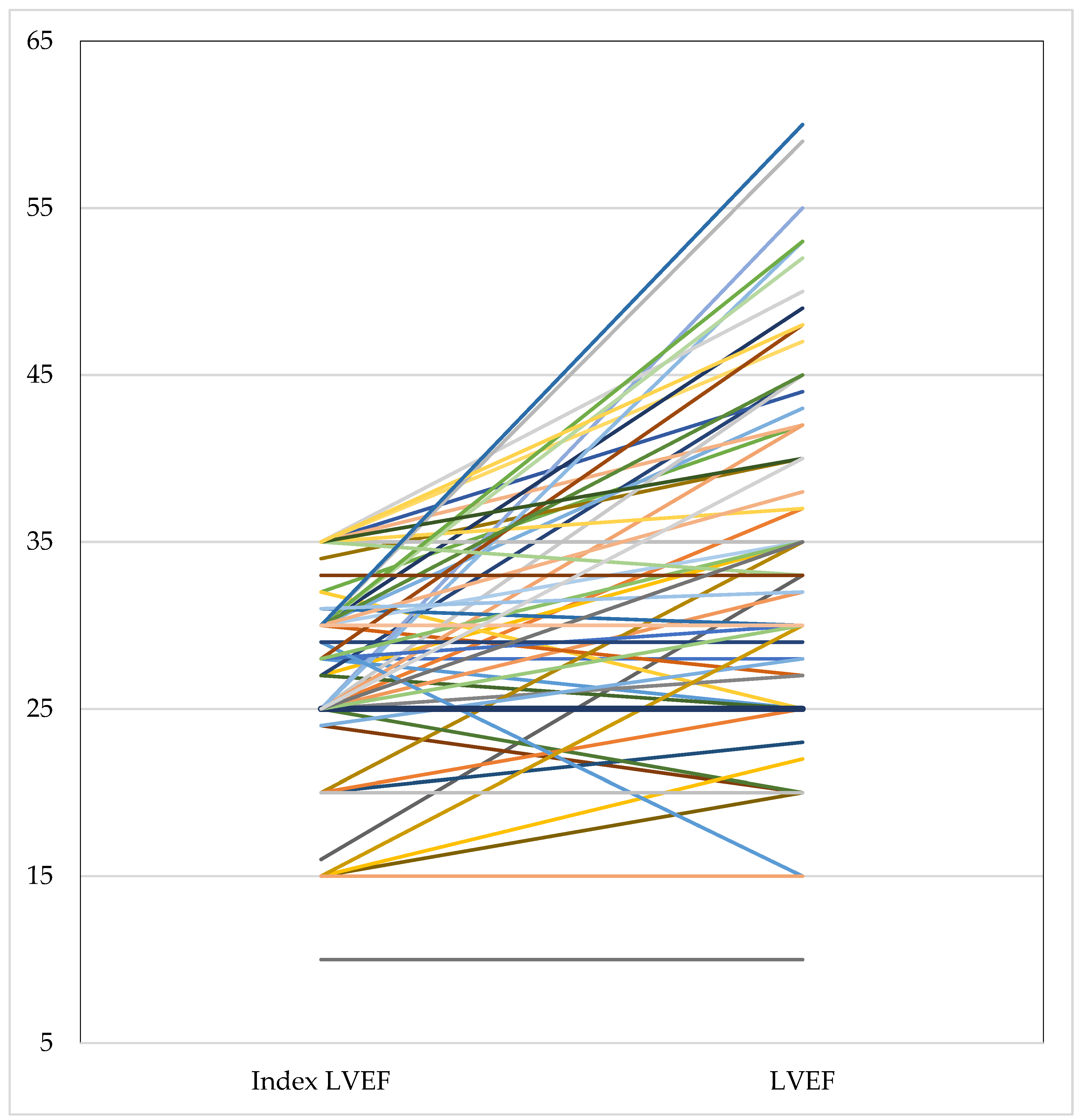
3.6. Arrhythmic Mortality
3.7. Follow-Up
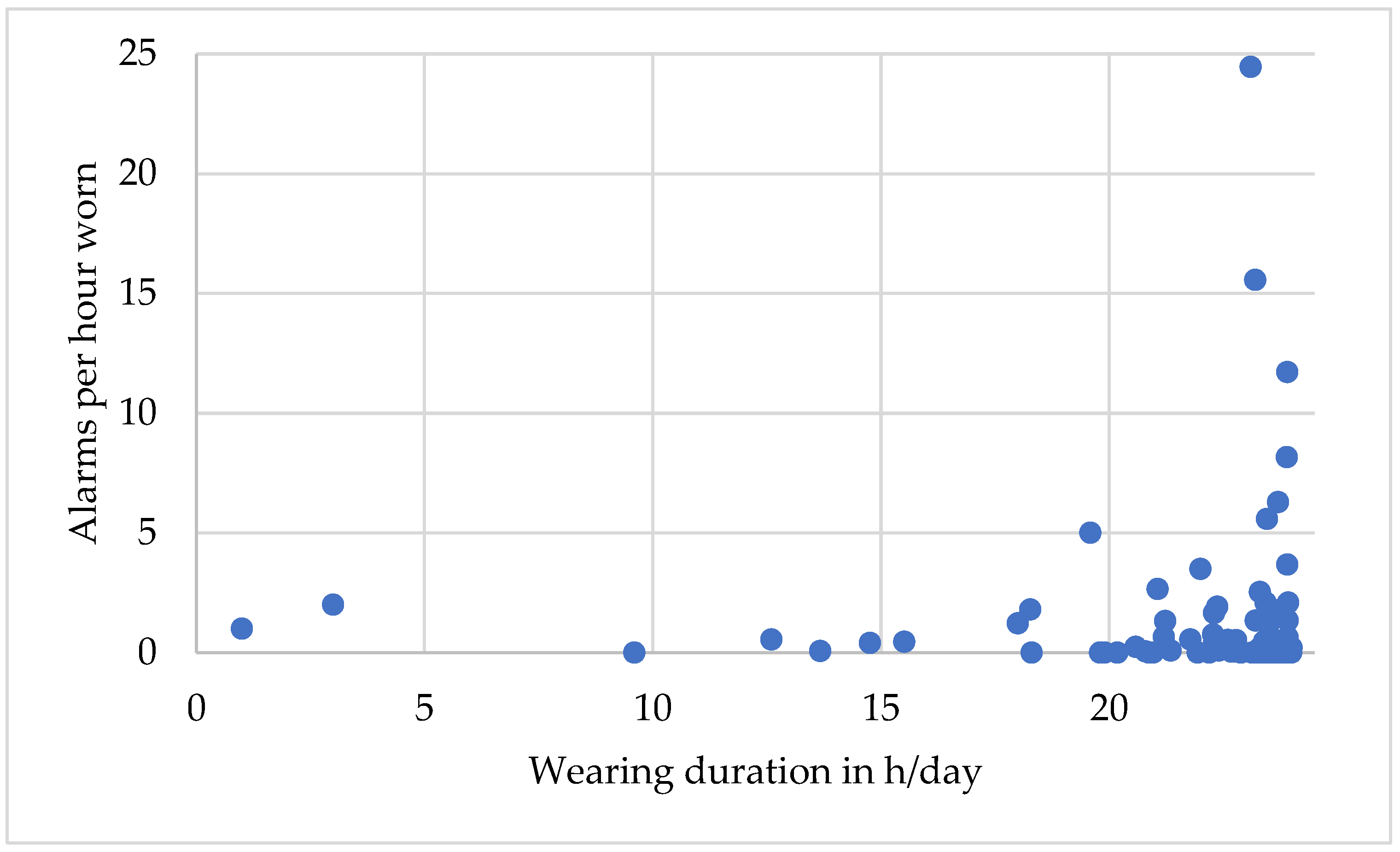
3.8. Alarms
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Solomon, S.D.; Zelenkofske, S.; McMurray, J.J.; Finn, P.V.; Velazquez, E.; Ertl, G.; Harsanyi, A.; Rouleau, J.L.; Maggioni, A.; Kober, L.; et al. Sudden death in patients with myocardial infarction and left ventricular dysfunction, heart failure, or both. N. Engl. J. Med. 2005, 352, 2581–2588. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; de Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [CrossRef] [PubMed]
- Hohnloser, S.H.; Kuck, K.H.; Dorian, P.; Roberts, R.S.; Hampton, J.R.; Hatala, R.; Fain, E.; Gent, M.; Connolly, S.J.; Investigators, D. Prophylactic use of an implantable cardioverter-defibrillator after acute myocardial infarction. N. Engl. J. Med. 2004, 351, 2481–2488. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgartner, F.; et al. Defibrillator implantation early after myocardial infarction. N. Engl. J. Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef] [PubMed]
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter-Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Olgin, J.E.; Lee, B.K.; Vittinghoff, E.; Morin, D.P.; Zweibel, S.; Rashba, E.; Chung, E.H.; Borggrefe, M.; Hulley, S.; Lin, F.; et al. Impact of wearable cardioverter-defibrillator compliance on outcomes in the VEST trial: As-treated and per-protocol analyses. J. Cardiovasc. Electrophysiol. 2020, 31, 1009–1018. [Google Scholar] [CrossRef]
- Bhatt, A.G.; Mittal, S. The wearable cardioverter-defibrillator is not needed for most high-risk patients. Heart Rhythm O2 2020, 1, 230–233. [Google Scholar] [CrossRef]
- Odeneg, T.; Ebner, C.; Mörtl, D.; Keller, H.; Dirninger, A.; Stix, G.; Föger, B.; Grimm, G.; Steinwender, C.; Gebetsberger, F.; et al. Indications for and outcome in patients with the wearable cardioverter-defibrillator in a nurse-based training programme: Results of the Austrian WCD Registry. Eur. J. Cardiovasc. Nurs. 2019, 18, 75–83. [Google Scholar] [CrossRef]
- Sun, S.; Johnson, J.; Degroot, P.; Brown, M.L.; Obel, O. Effect of ICD Therapies on Mortality in the OMNI Trial. J. Cardiovasc. Electrophysiol. 2016, 27, 192–199. [Google Scholar] [CrossRef]
- MacIntyre, C.J.; Sapp, J.L.; Abdelwahab, A.; Al-Harbi, M.; Doucette, S.; Gray, C.; Gardner, M.J.; Parkash, R. The Effect of Shock Burden on Heart Failure and Mortality. CJC Open 2019, 1, 161–167. [Google Scholar] [CrossRef]
- Sood, N.; Ruwald, A.C.; Solomon, S.; Daubert, J.P.; McNitt, S.; Polonsky, B.; Jons, C.; Clyne, C.A.; Zareba, W.; Moss, A.J. Association between myocardial substrate, implantable cardioverter defibrillator shocks and mortality in MADIT-CRT. Eur. Heart J. 2014, 35, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Barraud, J.; Pinon, P.; Laine, M.; Cautela, J.; Orabona, M.; Koutbi, L.; Pinto, J.; Thuny, F.; Franceschi, F.; Paganelli, F.; et al. Ventricular Arrhythmia Occurrence and Compliance in Patients Treated With the Wearable Cardioverter Defibrillator Following Percutaneous Coronary Intervention. Heart Lung Circ. 2018, 27, 984–988. [Google Scholar] [CrossRef]
- Barsheshet, A.; Kutyifa, V.; Vamvouris, T.; Moss, A.J.; Biton, Y.; Chen, L.; Storozynsky, E.; Wan, C.; Szymkiewicz, S.J.; Goldenberg, I. Study of the wearable cardioverter defibrillator in advanced heart-failure patients (SWIFT). J. Cardiovasc. Electrophysiol. 2017, 28, 778–784. [Google Scholar] [CrossRef] [PubMed]
- Erath, J.W.; Vamos, M.; Sirat, A.S.; Hohnloser, S.H. The wearable cardioverter-defibrillator in a real-world clinical setting: Experience in 102 consecutive patients. Clin. Res. Cardiol. 2017, 106, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Epstein, A.E.; Abraham, W.T.; Bianco, N.R.; Kern, K.B.; Mirro, M.; Rao, S.V.; Rhee, E.K.; Solomon, S.D.; Szymkiewicz, S.J. Wearable cardioverter-defibrillator use in patients perceived to be at high risk early post-myocardial infarction. J. Am. Coll. Cardiol. 2013, 62, 2000–2007. [Google Scholar] [CrossRef]
- Kutyifa, V.; Moss, A.J.; Klein, H.; Biton, Y.; McNitt, S.; MacKecknie, B.; Zareba, W.; Goldenberg, I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the Prospective Registry of Patients Using the Wearable Cardioverter Defibrillator (WEARIT-II Registry). Circulation 2015, 132, 1613–1619. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.K.; Szymkiewicz, S.J.; Shao, M.; Zishiri, E.; Niebauer, M.J.; Lindsay, B.D.; Tchou, P.J. Aggregate national experience with the wearable cardioverter-defibrillator: Event rates, compliance, and survival. J. Am. Coll. Cardiol. 2010, 56, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Leyton-Mange, J.S.; Hucker, W.J.; Mihatov, N.; Reynolds, M.; Albert, C.; Lubitz, S.A.; Milan, D.J. Experience with Wearable Cardioverter-Defibrillators at 2 Academic Medical Centers. JACC Clin. Electrophysiol. 2018, 4, 231–239. [Google Scholar] [CrossRef]
- Hioki, H.; Kozuma, K.; Kobayashi, Y.; Ando, K.; Morino, Y.; Kishihara, J.; Ako, J.; Ikari, Y. Wearable cardioverter-defibrillators after myocardial infarction: A review of its clinical utility and unmet needs in current clinical practice. Cardiovasc. Interv. Ther. 2022, 37, 53–59. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L.; et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef]
- Zareba, W.; Moss, A.J.; Jackson Hall, W.; Wilber, D.J.; Ruskin, J.N.; McNitt, S.; Brown, M.; Wang, H.; Investigators, M.I. Clinical course and implantable cardioverter defibrillator therapy in postinfarction women with severe left ventricular dysfunction. J. Cardiovasc. Electrophysiol. 2005, 16, 1265–1270. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Oertelt-Prigione, S.; Prescott, E.; Franconi, F.; Gerdts, E.; Foryst-Ludwig, A.; Maas, A.H.; Kautzky-Willer, A.; Knappe-Wegner, D.; Kintscher, U.; et al. Gender in cardiovascular diseases: Impact on clinical manifestations, management, and outcomes. Eur. Heart J. 2016, 37, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Ibánez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Rev. Esp. Cardiol. (Engl. Ed.) 2017, 70, 1082. [Google Scholar] [CrossRef] [PubMed]
- Erath, J.W.; Assmus, B.; Burch, A.; Bondermann, D.; Russo, A.M.; Kutyifa, V. Sustained Ventricular Tachyarrhythmia Termination in a Large Cohort of Women Using Wearable Cardioverter-Defibrillators. JACC Clin. Electrophysiol. 2020, 6, 1187–1188. [Google Scholar] [CrossRef]
- Masri, A.; Altibi, A.M.; Erqou, S.; Zmaili, M.A.; Saleh, A.; Al-Adham, R.; Ayoub, K.; Baghal, M.; Alkukhun, L.; Barakat, A.F.; et al. Wearable Cardioverter-Defibrillator Therapy for the Prevention of Sudden Cardiac Death: A Systematic Review and Meta-Analysis. JACC Clin. Electrophysiol. 2019, 5, 152–161. [Google Scholar] [CrossRef]
- Alsamman, M.; Prashad, A.; Abdelmaseih, R.; Khalid, T.; Prashad, R. Update on Wearable Cardioverter Defibrillator: A Comprehensive Review of Literature. Cardiol. Res. 2022, 13, 185–189. [Google Scholar] [CrossRef]
- Israel, C.; Staudacher, I.; Leclercq, C.; Botto, G.L.; Scherr, D.; Fach, A.; Duru, F.; Zylla, M.M.; Katus, H.A.; Thomas, D. Sudden cardiac death while waiting: Do we need the wearable cardioverter-defibrillator? Clin. Res. Cardiol. 2022, 111, 1189–1197. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Goetz, G.; Wernly, B.; Wild, C. Wearable cardioverter defibrillator for preventing sudden cardiac death in patients at risk: An updated systematic review of comparative effectiveness and safety. Int. J. Cardiol. Heart Vasc. 2023, 45, 101189. [Google Scholar] [CrossRef]
- Goldenberg, I.; Erath, J.W.; Russo, A.M.; Burch, A.E.; Assmus, B.; Bonderman, D.; McNitt, S.; Kutyifa, V. Sex differences in arrhythmic burden with the wearable cardioverter-defibrillator. Heart Rhythm 2021, 18, 404–410. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Austrian WCD ‖ Cohort | VEST § WCD ‖ Cohort [5] | p-Value | |
|---|---|---|---|
| Number of patients | 105 | 1524 | - |
| Age | 64 ± 11 years | 61 ± 13 years | n.s. |
| Female | 13/105 (12%) | 413/1521 (27%) | <0.01 |
| Arterial hypertension | 96/105 (91%) | 994/1521 (65%) | <0.001 |
| Diabetes mellitus | 27/105 (25%) | 497/1521 (32.7%) | n.s. |
| PCI ‡ | 103/105 (98%) | 1275/1513 (84.3%) | <0.001 |
| CABG * | 1/105 (1%) | 14/1513 (0.9%) | n.s. |
| Thrombolytics | 0/105 (0%) | 118/1513 (7.8%) | <0.01 |
| LVEF † baseline | 28 ± 6% | 28.2 ± 6.1% | n.s. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rohrer, U.; Manninger, M.; Fiedler, L.; Steinwender, C.; Binder, R.K.; Stühlinger, M.; Zirngast, B.; Zweiker, D.; Zirlik, A.; Scherr, D., on behalf of the Austrian WCD Study Group. Prevention of Early Sudden Cardiac Death after Myocardial Infarction Using the Wearable Cardioverter Defibrillator—Results from a Real-World Cohort. J. Clin. Med. 2023, 12, 5029. https://doi.org/10.3390/jcm12155029
Rohrer U, Manninger M, Fiedler L, Steinwender C, Binder RK, Stühlinger M, Zirngast B, Zweiker D, Zirlik A, Scherr D on behalf of the Austrian WCD Study Group. Prevention of Early Sudden Cardiac Death after Myocardial Infarction Using the Wearable Cardioverter Defibrillator—Results from a Real-World Cohort. Journal of Clinical Medicine. 2023; 12(15):5029. https://doi.org/10.3390/jcm12155029
Chicago/Turabian StyleRohrer, Ursula, Martin Manninger, Lukas Fiedler, Clemens Steinwender, Ronald K. Binder, Markus Stühlinger, Birgit Zirngast, David Zweiker, Andreas Zirlik, and Daniel Scherr on behalf of the Austrian WCD Study Group. 2023. "Prevention of Early Sudden Cardiac Death after Myocardial Infarction Using the Wearable Cardioverter Defibrillator—Results from a Real-World Cohort" Journal of Clinical Medicine 12, no. 15: 5029. https://doi.org/10.3390/jcm12155029
APA StyleRohrer, U., Manninger, M., Fiedler, L., Steinwender, C., Binder, R. K., Stühlinger, M., Zirngast, B., Zweiker, D., Zirlik, A., & Scherr, D., on behalf of the Austrian WCD Study Group. (2023). Prevention of Early Sudden Cardiac Death after Myocardial Infarction Using the Wearable Cardioverter Defibrillator—Results from a Real-World Cohort. Journal of Clinical Medicine, 12(15), 5029. https://doi.org/10.3390/jcm12155029






