1. Introduction
The persistent shortage of donor kidneys has led to increased utility of kidneys from expanded criteria donors (ECD). Despite an elevated risk of delayed graft function (DGF) and a 70% increased risk of graft failure compared to recipients of standard criteria donor (SCD) kidneys [
1,
2], recipients of the ECD kidneys have an increased survival rate as compared to being on dialysis. ECD-grafts translate into poorer long-term graft and patient survival rates as compared to SCD grafts, with 5-year graft survival of 49–80% according to a recent overview, although this varies greatly between centers [
3,
4]. These grafts are also more susceptible to ischemic-reperfusion. Therefore, limiting injury is important Static cold storage (SCS) was developed during the early years of transplantation, and is the gold standard for organ preservation [
5,
6]. The University of Wisconsin (UW) solution was later introduced and proven to be effective due to its cell impermeant agents that prevent the cells from swelling during cold ischemic storage. Even though 20–30% of kidneys do not function immediately after transplantation, organ preservation has been mainly unchanged until the introduction of hypothermic machine perfusion (HMP), developed as an improvement to SCS. During HMP, a pump is connected to the major vessels of the graft, perfusing it with a protective solution. HMP is standard at many centers for ECD kidneys owing to its well-established advantage over SCS, as a landmark randomized controlled trial first suggested in 2009 [
7]. In this study, HMP significantly decreased the risk of DGF and increased 1- and 3-year graft survival in deceased donor kidneys compared with SCS [
8,
9]. The graft survival benefit was even more pronounced in ECD kidneys (3-year 86% for HMP vs. 76% for SCS) [
7,
9]. These results have since been validated to varying degrees by multiple studies, most consistently in terms of reducing DGF-rates [
4,
10]. However, there is a paucity of sound evidence on the effect of HMP on long-term graft survival and function [
10].
During SCS and HMP alike the kidneys sustain cold ischemic injury, and increased cold ischemia time (CIT) is an established risk factor for developing DGF and decreased graft survival [
11]. Therefore, a current challenge for transplant centers is to limit the CIT. HMP has also been shown to be most effective for kidneys developing DGF, and research is aimed at identifying these kidneys at risk for DGF prior to transplantation [
8]. Donations after cardiac death (DCD) are in many regions the fastest growing donor pool, and increasingly a subject of research trying to alleviate their high risk of DGF. Lately, promising research has been published investigating alterations to the preservation technique, limiting the CIT by either oxygenating the perfusate or perfusing the kidney at normothermic temperatures [
12,
13]. Whether these methods are effective in ECD-kidney transplantation has not yet been thoroughly investigated.
Death-censored graft survival rates in Sweden are reportedly on the higher end, consistently > 90% at 5 years for kidneys from deceased donors [
14,
15]. HMP has been used at Sahlgrenska University Hospital (SU) since 2014 for ECD kidneys, but no study from our center has been conducted to date to evaluate the results. There are also limited reports on HMP efficacy in populations with relatively high graft survival rates. This exploratory study aimed to investigate whether HMP is better than SCS in preventing DGF and improving graft survival in ECD kidney transplantation performed at SU.
4. Discussion
HMP has consistently yielded better short-term results than SCS in ECD kidney transplantation, but the magnitude varies widely depending on the transplant center. However, this short-term benefit does not seem to unanimously translate into better graft function in the long term; in fact, most evidence suggests that it does not [
10]. There are also limited studies comparing the two preservation methods in transplant populations with relatively high rates of graft survival, such as in Sweden [
14]. Our matched retrospective study revealed a relatively low DGF-rate in both groups: 9.2% in HMP and 16.1% in SCS (
Table 4). Although this difference was not statistically significant in the primary analysis (
p = 0.063), it suggested a trend for DGF-rate reduction when using HMP. This result was strengthened by the significant benefit observed in both sensitivity analyses, suggesting that HMP may be effective in reducing DGF-rates even when the overall DGF-rate is low. With that said, it is not evident that HMP makes sense from an economic standpoint, due to the already low DGF-rates, and therefore, relatively small effect size. A follow-up study including more participants could bring more clarity. Also, cost-effectiveness and harm-benefit analyses would be of interest to further navigate the future use of HMP in ECD kidney transplantation at our center.
The trend found with HMP on DGF-rate in our study is similar to those found in studies from other centers [
8,
10,
24]. The risk reduction for DGF of 55% with HMP (adjusted OR = 0.45,
p = 0.012) using logistic regression adjusted for confounders in the entire cohort, as well as the logistic regression adjusted for donor age in the marginal-matched cohort (adjusted OR = 0.53,
p = 0.045) is comparable to the adjusted OR of 0.46 reported by Treckmann et al. in their RCT in 2011 [
8] and the OR of 0.485, from another matched study on donation after brain death (DBD)-kidneys by Gasteiger et al. in 2020 [
25]. This comparability is interesting because the DGF-rates found in our study are at the lower end of the spectrum: 16.1% for the SCS group and 9.2% for the HMP group, compared to 29.7% and 22% reported by Treckmann et al. with similar CIT and donor age. Another Swedish study by Sedigh et al. reported a 20.3% DGF-rate in the SCS group and 16.7% in the HMP group in ECD-kidneys at a slightly shorter CIT [
24]. Our DGF-rate in SCS kidneys was low compared to these studies. Interestingly, our results differ from an earlier single-center study at our center, which reported a DGF-rate of 24.1% in DBD kidneys between 2007 and 2009, of which approximately 60% were ECD [
26]. The reason for the higher DGF-rates in this previous study compared to ours is not clear.
This study was not designed and did not reveal the reason for the relatively low incidence of DGF in our groups. The definition of DGF may have had an impact. DGF is mostly defined as the need for dialysis in the first week post-transplantation. Dialysis within the first 24 h post-transplantation was excluded according to the DGF definition used in our study. It is probable that using the more common definition would result in higher DGF-rates and better comparisons with other studies. In a single-center UK-based study investigating the effect of different DGF-definitions on the outcomes, DGF-rates using our definition reached 32.4%, compared to 40.0% when using the more traditional definition in a DBD cohort [
27]. Therefore, we expect our DGF-rates to be underestimated only marginally and the overall DGF-rates to remain relatively low. If the different treatment arms were affected differently, there is a possibility that this may have affected the outcome. Unfortunately, there are no studies to our knowledge that have investigated this.
Our marginal-matching procedure yielded an SCS group that was 2.5 years older (mean) and had 1 h longer CIT (mean and median) than the unmatched cohort. Matching also resulted in a more comparable KDPI between the groups, which was favorable in our graft survival analyses. Donor age and CIT are important risk factors for DGF, and we assumed increasing these would increase the DGF-rates [
28]. Surprisingly, this was not the case; instead, the DGF-rate decreased slightly from 17.3% to 16.1% after matching. We used only donor age, CIT, and graft number as matching variables, and although they are important, there is a possibility that other risk factors were unevenly excluded in our matching procedure, causing this small decrease in the DGF-rate, yielding the main analysis insignificant. However, there was no obvious skewness comparing baseline characteristics before and after matching (
Table 2 and
Table 3) as SMD was consistently very low and no difference between the groups reached significance, except for donor terminal creatinine and eGFR. Although our collection of risk factors was extensive, the possibility remains that unmeasured variables were significantly different between the groups, influencing the risk of DGF. Nevertheless, because of the similarities between the groups after matching and the similar efficacy of DGF reduction in the primary and sensitivity analyses, we are confident in our matching and the results.
Our post-hoc interaction analysis, investigating whether the effect of HMP on DGF varies with donor age, did not show a statistically significant interaction (
p = 0.36) or better effect in any age group, although there was an indication of a better effect for donors aged <70 years (OR 0.43) than for those aged ≥70 (OR 0.80). However, because of the relatively few cases of DGF and participants in these groups, these analyses have low power. This is also true in the survival analyses (
Figure 4a,b). Hence, studies with a greater number of participants are needed to draw any firm conclusions on whether HMP is more effective in kidneys donated by younger or older donors.
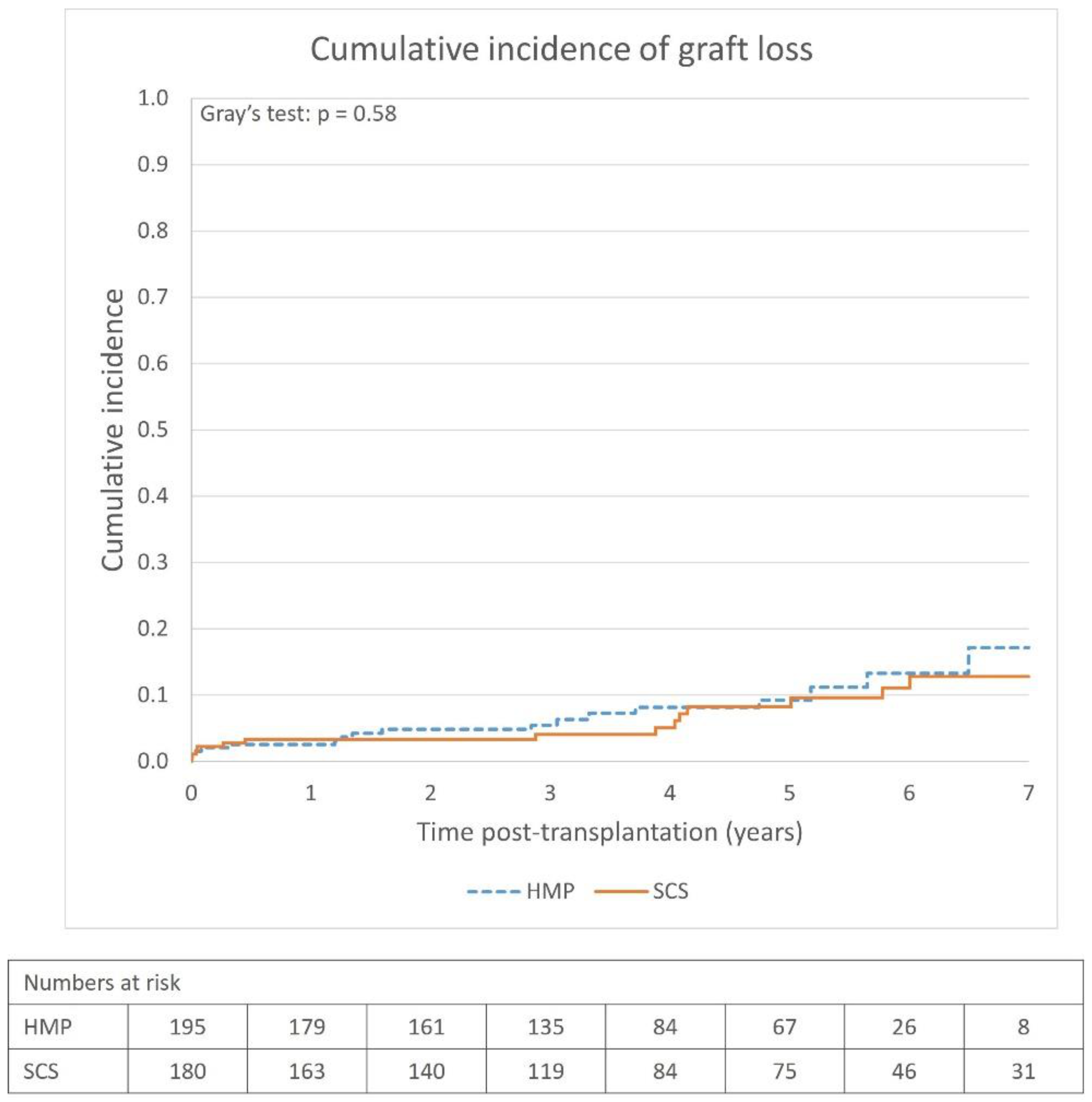
The overall graft survival rates were high (>90% at 5 years) in both the SCS and HMP groups, and there was no apparent survival benefit using HMP (
Figure 2). The most remarkable finding was the relatively high graft survival rate, especially in the SCS group (91.7% at 5-years). This is comparable to the results presented by other studies on Swedish populations. The SNR in their 2022 annual report reported similar survival rates: 93.3% at 5 years for kidneys from deceased donors [
14]. Another study of kidney transplantation in Sweden between 2005 and 2013, regardless of the donor type, reported 1-year and 5-year death-censored graft survival rates of 97.9% and 94.2%, respectively [
15], which are consistent with our results. This is relatively high compared to international studies in the last decade. For SCS, studies have reported survival rates of 70–97% at 1-year, 80–85% at 3 years, and approximately 80% at 5 years [
10,
29,
30]. The previously mentioned international landmark RCT performed in Western European countries within Eurotransplant reported 1- and 3-year graft survival rates of 80% and 76%, respectively, in their SCS subgroup for ECD kidneys and a statistically significant survival benefit using HMP [
8,
9]. In contrast, a large French study found no graft survival benefit using HMP at 5 years [
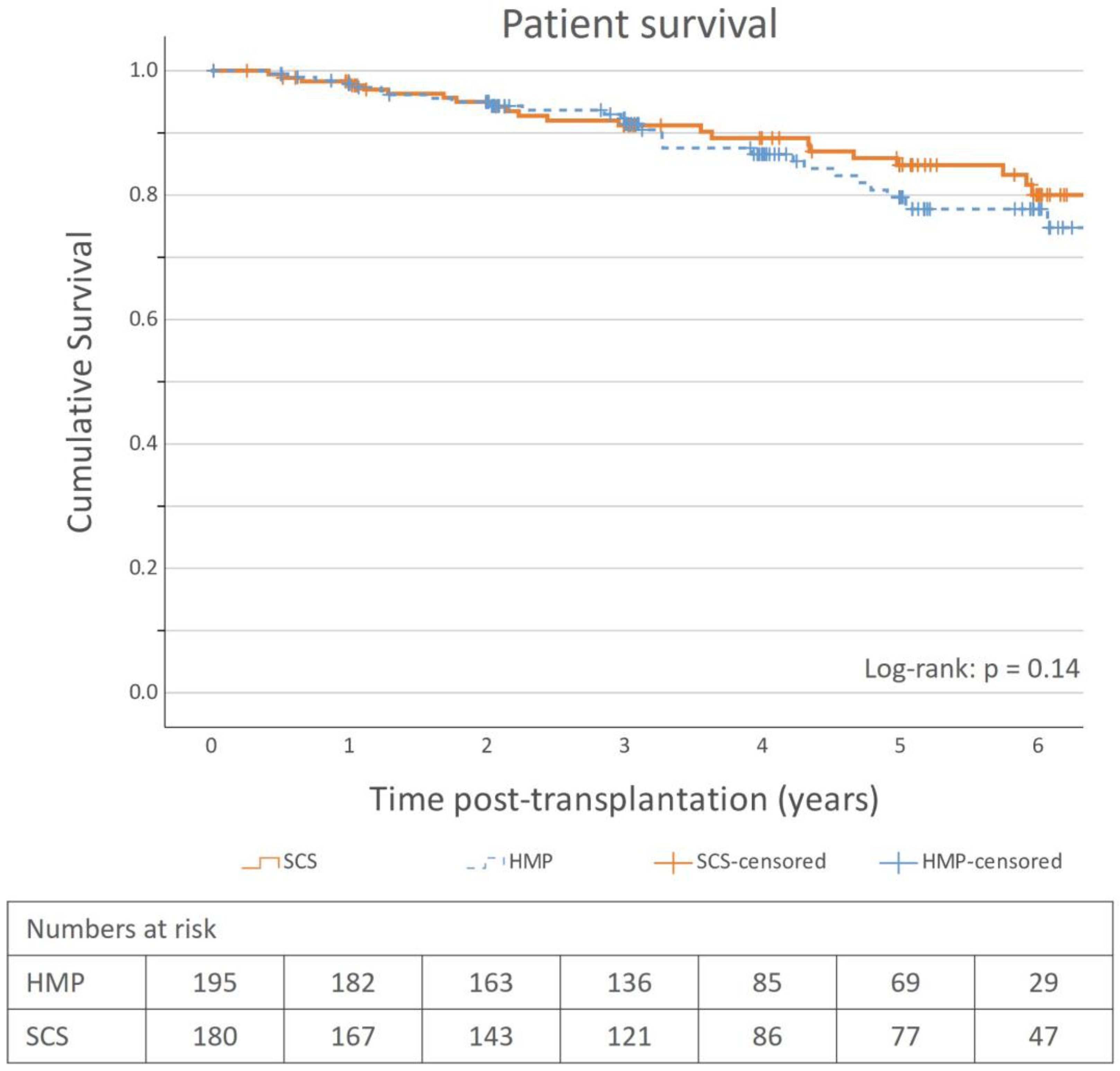
31]. There are limited data on long-term graft survival comparing HMP to SCS, and evidence for the benefit of using HMP is weak, which is also corroborated by our study. The cumulative incidence of graft loss, and subsequently the graft survival rates, was comparable between the groups, and no conclusion can be made regarding whether HMP affects graft survival with the dataset used in the study. This is also the case for patient survival, as no significant patient survival benefit was observed.
The reason for the good outcomes observed in Sweden is relatively unknown. Donor-related factors do not seem to explain it, indeed the KDPI was relatively high. It is therefore likely that several other factors, such as recipient-related factors, universal and equitable health coverage, organ allocation system, access to transplantation and immunosuppressive medications, good follow-up regimens as well as national health quality registries in Sweden that provide a unique opportunity to monitor quality and results may contribute. Unfortunately, this is mere speculation; to our knowledge, no studies have been conducted comparing these processes to other countries.
The post-transplantation graft function was similar between the groups. There was a trend, although very modest, of initially better graft function in the HMP group, although it only reached significance at 1 month (
Table 4). This could be due to less damage from the transplantation process and, therefore, quicker return of kidney function using HMP. However, since the difference was very small it should be interpreted with caution. Also, no long-term benefit was observed on kidney function, in accordance with current knowledge.
This study had a few limitations. The most obvious is the retrospective and non-randomized nature of the study, and although we performed a matching of good quality, the results can never reach the strength of an RCT. There are also several potential biases. As previously discussed, our DGF-definition, excluding recipients receiving dialysis the first 24 h post-transplantation may complicate comparisons to other studies. The sample size was also small to confidently reveal long-term differences between the groups, especially in the stratified analyses. The inclusion of SCS-kidneys in 2013 may have induced a time-period bias, although this is expected to be limited. The selection of the preservation method was not randomized, and although every ECD-kidney should receive HMP, this was not the case in 186 kidneys between 2014 and 2021. Whether this was due to logistical, donor, or recipient factors, there is potential for selection bias, although we tried to mitigate this to some extent in our matching procedures. Matching may cause clustering or dispersion of confounding factors not included in the matching model; however, we did not find any obvious disproportions of important variables due to matching and the robustness of the matching was also evaluated using our sensitivity analyses, suggesting adequate matching parameters. The results of this study are from a single center in Sweden and cannot be generalized to other populations.
The strengths of the study include an extensive and well-characterized cohort, with a low number of dropouts, and the use of the matching procedure that generated comparable groups of important risk factors for both DGF and graft survival, thus strengthening the results. The inclusion of more baseline characteristics in a multivariable analysis including all the participants was also performed to gauge their effect on the endpoints and support the results. We also used the cumulative incidence function for a more correct and accurate graft survival analysis [
22]. This being a single-center study, the comparability between the groups may be better, as it is likely that kidney transplantation and preservation procedures, DGF diagnostics, and immunosuppressive therapy are very similar.