Does Patient Compliance Influence Wearable Cardioverter Defibrillator Effectiveness? A Single-Center Experience
Abstract
1. Introduction
2. Materials and Methods
2.1. Aim
2.2. Participants
2.3. Study Design and Methods
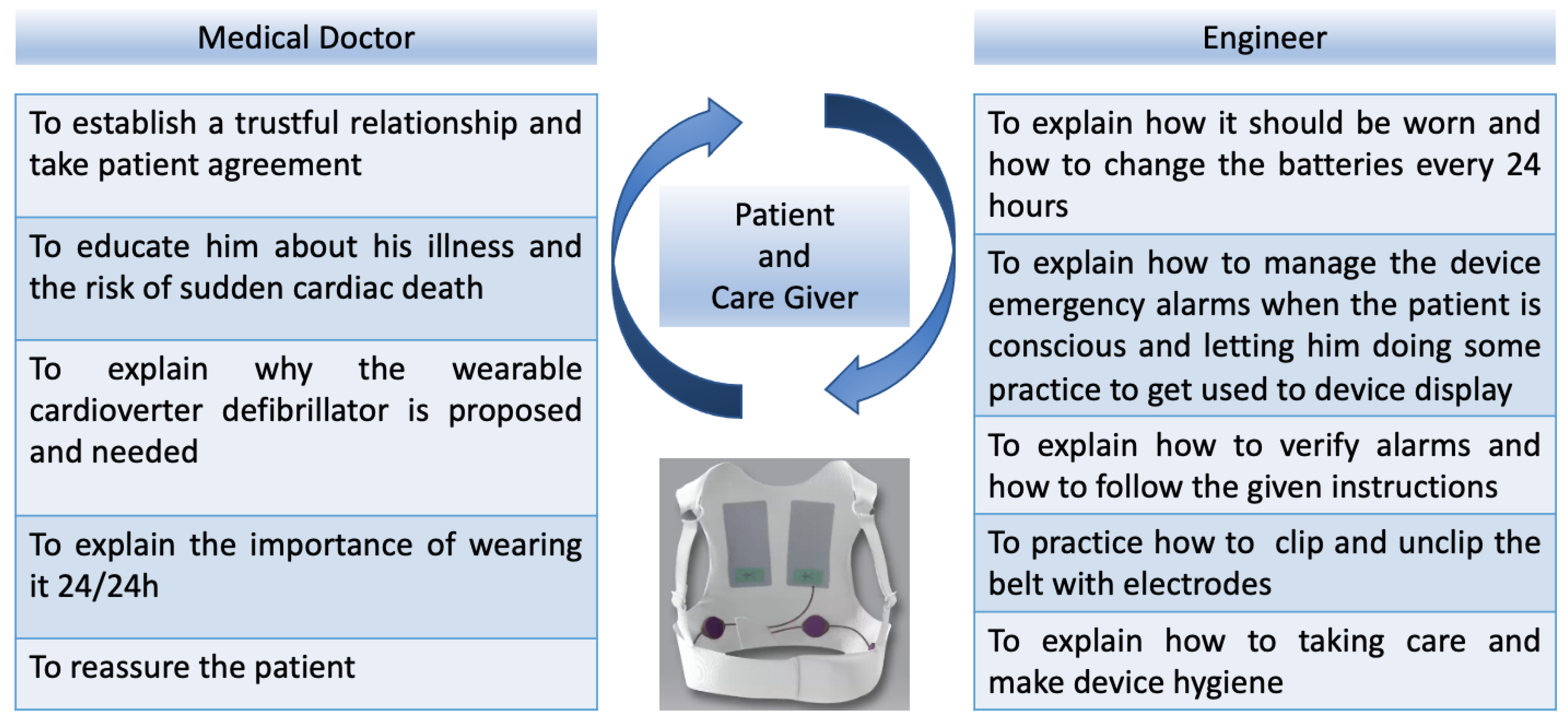
2.4. How We Train the Patient
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olgin, J.E.; Pletcher, M.J.; Vittinghoff, E.; Wranicz, J.; Malik, R.; Morin, D.P.; Zweibel, S.; Buxton, A.E.; Elayi, C.S.; Chung, E.H.; et al. Wearable Cardioverter–Defibrillator after Myocardial Infarction. N. Engl. J. Med. 2018, 379, 1205–1215. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Wittstein, I.S.; Prasad, A.; Sharkey, S.; Dote, K.; Akashi, Y.J.; Cammann, V.L.; Crea, F.; Galiuto, L.; Desmet, W.; et al. International expert consensus document on takotsubo syndrome (Part II): Diagnostic workup, outcome, and management. Eur. Heart J. 2018, 39, 2047–2062. [Google Scholar] [CrossRef] [PubMed]
- Sancassiani, F.; Carta, M.G.; Montisci, R.; Preti, A.; Machado, S.; Moro, M.F.; Marchetti, M.F.; Meloni, L. Takotsubo Syndrome is Associated with Mood Disorders and Antidepressants Use, not with Anxiety and Impairment of Quality of Life Due to the Psychiatric Disorder. Clin. Pract. Epidemiol. Ment. Health 2018, 14, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Steinbeck, G.; Andresen, D.; Seidl, K.; Brachmann, J.; Hoffmann, E.; Wojciechowski, D.; Kornacewicz-Jach, Z.; Sredniawa, B.; Lupkovics, G.; Hofgartner, F.; et al. Defibrillator implantation early after myo- cardial infarction. N. Engl. J. Med. 2009, 361, 1427–1436. [Google Scholar] [CrossRef]
- Buxton, A.E.; Lee, K.L.; Fisher, J.D.; Josephson, M.E.; Prystowsky, E.N.; Hafley, G. A Randomized study of the prevention of sudden death in patients with coronary artery disease. N. Engl. J. Med. 1999, 341, 1882–1890. [Google Scholar] [CrossRef]
- Moss, A.J.; Hall, W.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Klein, H.; Levine, J.H.; Saksena, S.; Waldo, A.L.; Wilber, D.; et al. Improved survival with an implanted defibrillator in patients with coronary disease at high risk for ventricular arrhythmia. N. Engl. J. Med. 1996, 335, 1933–1940. [Google Scholar] [CrossRef]
- Moss, A.J.; Zareba, W.; Hall, W.J.; Klein, H.; Wilber, D.J.; Cannom, D.S.; Daubert, J.P.; Higgins, S.L.; Brown, M.W.; Andrews, M.L. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N. Engl. J. Med. 2002, 346, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.S.; McMurray, J.J.; Packer, M.; Swedberg, K.; Rouleau, J.L.; Chen, F.; Gong, J.; Rizkala, A.R.; Brahimi, A.; Claggett, B.; et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur. Heart J. 2015, 36, 1990–1997. [Google Scholar] [CrossRef]
- Curtain, J.P.; Docherty, K.F.; Jhund, P.S.; Petrie, M.C.; E Inzucchi, S.; Køber, L.; Kosiborod, M.N.; A Martinez, F.; Ponikowski, P.; Sabatine, M.S.; et al. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur. Heart J. 2021, 42, 3727–3738. [Google Scholar] [CrossRef]
- Casolo, G.; Gulizia, M.M.; Aschieri, D.; Chinaglia, A.; Corda, M.; Nassiacos, D.; Caico, S.I.; Chimenti, C.; Giaccardi, M.; Gotti, E.; et al. ANMCO Position paper: Wearable cardioverter defibrillator appropriate use guidance for the management of patients at high transient risk of sudden cardiac death. G. Ital. Cardiol. 2023, 24, 394–411. [Google Scholar] [CrossRef]
- Priori, S.G.; Blomström-Lundqvist, C.; Mazzanti, A.; Blom, N.; Borggrefe, M.; Camm, J.; Elliott, P.M.; Fitzsimons, D.; Hatala, R.; Hindricks, G.; et al. ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: The task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2015, 36, 2793–2867. [Google Scholar] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Garcia, R.; Combes, N.; Defaye, P.; Narayanan, K.; Guedon-Moreau, L.; Boveda, S.; Blangy, H.; Bouet, J.; Briand, F.; Chevalier, P.; et al. Wearable cardioverter-defibrillator in patients with a transient risk of sudden cardiac death: The WEARIT-France cohort study. Europace 2020, 23, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Feldman, A.M.; Klein, H.; Tchou, P.; Murali, S.; Hall, W.J.; Mancini, D.; Boehmer, J.; Harvey, M.; Heilman, M.S.; Szymkiewicz, S.J.; et al. Use of a wearable defibrillator in terminating tachyarrhythmias in patients at high risk for sudden death:. Results of WEARIT/BIROAD. Pacing Clin. Electrophysiol. 2004, 27, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Kutyifa, V.; Moss, A.J.; Klein, H.; Biton, Y.; McNitt, S.; MacKecknie, B.; Zareba, W.; Goldenberg, I. Use of the wearable cardioverter defibrillator in high-risk cardiac patients: Data from the prospective registry of patients using the wearable cardioverter defibrillator (WEARIT-II registry). Circulation 2015, 132, 1613–1619. [Google Scholar] [CrossRef]
- Chung, M.K.; Szymkiewicz, S.J.; Shao, M.; Zishiri, E.; Niebauer, M.J.; Lindsay, B.D.; Tchou, P.J. Aggregate national experience with the wearable cardioverter-defibrillator: Event rates, compliance, and survival. J. Am. Coll. Cardiol. 2010, 56, 194–203. [Google Scholar] [CrossRef]
- Epstein, A.E.; Abraham, W.T.; Bianco, N.R.; Kern, K.B.; Mirro, M.; Rao, S.V.; Rhee, E.K.; Solomon, S.D.; Szymkiewicz, S.J. Wearable cardioverter-defibrillator use inpatients perceived to be at high risk early post-myocardial infarction. J. Am. Coll. Cardiol. 2013, 62, 2000–2007. [Google Scholar] [CrossRef]
- Wäßnig, N.K.; Günther, M.; Quick, S.; Pfluecke, C.; Rottstädt, F.; Szymkiewicz, S.J.; Ringquist, S.; Strasser, R.H.; Speiser, U. Experience with the wearable cardioverter-defibrillator in patients at high risk for sudden cardiac death. Circulation 2016, 134, 635–643. [Google Scholar] [CrossRef]
- Osto, E.; Tona, F.; Angelini, A.; Montisci, R.; Ruscazio, M.; Vinci, A.; Tarantini, G.; Ramondo, A.; Gambino, A.; Thiene, G.; et al. Determinants of coronary flow reserve in heart transplantation: A study performed with contrast-enhanced echocardiography. J. Heart Lung Transplant. 2009, 28, 453–460. [Google Scholar] [CrossRef]
- Montisci, R.; Cecchetto, G.; Ruscazio, M.; Snenghi, R.; Portale, A.; Viel, G.; Nalesso, A.; Paoli, A.; Iliceto, S.; Meloni, L.; et al. Early myocardial dysfunction after chronic use of anabolic androgenic steroids: Combined pulsed-wave tissue doppler imaging and ultrasonic integrated backscatter cyclic variations analysis. J. Am. Soc. Echocardiogr. 2010, 23, 516–522. [Google Scholar] [CrossRef]
- Reek, S.; Burri, H.; Roberts, P.R.; Perings, C.; Epstein, A.E.; Klein, H.U.; Lip, G.; Gorenek, B.; Sticherling, C.; Fauchier, L.; et al. The wearable cardioverter-defibrillator: Current technology and evolving indications. Europace 2016, 19, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.; Burri, H.; Buehler, A.; Reek, S.; Sticherling, C.; Schaer, B.; Linka, A.; Ammann, P.; Müller, A.S.; Dzemali, O.; et al. High Incidence of Inappropriate Alarms in Patients with Wearable Cardioverter-Defibrillators: Findings from the Swiss WCD Registry. J. Clin. Med. 2021, 10, 3811. [Google Scholar] [CrossRef] [PubMed]
- Zoppo, F.; Mugnai, G.; Lupo, A.; Zerbo, F. Bridge to avoid ICD implantation with wearable cardioverter defibrillators. Adv. Interv. Cardiol. 2020, 16, 227–228. [Google Scholar] [CrossRef]
- Di Marco, A.; Anguera, I.; Schmitt, M.; Klem, I.; Neilan, T.G.; White, J.A.; Sramko, M.; Masci, P.G.; Barison, A.; Makenna, P.; et al. Late gadolinium enhancement and the risk for ventricular arrhythmias or sudden death in dilated cardiomyopathy: Systematic review and meta-analysis. JACC Heart Fail. 2017, 5, 28–38. [Google Scholar] [CrossRef]
- Halliday, B.P.; Baksi, A.J.; Gulati, A.; Ali, A.; Newsome, S.; Izgi, C.; Arzanauskaite, M.; Lota, A.; Tayal, U.; Vassiliou, V.; et al. Outcome in dilated cardiomyopathy related to the extent, location, and pattern of late gadolinium enhancement. JACC Cardiovasc. Imaging 2019, 12, 1645–1655. [Google Scholar] [CrossRef]
- Guaricci, A.I.; Masci, P.G.; Muscogiuri, G.; Guglielmo, M.; Baggiano, A.; Fusini, L.; Lorenzoni, V.; Martini, C.; Andreini, D.; Pabon, A.G.; et al. Cardiac magnetic Resonance for prophylactic Implantable cardioverter defibrillator therapy in non ischaemic dilated cardiomyopathy: An international Registry. Europace 2021, 23, 1072–1083. [Google Scholar] [CrossRef]
- Piaserico, S.; Osto, E.; Famoso, G.; Montisci, R.; De Michieli, L.; Zanetti, I.; Iliceto, S.; Tona, F. Long-term prognostic value of coronary flow reserve in psoriasis patients. Atherosclerosis 2019, 289, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Dagres, N.; Peek, N.; Leclercq, C.; Hindricks, G. The PROFID project. Eur. Heart J. 2020, 41, 3781–3782. [Google Scholar] [CrossRef]
- Zeppenfeld, K.; Tfelt-Hansen, J.; De Riva, M.; Winkel, B.G.; Behr, E.R.; Blom, N.A.; Charron, P.; Corrado, D.; Dagres, N.; de Chillou, C.; et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death Developed by the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC) Endorsed by the Association for European Paediatric and Congenital Cardiology (AEPC). Eur. Heart J. 2022, 43, 3997–4126. [Google Scholar] [PubMed]
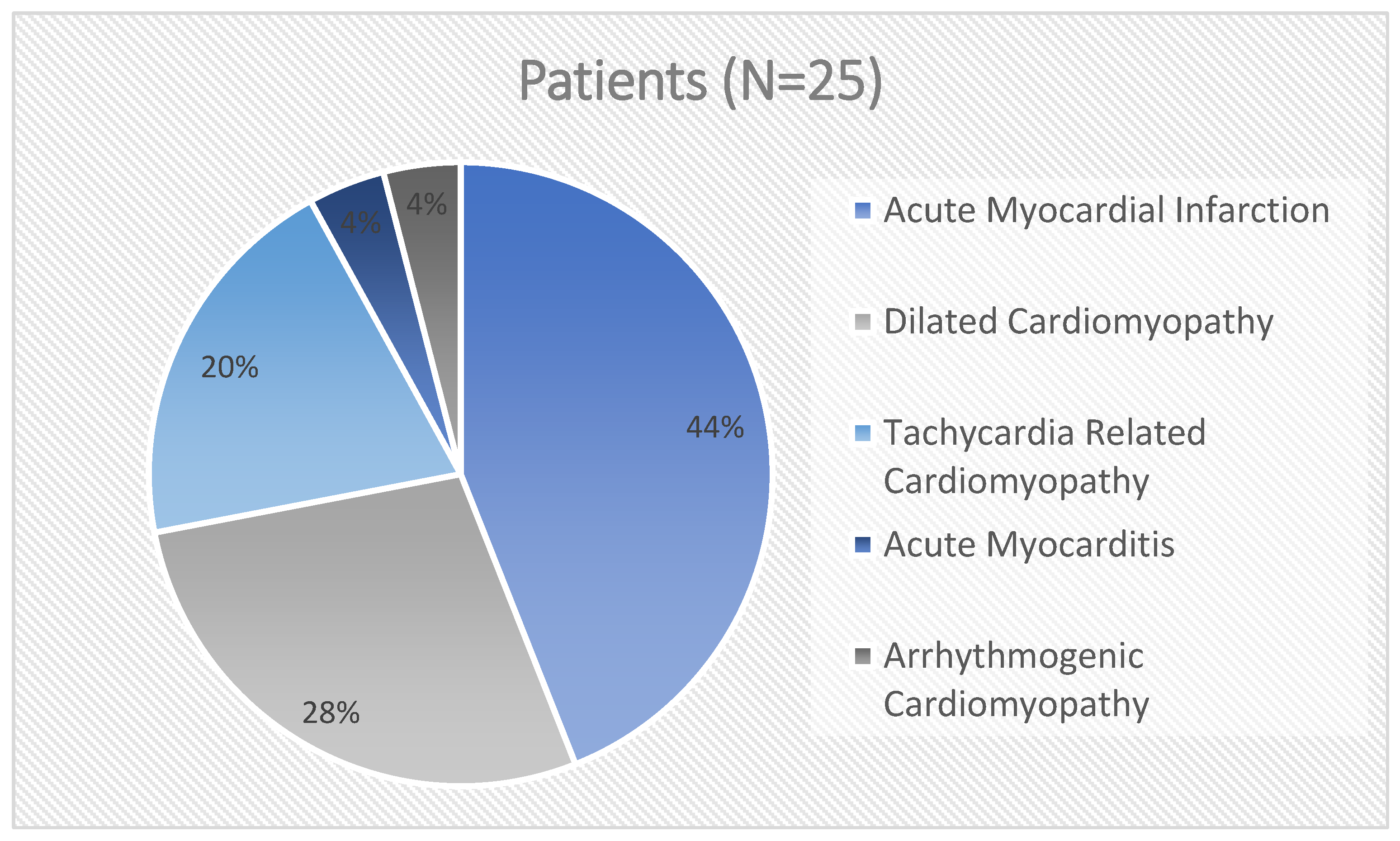
| Baseline Characteristics | Patients (N = 25) |
|---|---|
| Age (year)_Mean ± SD | 63.6 ± 14.6 |
| Female sex | 16% |
| BMI (kg/m2)_Mean ± SD | 26.0 ± 5.8 |
| Hypertension | 56% |
| Dyslipidemia | 64% |
| Diabetes | 36% |
| Tobacco use | 28% |
| Coronary artery disease | 52% |
| Right bundle branch block | 16% |
| Left bundle branch block | 12% |
| Dual antiplatelets therapy | 24% |
| ACEi | 32% |
| ARB | 64% |
| MRA | 68% |
| Beta blockers | 96% |
| Loop diuretics | 72% |
| Follow-Up Analysis | ICD Not Implanted Group (19) | ICD Implanted Group (6) | p-Value |
|---|---|---|---|
| Age_year (Mean (SD)) | 63.8 (15.1) | 63.1 (14.3) | 0.93 |
| Sex_males (N (%)) | 15 (79%) | 6 (100%) | 0.54 |
| BMI_kg/m2 (Mean (SD)) | 25.7 (5.8) | 27.1 (6.7) | 0.67 |
| LVEF Simpson_% (Mean (SD)) | 46.4 (8.5) | 35.8 (12.8) | 0.028 |
| Left ventricular end-diastolic dimension_mm (Mean (SD)) | 59.7 (9.4) | 55.0 (8.3) | 0.27 |
| Left ventricular end-diastolic volume_mL (Mean (SD)) | 132.5 (52.8) | 123.5 (113.9) | 0.85 |
| Left ventricular indexed end-diastolic volume_mL/m2 (Mean (SD)) | 70.7 (21.2) | 108.0 (52.2) | 0.024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fazzini, L.; Marchetti, M.F.; Perra, F.; Biddau, M.; Massazza, N.; Nissardi, V.; Agus, E.; Demelas, R.; Montisci, R. Does Patient Compliance Influence Wearable Cardioverter Defibrillator Effectiveness? A Single-Center Experience. J. Clin. Med. 2023, 12, 4743. https://doi.org/10.3390/jcm12144743
Fazzini L, Marchetti MF, Perra F, Biddau M, Massazza N, Nissardi V, Agus E, Demelas R, Montisci R. Does Patient Compliance Influence Wearable Cardioverter Defibrillator Effectiveness? A Single-Center Experience. Journal of Clinical Medicine. 2023; 12(14):4743. https://doi.org/10.3390/jcm12144743
Chicago/Turabian StyleFazzini, Luca, Maria Francesca Marchetti, Ferdinando Perra, Mattia Biddau, Nicola Massazza, Vincenzo Nissardi, Elena Agus, Roberta Demelas, and Roberta Montisci. 2023. "Does Patient Compliance Influence Wearable Cardioverter Defibrillator Effectiveness? A Single-Center Experience" Journal of Clinical Medicine 12, no. 14: 4743. https://doi.org/10.3390/jcm12144743
APA StyleFazzini, L., Marchetti, M. F., Perra, F., Biddau, M., Massazza, N., Nissardi, V., Agus, E., Demelas, R., & Montisci, R. (2023). Does Patient Compliance Influence Wearable Cardioverter Defibrillator Effectiveness? A Single-Center Experience. Journal of Clinical Medicine, 12(14), 4743. https://doi.org/10.3390/jcm12144743






