Impact of T Lymphocytes Isolated from Liver Perfusate of Deceased Brain Donors on Kidney Transplantation: Preliminary Evidence and Future Directions
Abstract
1. Introduction
2. Materials and Methods
2.1. Donor Cohort and Liver Perfusate Fraction Analysis
- -
- Pregnancy or active breast-feeding,
- -
- Allergy or autoimmune disease,
- -
- Donors requiring systemic immunosuppressive drug at the time of procurement,
- -
- History of malignancy and of human immunodeficiency virus positivity,
- -
- Donor who had previously received an organ transplant.
2.2. Flow Cytometry Staining of Lymphocytes and Antibody
2.3. Recipient Cohort and Study Endpoints
- -
- Dual KT and/or combined solid organ transplantation recipients,
- -
- A recipient who underwent KT with a graft procured from a DBD affected with thrombotic micro-angiopathy,
- -
- Recipients of a living donor transplant.
- -
- Acute kidney injury (AKI) needs dialysis within 1 week of transplantation [1];
- -
- Incidence of early graft loss (EGL) at 6 months after KT.
2.4. Statistical Analysis
2.5. Ethical Validation
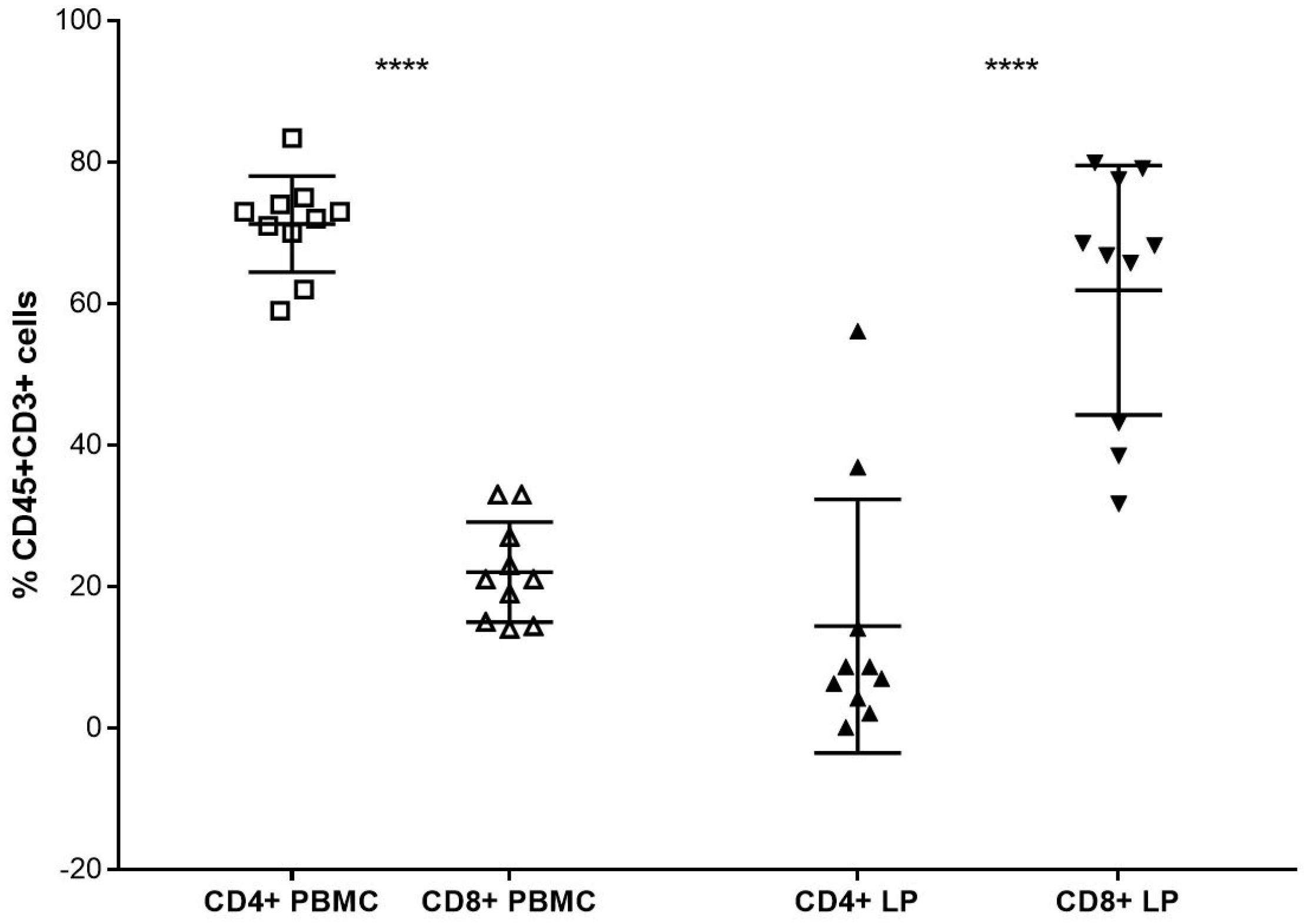
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Swanson, K.J.; Bhattarai, M.; Parajuli, S. Delayed graft function: Current status and future directions. Curr. Opin. Organ Transplant. 2023, 28, 1–7. [Google Scholar] [CrossRef]
- Lim, W.H.; Johnson, D.W.; Teixeira-Pinto, A.; Wong, G. Association Between Duration of Delayed Graft Function, Acute Rejection, and Allograft Outcome After Deceased Donor Kidney Transplantation. Transplantation 2019, 103, 412–419. [Google Scholar] [CrossRef] [PubMed]
- Morath, C.; Schmitt, A.; Kleist, C.; Daniel, V.; Opelz, G.; Süsal, C.; Ibrahim, E.; Kälble, F.; Speer, C.; Nusshag, C.; et al. Phase I trial of donor-derived modified immune cell infusion in kidney transplantation. J. Clin. Investig. 2020, 130, 2364–2376. [Google Scholar] [CrossRef]
- de Leur, K.; Dieterich, M.; Hesselink, D.A.; Corneth, O.B.J.; Dor, F.J.M.F.; de Graav, G.N.; Peeters, A.M.A.; Mulder, A.; Kimenai, H.J.A.N.; Claas, F.H.J.; et al. Characterization of donor and recipient CD8+ tissue-resident memory T cells in transplant nephrectomies. Sci. Rep. 2019, 9, 5984. [Google Scholar] [CrossRef] [PubMed]
- Jahn, N.; Sack, U.; Stehr, S.; Vöelker, M.T.; Laudi, S.; Seehofer, D.; Atay, S.; Zgoura, P.; Viebahn, R.; Boldt, A.; et al. The Role of Innate Immune Cells in the Prediction of Early Renal Allograft Injury Following Kidney Transplantation. J. Clin. Med. 2022, 11, 6148. [Google Scholar] [CrossRef]
- Jayachandran, R.; Gumienny, A.; Bolinger, B.; Ruehl, S.; Lang, M.J.; Fucile, G.; Mazumder, S.; Tchang, V.; Woischnig, A.K.; Stiess, M.; et al. Disruption of Coronin 1 Signaling in T Cells Promotes Allograft Tolerance while Maintaining Anti-Pathogen Immunity. Immunity 2019, 50, 152–165.e8. [Google Scholar] [CrossRef]
- Zito, G.; Miceli, V.; Carcione, C.; Busà, R.; Bulati, M.; Gallo, A.; Iannolo, G.; Pagano, D.; Conaldi, P.G. Human Amnion-Derived Mesenchymal Stromal/Stem Cells Pre-Conditioning Inhibits Inflammation and Apoptosis of Immune and Parenchymal Cells in an In Vitro Model of Liver Ischemia/Reperfusion. Cells 2022, 11, 709. [Google Scholar] [CrossRef]
- Safinia, N.; Afzali, B.; Atalar, K.; Lombardi, G.; Lechler, R.I. T-cell alloimmunity and chronic allograft dysfunction. Kidney Int. Suppl. 2010, 78, S2–S12. [Google Scholar] [CrossRef]
- Scandling, J.D.; Busque, S.; Dejbakhsh-Jones, S.; Benike, C.; Millan, M.T.; Shizuru, J.A.; Hoppe, R.T.; Lowsky, R.; Engleman, E.G.; Strober, S. Tolerance and chimerism after renal and hematopoietic-cell transplantation. N. Engl. J. Med. 2008, 358, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Noris, M.; Azzollini, N.; Mister, M.; Pezzotta, A.; Piccinini, G.; Casiraghi, F.; Cugini, D.; Perico, N.; Orisio, S.; Remuzzi, G. Peripheral donor leukocytes prolong survival of rat renal allografts. Kidney Int. 1999, 56, 1101–1112. [Google Scholar] [CrossRef]
- Salgar, S.K.; Shapiro, R.; Dodson, F.; Corry, R.; McCurry, K.; Zeevi, A.; Pham, S.; Abu-Elmagd, K.; Reyes, J.; Jordan, M.; et al. Infusion of donor leukocytes to induce tolerance in organ allograft recipients. J. Leukoc. Biol. 1999, 66, 310–314. [Google Scholar] [CrossRef] [PubMed]
- van Besouw, N.M.; van der Mast, B.J.; de Kuiper, P.; Smak Gregoor, P.J.; Vaessen, L.M.; IJzermans, J.N.; van Gelder, T.; Weimar, W. Donor-specific T-cell reactivity identifies kidney transplant patients in whom immunosuppressive therapy can be safely reduced. Transplantation 2000, 70, 136–143. [Google Scholar]
- Pagano, D.; Badami, E.; Conaldi, P.G.; Seidita, A.; Tuzzolino, F.; Barbàra, M.; di Francesco, F.; Tropea, A.; Liotta, R.; Chiarello, G.; et al. Liver Perfusate Natural Killer Cells from Deceased Brain Donors and Association with Acute Cellular Rejection after Liver Transplantation: A Time-to-Rejection Analysis. Transplantation 2019, 103, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Badami, E. NK-Mediated Immunotherapy and Uses Thereof. U.S. Patent 20190322985, 24 October 2019. [Google Scholar]
- Carreca, A.P.; Gaetani, M.; Busà, R.; Francipane, M.G.; Gulotta, M.R.; Perricone, U.; Iannolo, G.; Russelli, G.; Carcione, C.; Conaldi, P.G.; et al. Galectin-9 and Interferon-Gamma Are Released by Natural Killer Cells upon Activation with Interferon-Alpha and Orchestrate the Suppression of Hepatitis C Virus Infection. Viruses 2022, 14, 1538. [Google Scholar] [CrossRef]
- Bonsignore, P.; Pagano, D.; Piazza, S.; Ricotta, C.; di Francesco, F.; Cintorino, D.; Li Petri, S.; Canzonieri, M.; Tropea, A.; Calamia, S.; et al. Crucial Role of Extended Criteria Donors in Deceased Donor Single Kidney Transplantation to Face Chronic Shortage in the Heart of the Mediterranean Basin: A Single-Center Experience. Transplant. Proc. 2019, 51, 2868–2872. [Google Scholar] [CrossRef]
- Angelico, M.; Nardi, A.; Romagnoli, R.; Marianelli, T.; Corradini, S.G.; Tandoi, F.; Gavrila, C.; Salizzoni, M.; Pinna, A.D.; Cillo, U.; et al. A Bayesian methodology to improve prediction of early graft loss after liver transplantation derived from the liver match study. Dig. Liver Dis. 2014, 46, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Logan, A.; Schenk, A.; Bumgardner, G.; Brock, G.; El-Hinnawi, A.; Rajab, A.; Washburn, K. Machine perfusion of kidney allografts affects early but not late graft function. Am. J. Surg. 2022, 223, 804–811. [Google Scholar] [CrossRef]
- Rhee, E.P. Kidney-specific metabolomic profiling in machine perfusate. Kidney Int. 2023, 103, 661–663. [Google Scholar] [CrossRef]
- Bellini, M.I.; Bonaccorsi Riani, E.; Giorgakis, E.; Kaisar, M.E.; Patrono, D.; Weissenbacher, A. Organ Reconditioning and Machine Perfusion in Transplantation. Transpl. Int. 2023, 36, 11100. [Google Scholar] [CrossRef]
- Malinoski, D.; Saunders, C.; Swain, S.; Groat, T.; Wood, P.R.; Reese, J.; Nelson, R.; Prinz, J.; Kishish, K.; Van De Walker, C.; et al. Hypothermia or Machine Perfusion in Kidney Donors. N. Engl. J. Med. 2023, 388, 418–426. [Google Scholar] [CrossRef]
- Schlegel, A.; Mueller, M.; Muller, X.; Eden, J.; Panconesi, R.; von Felten, S.; Steigmiller, K.; Sousa Da Silva, R.X.; de Rougemont, O.; Mabrut, J.Y.; et al. A multicenter randomized-controlled trial of hypothermic oxygenated perfusion (HOPE) for human liver grafts before transplantation. J. Hepatol. 2023, 78, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Mazilescu, L.I.; Urbanellis, P.; Kim, S.J.; Goto, T.; Noguchi, Y.; Konvalinka, A.; Reichman, T.W.; Sayed, B.A.; Mucsi, I.; Lee, J.Y.; et al. Normothermic Ex Vivo Kidney Perfusion for Human Kidney Transplantation: First North American Results. Transplantation 2022, 106, 1852–1859. [Google Scholar] [CrossRef] [PubMed]
- Mawad, H.; Pinard, L.; Medani, S.; Chagnon, M.; Boucquemont, J.; Turgeon, J.; Dieudé, M.; Hamelin, K.; Rimbaud, A.K.; Belayachi, A.; et al. Hypothermic Perfusion Modifies the Association Between Anti-LG3 Antibodies and Delayed Graft Function in Kidney Recipients. Transpl. Int. 2023, 36, 10749. [Google Scholar] [CrossRef]
- Liu, R.X.; Koyawala, N.; Thiessen-Philbrook, H.R.; Doshi, M.D.; Reese, P.P.; Hall, I.E.; Mohan, S.; Parikh, C.R. Untargeted metabolomics of perfusate and their association with hypothermic machine perfusion and allograft failure. Kidney Int. 2023, 103, 762–771. [Google Scholar] [CrossRef] [PubMed]
- Peris, A.; Fulceri, G.E.; Lazzeri, C.; Bonizzoli, M.; Li Marzi, V.; Serni, S.; Cirami, L.; Migliaccio, M.L. Delayed graft function and perfusion parameters of kidneys from uncontrolled donors after circulatory death. Perfusion 2021, 36, 299–304. [Google Scholar] [CrossRef]
- Stringer, D.; Gardner, L.; Shaw, O.; Clarke, B.; Briggs, D.; Worthington, J.; Buckland, M.; Danzi, G.; Hilton, R.; Picton, M.; et al. Optimized immunosuppression to prevent graft failure in renal transplant recipients with HLA antibodies (OuTSMART): A randomised controlled trial. eClinicalMedicine 2023, 56, 101819. [Google Scholar] [CrossRef]
- Brulé, N.; Canet, E.; Péré, M.; Feuillet, F.; Hourmant, M.; Asehnoune, K.; Rozec, B.; Duveau, A.; Dube, L.; Pierrot, M.; et al. Impact of targeted hypothermia in expanded-criteria organ donors on recipient kidney-graft function: Study protocol for a multicentre randomised controlled trial (HYPOREME). BMJ Open 2022, 12, e052845. [Google Scholar] [CrossRef]
- Morath, C.; Schmitt, A.; Schmitt, M.; Wang, L.; Kleist, C.; Opelz, G.; Süsal, C.; Tran, T.H.; Scherer, S.; Schwenger, V.; et al. Individualised immunosuppression with intravenously administered donor-derived modified immune cells compared with standard of care in living donor kidney transplantation (TOL-2 Study): Protocol for a multicentre, open-label, phase II, randomised controlled trial. BMJ Open 2022, 12, e066128. [Google Scholar] [CrossRef] [PubMed]
- Pietrosi, G.; Vizzini, G.B.; Gruttadauria, S.; Gridelli, B. Clinical applications of hepatocyte transplantation. World J. Gastroenterol. 2009, 15, 2074–2077. [Google Scholar] [CrossRef]
- Gruttadauria, S.; di Francesco, F.; Vizzini, G.B.; Luca, A.; Spada, M.; Cintorino, D.; Li Petri, S.; Pietrosi, G.; Pagano, D.; Gridelli, B. Early graft dysfunction following adult-to-adult living-related liver transplantation: Predictive factors and outcomes. World J. Gastroenterol. 2009, 15, 4556–4560. [Google Scholar] [CrossRef]
| Donors’ Characteristics (n = 42) | |
|---|---|
| Gender, male (%) | 26 (62) |
| Age (years), ±dev. standard | 58.07 ± 14.2 |
| Hypertension (%) | 2 (5) |
| Body mass index (Kg/m2), ±dev. standard | 25.2 ± 3.9 |
| Type II diabetes mellitus, n (%) | 0 (0) |
| Creatininaemia (mg/dL), ±standard deviation | 0.9 ± 0.8 |
| Cause of death | |
| Cerebro-vascular, n (%) | 21 (50) |
| Trauma, n (%) | 11 (26) |
| Hypoxia, n (%) | 8 (19) |
| Others, n (%) | 2 (5) |
| Infectious risk | |
| Bacteremia, n (%) | 2 (5) |
| HBcAb+, n (%) | 1 (2) |
| HBsAg+, n (%) | 0 (0) |
| Hepatitis C virus infection, n (%) | 1 (2) |
| HCV and HBcAb + infection, n (%) | 0 (0) |
| Cancer risk, n (%) | 2 (5) |
| Recipients’ Characteristics (n = 42) | |
| Gender, male (%) | 27 (59) |
| Age (years), ±standard dev. | 55.06 ± 11.3 |
| Body mass index (Kg/m2), ±dev. std. | 26.6 ± 3.1 |
| Previous kidney transplant, n (%) | 7 (15.2) |
| Etiology Chronic Renal Failure | |
| Polycystic kidney | 14 (30.4) |
| Unknown etiology | 18 (39.1) |
| IgA deposits | 3 (6.5) |
| Other | 11 (24) |
| CMV IgG, n (%) | 40 (87) |
| Primary CMV infection, n (%) | 5 (10.8) |
| CMV infection, n (%) | 14 (30.4) |
| New onset diabetes mellitus, n (%) | 9 (19.5) |
| Induction | |
| Thymoglobulins, n (%) | 17 (37) |
| Basiliximab, n (%) | 29 (63) |
| HLA mismatch | |
| I level (0–1), n | 5 |
| II level (2–4), n | 41 |
| III level (5–6), n | 0 |
| Acute rejection, n, (%) | 10 (21.7) |
| Graft loss within one year, n, (%) | 11 (24) |
| Delayed graft function, n (%) | 23 (50) |
| Mortality at one year, n (%) | 3 (6.5) |
| Variable | Mean Diff (1–2) | LowerCLMean | UpperCLMean | StdDev | p-Value |
|---|---|---|---|---|---|
| HEIGHT (cm) | 0.3883 | −5.0206 | 5.7973 | 9.4085 | 0.8858 |
| WEIGHT (kg) | 4.5683 | −3.0132 | 12.1498 | 13.1875 | 0.2315 |
| AGE (years) | −2.3267 | −13.6716 | 9.0183 | 19.7337 | 0.6818 |
| BMI (kg/m2) | 1.3088 | −0.5287 | 3.1464 | 3.1962 | 0.1585 |
| ICU STAY (day) | 0.4673 | −1.2128 | 2.1474 | 2.8535 | 0.5781 |
| Na+ | −5.4692 | −12.8349 | 1.8965 | 12.533 | 0.1418 |
| AST | 10.1583 | −17.9589 | 38.2755 | 48.3464 | 0.4708 |
| ALT | 8 | −21.3838 | 37.3838 | 49.0093 | 0.5838 |
| TOT BILIRUBIN | −0.0201 | −0.5702 | 0.5301 | 0.9615 | 0.9416 |
| GGT | −3.0227 | −53.5032 | 47.4578 | 80.8431 | 0.9043 |
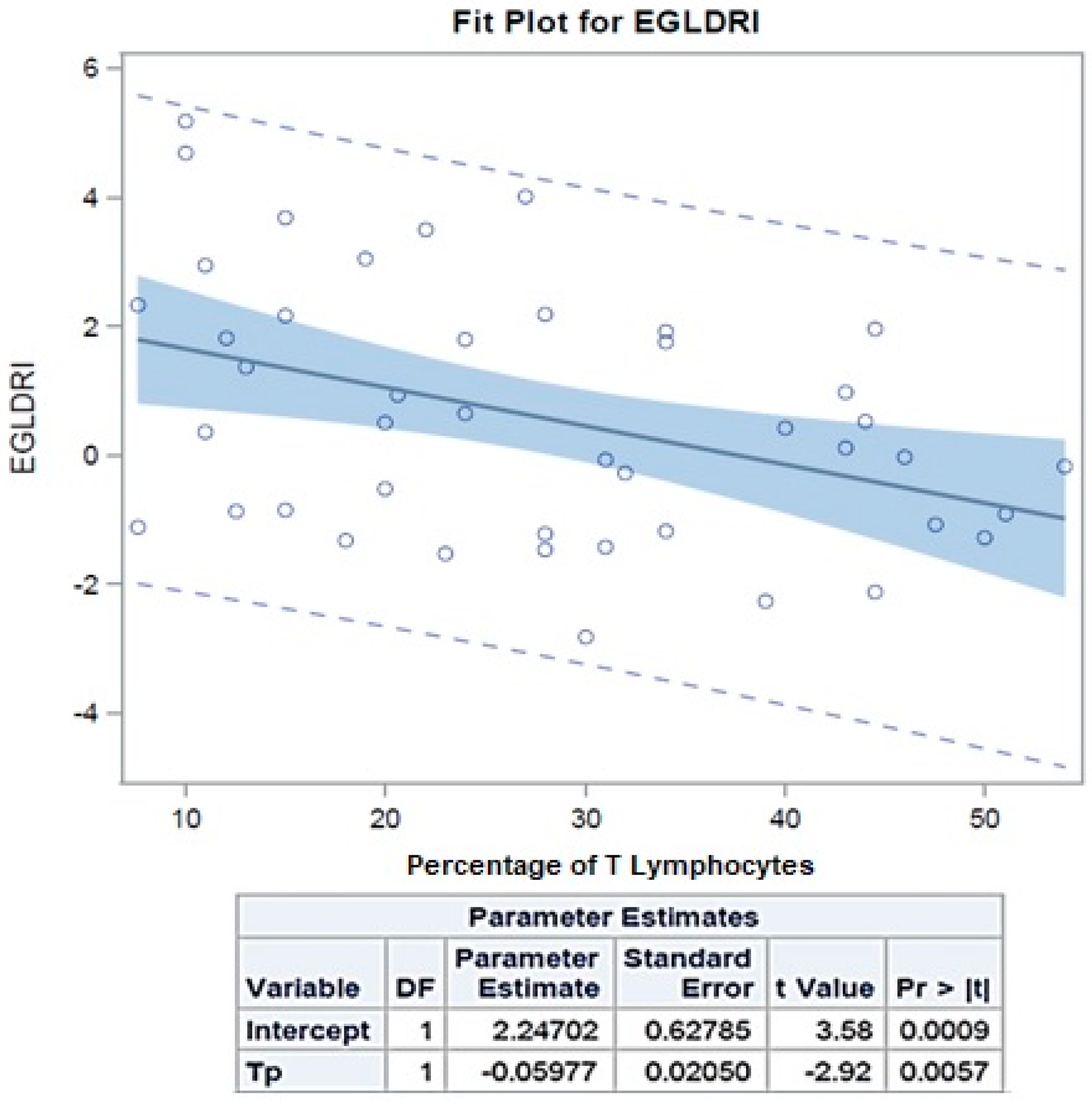
| EGL-DRI | 1.4751 | 0.3572 | 2.593 | 1.8353 | 0.0109 |
| C.I.T. (min) | 78.3437 | 6.6644 | 150 | 117.7 | 0.0329 |
| CD3CD4 | −10.6601 | −25.758 | 4.4378 | 17.7455 | 0.1580 |
| CD3CD8 | 8.232 | −5.3453 | 21.8093 | 15.9582 | 0.2229 |
| CD56CD3NKG2D | −11.7829 | −48.5 | 24.9343 | 33.4039 | 0.5075 |
| CD56CD3NKp30 | −25.2182 | −47.5861 | −2.8502 | 19.3359 | 0.0298 |
| CD56CD3NKp44 | −3.15 | −16.9595 | 10.6595 | 15.9714 | 0.6219 |
| CD56CD3NKp46 | 4.6236 | −29.7991 | 39.0464 | 29.7566 | 0.7775 |
| MAIT (%) | −0.7961 | −5.5382 | 3.946 | 4.6266 | 0.7266 |
| NKT (%) | −3.6128 | −9.9881 | 2.7624 | 11.0892 | 0.2600 |
| NK/NKT | 1.3599 | −0.6443 | 3.3641 | 3.4861 | 0.1788 |
| NK (%) | 8.9885 | 1.2376 | 16.7394 | 13.482 | 0.0240 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagano, D.; Badami, E.; Zito, G.; Conaldi, P.G.; Vella, I.; Buscemi, B.; Amico, G.; Busà, R.; Salis, P.; Li Petri, S.; et al. Impact of T Lymphocytes Isolated from Liver Perfusate of Deceased Brain Donors on Kidney Transplantation: Preliminary Evidence and Future Directions. J. Clin. Med. 2023, 12, 4786. https://doi.org/10.3390/jcm12144786
Pagano D, Badami E, Zito G, Conaldi PG, Vella I, Buscemi B, Amico G, Busà R, Salis P, Li Petri S, et al. Impact of T Lymphocytes Isolated from Liver Perfusate of Deceased Brain Donors on Kidney Transplantation: Preliminary Evidence and Future Directions. Journal of Clinical Medicine. 2023; 12(14):4786. https://doi.org/10.3390/jcm12144786
Chicago/Turabian StylePagano, Duilio, Ester Badami, Giovanni Zito, Pier Giulio Conaldi, Ivan Vella, Barbara Buscemi, Giandomenico Amico, Rosalia Busà, Paola Salis, Sergio Li Petri, and et al. 2023. "Impact of T Lymphocytes Isolated from Liver Perfusate of Deceased Brain Donors on Kidney Transplantation: Preliminary Evidence and Future Directions" Journal of Clinical Medicine 12, no. 14: 4786. https://doi.org/10.3390/jcm12144786
APA StylePagano, D., Badami, E., Zito, G., Conaldi, P. G., Vella, I., Buscemi, B., Amico, G., Busà, R., Salis, P., Li Petri, S., di Francesco, F., Calamia, S., Bonsignore, P., Tropea, A., Accardo, C., Piazza, S., & Gruttadauria, S. (2023). Impact of T Lymphocytes Isolated from Liver Perfusate of Deceased Brain Donors on Kidney Transplantation: Preliminary Evidence and Future Directions. Journal of Clinical Medicine, 12(14), 4786. https://doi.org/10.3390/jcm12144786










