The Effectiveness of Three-Dimensional Osteosynthesis Plates versus Conventional Plates for the Treatment of Skeletal Fractures: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Protocol Development
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
- Type of Patients or population: adult patients with skeletal fractures requiring surgical fixation treatment.
- Type of intervention: fixation with preformed osteosynthesis plates (including PSIs).
- Comparison of the control group: fixation with conventional plates.
- Primary outcome: anatomical reduction.
- Secondary outcomes: stability and complications, days spent in the hospital, operation time, and patient satisfaction.
- Study design: randomised clinical trials (RCTs), prospective controlled clinical trials (CCTs), and prospective and retrospective cohort studies.
- Study language: there were no language restrictions.
- Studies with fewer than 10 patients;
- Use of biodegradable systems (only materials such as titanium or RVS were included);
- Pathological fractures;
- Case reports, case series, experts’ opinions, conference abstracts, letters to the editor, animal studies, reviews, and systematic reviews.
2.4. Screening Methods
2.5. Data Extraction
2.6. Evaluation of Study Quality and Risk of Bias
2.7. Statistical Analysis
3. Results
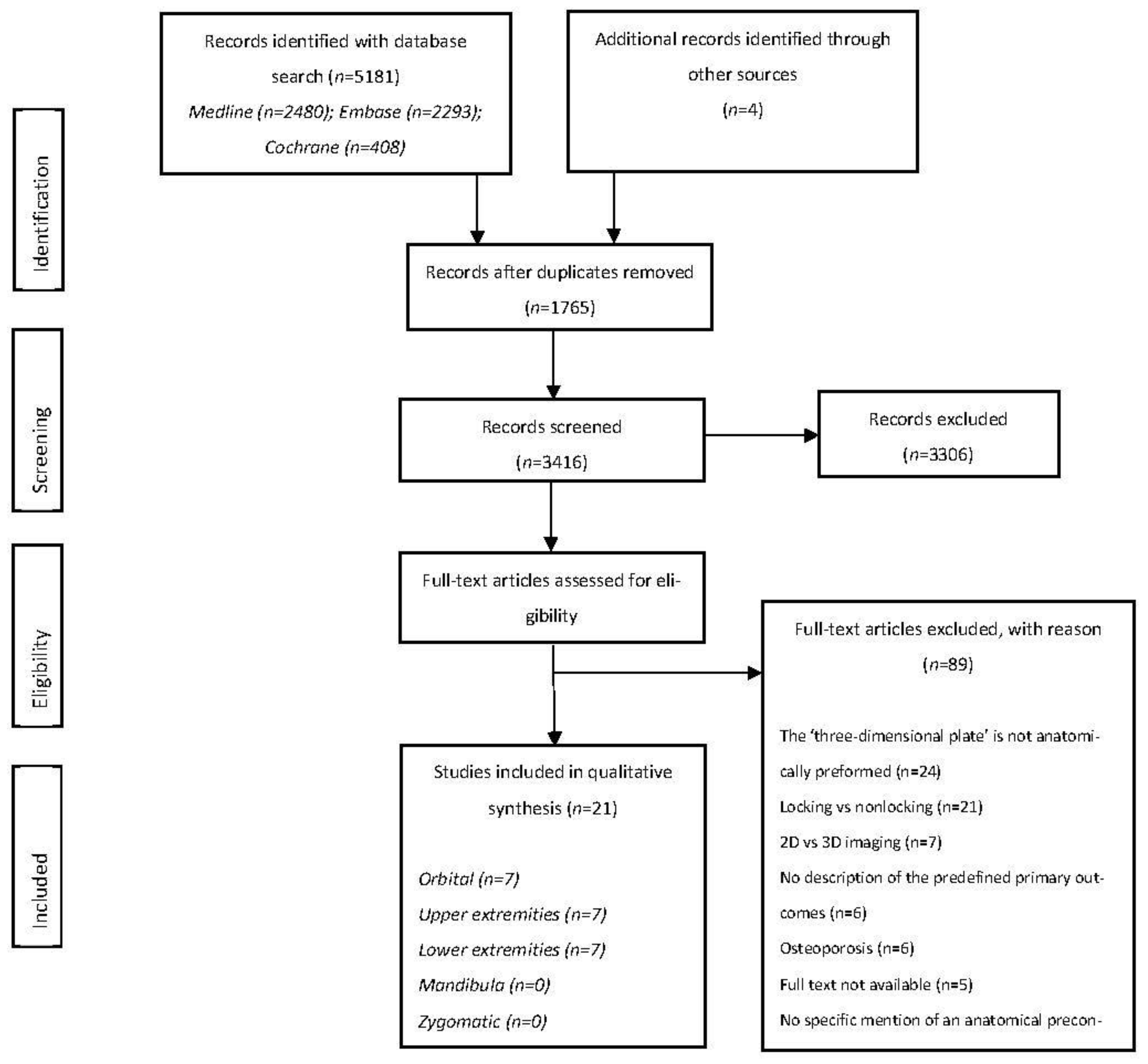
3.1. Study Selection
3.2. Study Characteristics
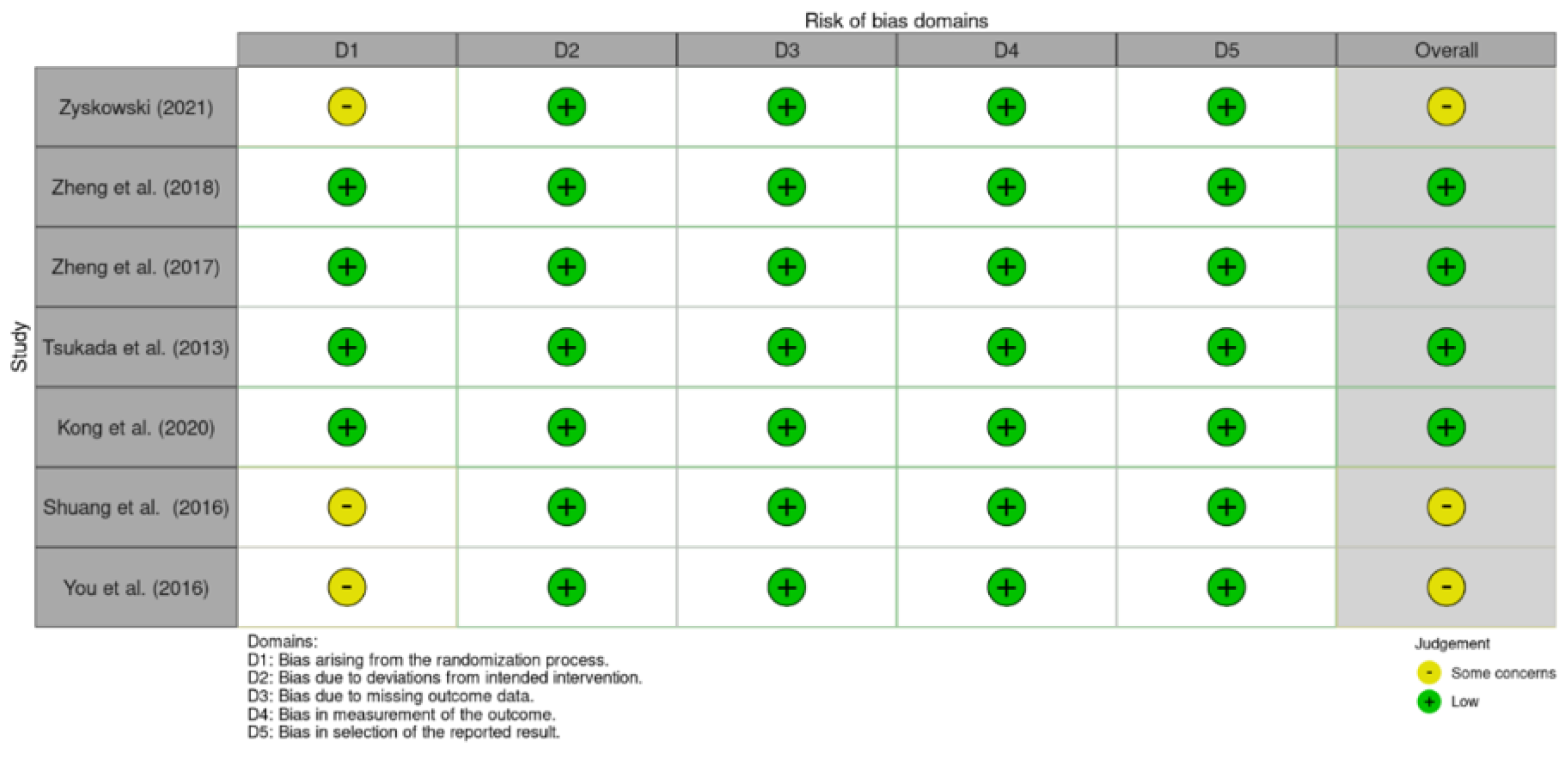
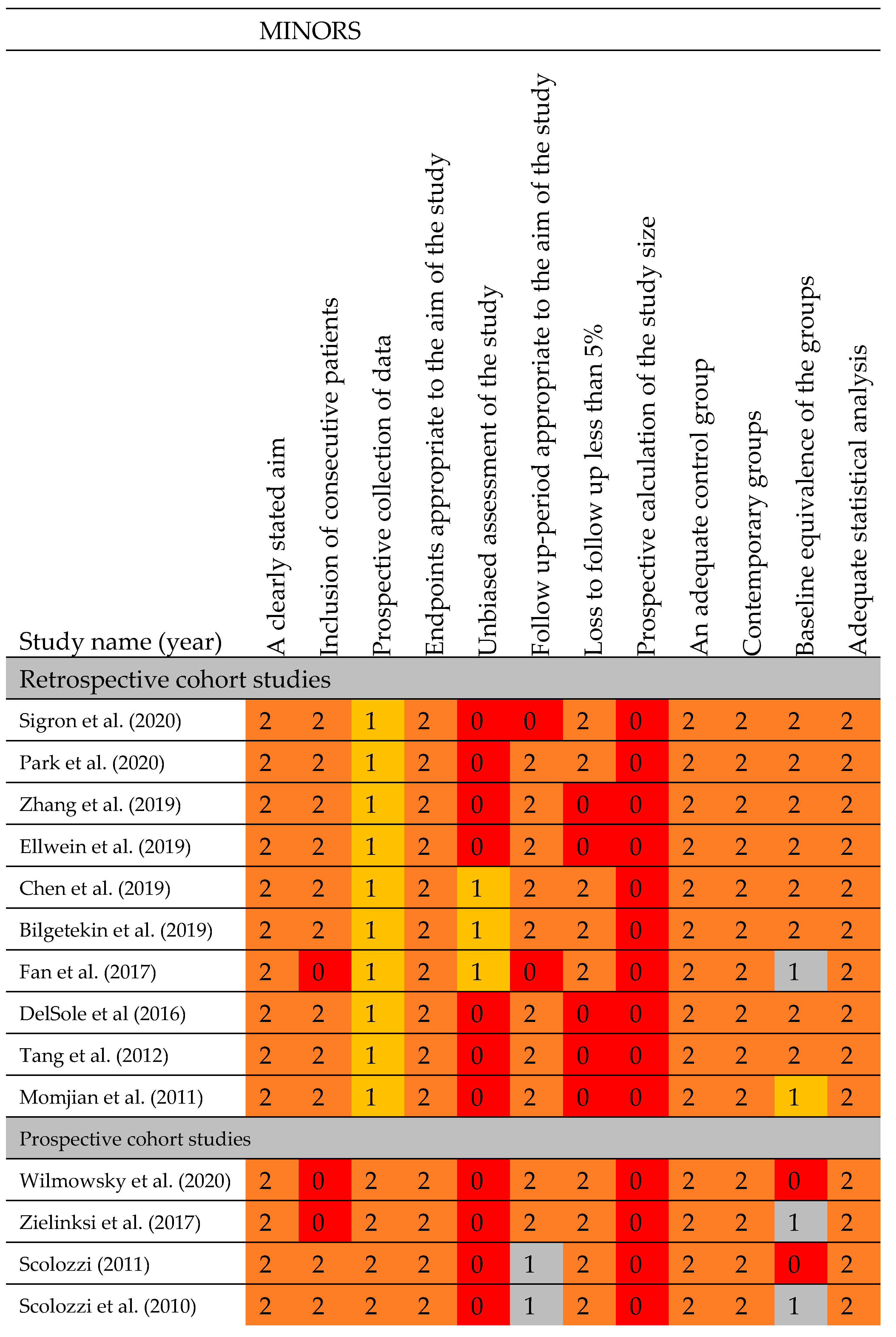
3.3. Assessment of Methodological Quality
3.4. Outcome Measures
3.4.1. Orbital Fractures
3.4.2. Upper Limb Fractures
| Parameter I: Stability and Reduction | Bone Union (N/Total Population) | Loss of Reduction (N/Total Population) | Time to Bone Union (Mean Weeks ± SD, Total Population) | Stability (N/Total Population) |
|---|---|---|---|---|
| Upper Limb Fractures | ||||
| Kong (2020) [49] | 2D: 1/16; 6.25% 3D: 1/16; 6.25% | |||
| Ellwein (2019) [43] | 2D: 18/18; 100% 3D: 10/10; 100% | |||
| Chen (2019) [48] | 1 | 2 | ||
| DelSole (2016) [47] | 2D: 14/14; 100% 3D: 8/8; 100% | |||
| You (2016) [45] | 2D: 32/32; 100% 3D: 34/34; 100% | 2D: 8.50 ± 1.22 (32) 3D: 8.36 ± 1.00 (34) | ||
| Tang (2012) [44] | 2D: 0/17; 0% 3D: 0/16; 0% | 2D: 11.76 (range: 9–19) (17) 3D: 12.93 (range: 10–17) (16) | ||
3.4.3. Lower Limb Fractures
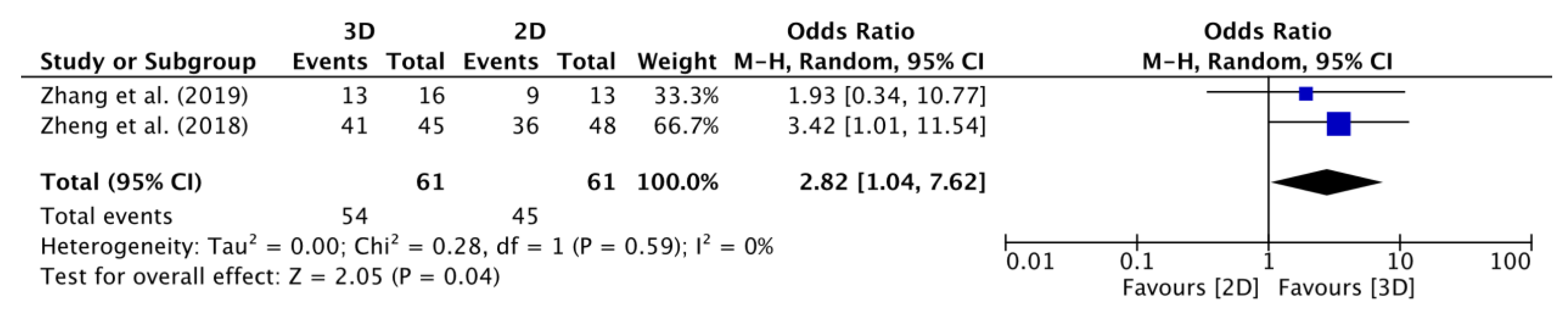
3.5. Meta-Analysis
3.5.1. Primary Outcome: Adequate Anatomical Reduction
3.5.2. Secondary Outcome: Operation Time
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jay, R.; Lieberman, G.E.F. Bone Regeneration and Repair: Biology and Clinical Applications; Humana Press: Totowa, NJ, USA, 2005; Volume 1, pp. XII, 398. [Google Scholar]
- Oryan, A.; Monazzah, S.; Bigham-Sadegh, A. Bone injury and fracture healing biology. Biomed. Environ. Sci. 2015, 28, 57–71. [Google Scholar] [CrossRef]
- Parmet, S.; Lynm, C.; Glass, R.M. JAMA patient page. Bone fractures. JAMA 2004, 291, 2160. [Google Scholar] [CrossRef]
- Van den Bergh, B.; Karagozoglu, K.H.; Heymans, M.W.; Forouzanfar, T. Aetiology and incidence of maxillofacial trauma in Amsterdam: A retrospective analysis of 579 patients. J. Craniomaxillofac. Surg. 2012, 40, e165–e169. [Google Scholar] [CrossRef]
- Amin, S.; Achenbach, S.J.; Atkinson, E.J.; Khosla, S.; Melton, L.J., 3rd. Trends in fracture incidence: A population-based study over 20 years. J. Bone Miner. Res. 2014, 29, 581–589. [Google Scholar] [CrossRef]
- Joseph Schatzker, M.T. The Rationale of Operative Fracture Care; Sunnybrook Medical Centre: Toronto, ON, Canada, 2013; Volume 3, p. 444. [Google Scholar]
- Richard, B.; Birrer, R.L.K. Field Guide to Fracture Management; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2005; Volume 1, 136p. [Google Scholar]
- Sheen, J.R.; Garla, V.V. Fracture Healing Overview. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- McKinley, T. Principles of Fracture Healing. Surgery 2003, 21, 209–212. [Google Scholar] [CrossRef]
- Tarantino, U.; Cerocchi, I.; Celi, M.; Scialdoni, A.; Saturnino, L.; Gasbarra, E. Pharmacological agents and bone healing. Clin. Cases Miner. Bone Metab. 2009, 6, 144–148. [Google Scholar]
- Herren, D.B.; Nagy, L.; Campbell, D.A. Osteosynthesis in the Hand: Current Concepts; S Karger Ag: Basel, Switzerland, 2008; Volume 1. [Google Scholar]
- Moya, M.; Mayberry, J. Rib Fracture Management: A Practical Manual; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Bryant, T. A Manual for the Practice of Surgery; J. & A. Churchill: London, UK, 1879; Volume 2. [Google Scholar]
- DeYulis, M.; Hinson, J.W. Joint Immobilization. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Horn, C.; Dobele, S.; Vester, H.; Schaffler, A.; Lucke, M.; Stockle, U. Combination of interfragmentary screws and locking plates in distal meta-diaphyseal fractures of the tibia: A retrospective, single-centre pilot study. Injury 2011, 42, 1031–1037. [Google Scholar] [CrossRef]
- Kroon, F.H.M.; van Beek, G.J.; Van Damme, P.A. Traumatologie van het aangezicht. Ned. Tijdschr. Tandheelkd. 2007, 214, 23–33. [Google Scholar]
- O’Brien, C.L.; Menon, M.; Jomha, N.M. Controversies in the management of open fractures. Open Orthop. J. 2014, 8, 178–184. [Google Scholar] [CrossRef]
- Sauerbier, S.; Schon, R.; Otten, J.E.; Schmelzeisen, R.; Gutwald, R. The development of plate osteosynthesis for the treatment of fractures of the mandibular body—A literature review. J. Craniomaxillofac. Surg. 2008, 36, 251–259. [Google Scholar] [CrossRef]
- Bell, R.B.; Kindsfater, C.S. The Use of Biodegradable Plates and Screws to Stabilize Facial Fractures. J. Oral Maxillofac. Surg. 2006, 64, 31–39. [Google Scholar]
- Gareb, B.; van Bakelen, N.B.; Dijkstra, P.U.; Vissink, A.; Bos, R.R.M.; van Minnen, B. Biodegradable versus titanium osteosynthesis in maxillofacial traumatology: A systematic review with meta-analysis and trial sequential analysis. Int. J. Oral Maxillofac. Surg. 2020, 49, 914–931. [Google Scholar] [CrossRef]
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef]
- Parks, E.T.H. Practical Office Orthopedics; Mcgraw-Hill Education: Columbus, OH, USA, 2017; Volume 1, p. 288. [Google Scholar]
- Wang, Y.T.; Chen, C.H.; Wang, P.F.; Lin, C.L. Development of a novel anatomical thin titanium mesh plate with reduction guidance and fixation function for Asian zygomatic-orbitomaxillary complex fracture. J. Craniomaxillofac. Surg. 2018, 46, 547–557. [Google Scholar] [CrossRef]
- Lin, A.S.; Fechter, C.M.; Magill, M.; Wipf, F.; Moore, T.; Guldberg, R.E. The effect of contouring on fatigue resistance of three types of fracture fixation plates. J. Orthop. Surg. Res. 2016, 11, 107. [Google Scholar] [CrossRef]
- Poxleitner, P.; Steybe, D.; Bublitz, B.; Schlager, S.; Fuessinger, M.A.; Voss, P.J.; Schmelzeisen, R.; Cornelius, C.P.; Metzger, M. Analysis of the accuracy of a novel preformed osteosynthesis plate for the reduction and fixation of zygomaticomaxillary complex fractures. J. Craniomaxillofac. Surg. 2019, 47, 951–958. [Google Scholar] [CrossRef]
- Yang, W.F.; Choi, W.S.; Wong, M.C.; Powcharoen, W.; Zhu, W.Y.; Tsoi, J.K.; Chow, M.; Kwok, K.W.; Su, Y.X. Three-Dimensionally Printed Patient-Specific Surgical Plates Increase Accuracy of Oncologic Head and Neck Reconstruction Versus Conventional Surgical Plates: A Comparative Study. Ann. Surg. Oncol. 2020, 28, 363–375. [Google Scholar] [CrossRef]
- Ambellan, F.; Lamecker, H.; von Tycowicz, C.; Zachow, S. Statistical Shape Models: Understanding and Mastering Variation in Anatomy. Adv. Exp. Med. Biol. 2019, 1156, 67–84. [Google Scholar] [CrossRef]
- Vancleef, S.; Herteleer, M.; Carette, Y.; Herijgers, P.; Duflou, J.R.; Nijs, S.; Vander Sloten, J. Why off-the-shelf clavicle plates rarely fit: Anatomic analysis of the clavicle through statistical shape modeling. J. Shoulder Elb. Surg. 2019, 28, 631–638. [Google Scholar] [CrossRef]
- Helmers, R.; Klop, C.; Schreurs, R.; de Lange, J.; Dubois, L. Minimally Invasive Treatment With a Patient Specific Implant in Reconstruction of Isolated Anterior Wall Fracture of the Frontal Sinus. J. Craniofac Surg. 2021, 32, 341–344. [Google Scholar] [CrossRef]
- Du, R.; Su, Y.-X.; Yan, Y.; Choi, W.S.; Yang, W.-F.; Zhang, C.; Chen, X.; Curtin, J.P.; Ouyang, J.; Zhang, B. A Systematic Approach for Making 3D-Printed Patient-Specific Implants for Craniomaxillofacial Reconstruction. Engineering 2020, 6, 1291–1301. [Google Scholar] [CrossRef]
- Crowe, C.S.; Massenburg, B.B.; Morrison, S.D.; Chang, J.; Friedrich, J.B.; Abady, G.G.; Alahdab, F.; Alipour, V.; Arabloo, J.; Asaad, M.; et al. Global trends of hand and wrist trauma: A systematic analysis of fracture and digit amputation using the Global Burden of Disease 2017 Study. Inj. Prev. 2020, 26, i115–i124. [Google Scholar] [CrossRef]
- Xenodemetropoulos, T.; Davison, S.; Ioannidis, G.; Adachi, J.D. The impact of fragility fracture on health-related quality of life: The importance of antifracture therapy. Drugs Aging 2004, 21, 711–730. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Slim, K.; Nini, E.; Forestier, D.; Kwiatkowski, F.; Panis, Y.; Chipponi, J. Methodological index for non-randomized studies (minors): Development and validation of a new instrument. ANZ J. Surg. 2003, 73, 712–716. [Google Scholar] [CrossRef]
- Fan, B.; Chen, H.; Sun, Y.J.; Wang, B.F.; Che, L.; Liu, S.Y.; Li, G.Y. Clinical effects of 3-D printing-assisted personalized reconstructive surgery for blowout orbital fractures. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 2051–2057. [Google Scholar] [CrossRef]
- Scolozzi, P. Reconstruction of severe medial orbital wall fractures using titanium mesh plates placed using transcaruncular-transconjunctival approach: A successful combination of 2 techniques. J. Oral Maxillofac. Surg. 2011, 69, 1415–1420. [Google Scholar] [CrossRef]
- Scolozzi, P.; Momjian, A.; Heuberger, J. Computer-aided volumetric comparison of reconstructed orbits for blow-out fractures with nonpreformed versus 3-dimensionally preformed titanium mesh plates: A preliminary study. J. Comput. Assist. Tomogr. 2010, 34, 98–104. [Google Scholar] [CrossRef]
- Momjian, A.; Heuberger, J.; Scolozzi, P. Post-traumatic orbital reconstruction comparing preformed versus non preformed titanium mesh plates. Rev. Stomatol. Chir. Maxillofac. 2011, 112, 145–150. [Google Scholar] [CrossRef]
- Von Wilmowsky, C.; Schwertner, M.G.; Nkenke, E.; Moest, T.; Adler, W.; Ebker, T. Use of CAD-based pre-bent implants reduces theatre time in orbital floor reconstruction: Results of a prospective study. Br. J. Oral Maxillofac. Surg. 2020, 58, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Sigron, G.R.; Ruedi, N.; Chammartin, F.; Meyer, S.; Msallem, B.; Kunz, C.; Thieringer, F.M. Three-Dimensional Analysis of Isolated Orbital Floor Fractures Pre- and Post-Reconstruction with Standard Titanium Meshes and “Hybrid” Patient-Specific Implants. J. Clin. Med. 2020, 9, 1579. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, R.; Malinska, M.; Kozakiewicz, M. Classical versus custom orbital wall reconstruction: Selected factors regarding surgery and hospitalization. J. Craniomaxillofac. Surg. 2017, 45, 710–715. [Google Scholar] [CrossRef]
- Ellwein, A.; Argiropoulos, K.; DeyHazra, R.O.; Pastor, M.F.; Smith, T.; Lill, H. Clinical evaluation of double-plate osteosynthesis for olecranon fractures: A retrospective case-control study. Orthop. Traumatol. Surg. Res. 2019, 105, 1601–1606. [Google Scholar] [CrossRef] [PubMed]
- Tang, Z.; Yang, H.; Chen, K.; Wang, G.; Zhu, X.; Qian, Z. Therapeutic effects of volar anatomical plates versus locking plates for volar Barton’s fractures. Orthopedics 2012, 35, e1198–e1203. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Liu, L.J.; Chen, H.X.; Xiong, J.Y.; Wang, D.M.; Huang, J.H.; Ding, J.L.; Wang, D.P. Application of 3D printing technology on the treatment of complex proximal humeral fractures (Neer3-part and 4-part) in old people. Orthop. Traumatol. Surg. Res. 2016, 102, 897–903. [Google Scholar] [CrossRef]
- Shuang, F.; Hu, W.; Shao, Y.; Li, H.; Zou, H. Treatment of Intercondylar Humeral Fractures With 3D-Printed Osteosynthesis Plates. Medicine 2016, 95, e2461. [Google Scholar] [CrossRef]
- DelSole, E.M.; Egol, K.A.; Tejwani, N.C. Construct Choice for the Treatment of Displaced, Comminuted Olecranon Fractures: Are Locked Plates Cost Effective? Iowa Orthop. J. 2016, 36, 59–63. [Google Scholar]
- Chen, M.; Gittings, D.J.; Yang, S.; Liu, G.; Xia, T. Variable-Angle Locking Compression Plate Fixation of Distal Radius Volar Rim Fractures. Iowa Orthop. J. 2019, 39, 55–61. [Google Scholar]
- Kong, L.; Yang, G.; Yu, J.; Zhou, Y.; Li, S.; Zheng, Q.; Zhang, B. Surgical treatment of intra-articular distal radius fractures with the assistance of three-dimensional printing technique. Medicine 2020, 99, e19259. [Google Scholar] [CrossRef]
- Park, Y.H.; Song, J.H.; Choi, G.W.; Kim, H.J. Comparative analysis of clinical outcomes of fixed-angle versus variable-angle locking compression plate for the treatment of Lisfranc injuries. Foot Ankle Surg. 2020, 26, 338–342. [Google Scholar] [CrossRef] [PubMed]
- Bilgetekin, Y.G.; Catma, M.F.; Ozturk, A.; Unlu, S.; Ersan, O. Comparison of different locking plate fixation methods in lateral malleolus fractures. Foot Ankle Surg. 2019, 25, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Zyskowski, M.; Wurm, M.; Greve, F.; Pesch, S.; von Matthey, F.; Pfluger, P.; Cronlein, M.; Biberthaler, P.; Kirchhoff, C. Is early full weight bearing safe following locking plate ORIF of distal fibula fractures? BMC Musculoskelet. Disord. 2021, 22, 159. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Chen, C.; Zhang, C.; Tao, Z.; Cai, L. The Feasibility of 3D Printing Technology on the Treatment of Pilon Fracture and Its Effect on Doctor-Patient Communication. Biomed. Res. Int. 2018, 2018, 8054698. [Google Scholar] [CrossRef]
- Zheng, W.; Tao, Z.; Lou, Y.; Feng, Z.; Li, H.; Cheng, L.; Zhang, H.; Wang, J.; Guo, X.; Chen, H. Comparison of the Conventional Surgery and the Surgery Assisted by 3d Printing Technology in the Treatment of Calcaneal Fractures. J. Investig. Surg. 2018, 31, 557–567. [Google Scholar] [CrossRef]
- Tsukada, S.; Otsuji, M.; Shiozaki, A.; Yamamoto, A.; Komatsu, S.; Yoshimura, H.; Ikeda, H.; Hoshino, A. Locking versus non-locking neutralization plates for treatment of lateral malleolar fractures: A randomized controlled trial. Int. Orthop. 2013, 37, 2451–2456. [Google Scholar] [CrossRef]
- Zhang, Y.W.; Xiao, X.; Xiao, Y.; Chen, X.; Zhang, S.L.; Deng, L. Efficacy and Prognosis of 3D Printing Technology in Treatment of High-Energy Trans-Syndesmotic Ankle Fracture Dislocation—”Log-Splitter” Injury. Med. Sci. Monit. 2019, 25, 4233–4243. [Google Scholar] [CrossRef]
- Zhang, X.; Han, C.Y.; Dai, M.J.; Chen, J.L.; Zheng, X.H.; Long, J.; Tang, W.; Tian, W.D.; Liu, L. Application of computer-assisted surgery techniques in the management of zygomatic complex fractures. Chin. J. Traumatol. 2018, 21, 281–286. [Google Scholar] [CrossRef]
- Schreurs, R.; Becking, A.G.; Jansen, J.; Dubois, L. Advanced Concepts of Orbital Reconstruction: A Unique Attempt to Scientifically Evaluate Individual Techniques in Reconstruction of Large Orbital Defects. Atlas Oral Maxillofac. Surg. Clin. N. Am. 2021, 29, 151–162. [Google Scholar] [CrossRef]
- Jansen, J.; Schreurs, R.; Dubois, L.; Maal, T.J.J.; Gooris, P.J.J.; Becking, A.G. The advantages of advanced computer-assisted diagnostics and three-dimensional preoperative planning on implant position in orbital reconstruction. J. Craniomaxillofac. Surg. 2018, 46, 715–721. [Google Scholar] [CrossRef]
- Van Bakelen, N.B.; Buijs, G.J.; Jansma, J.; de Visscher, J.G.; Hoppenreijs, T.J.; Bergsma, J.E.; Stegenga, B.; Bos, R.R. Comparison of biodegradable and titanium fixation systems in maxillofacial surgery: A two-year multi-center randomized controlled trial. J. Dent. Res. 2013, 92, 1100–1105. [Google Scholar] [CrossRef] [PubMed]
- Song, R.; Murphy, M.; Li, C.; Ting, K.; Soo, C.; Zheng, Z. Current development of biodegradable polymeric materials for biomedical applications. Drug. Des. Dev. Ther. 2018, 12, 3117–3145. [Google Scholar] [CrossRef]
- Buijs, G.J.; van der Houwen, E.B.; Stegenga, B.; Bos, R.R.; Verkerke, G.J. Mechanical strength and stiffness of biodegradable and titanium osteofixation systems. J. Oral Maxillofac. Surg. 2007, 65, 2148–2158. [Google Scholar] [CrossRef] [PubMed]
- Strong, E.B.; Gary, C. Management of Zygomaticomaxillary Complex Fractures. Facial Plast. Surg. Clin. N. Am. 2017, 25, 547–562. [Google Scholar] [CrossRef] [PubMed]
- Ellis, E., 3rd; Perez, D. An algorithm for the treatment of isolated zygomatico-orbital fractures. J. Oral Maxillofac. Surg. 2014, 72, 1975–1983. [Google Scholar] [CrossRef]
- Luca Cristofolini, M.B.; Schileo, E.; Van Sint Jan, S.; Juszczyk, M.M.; Öhman, C.; Zwierzak, I.; Lefèvre, P.; Juszczyk, J.M.; Viceconti, M. Differences between contralateral bones of the human lower limbs: A multiscale investigation. J. Mech. Med. Biol. 2014, 14, 1450032. [Google Scholar] [CrossRef]
- Jansen, J.; Dubois, L.; Schreurs, R.; Gooris, P.J.J.; Maal, T.J.J.; Beenen, L.F.; Becking, A.G. Should Virtual Mirroring Be Used in the Preoperative Planning of an Orbital Reconstruction? J. Oral Maxillofac. Surg. 2018, 76, 380–387. [Google Scholar] [CrossRef]
- Mittal, Y.; Varghese, K.G.; Mohan, S.; Jayakumar, N.; Chhag, S. A Comparative Study of 3-Dimensional Titanium Versus 2-Dimensional Titanium Miniplates for Open Reduction and Fixation of Mandibular Parasymphysis Fracture. J. Maxillofac. Oral Surg. 2016, 15, 93–98. [Google Scholar] [CrossRef]
- Barde, D.H.; Mudhol, A.; Ali, F.M.; Madan, R.S.; Kar, S.; Ustaad, F. Efficacy of 3-Dimensional plates over Champys miniplates in mandibular anterior fractures. J. Int. Oral Health 2014, 6, 20–26. [Google Scholar]

| Database | Strategy |
|---|---|
| MEDLINE | (“Fractures, Bone”[Mesh] OR “Fracture Fixation”[Mesh] OR fractur*[tiab]) AND (“Fracture Fixation, Internal”[Mesh] OR “Bone Plates”[Mesh] OR fixat*[tiab] OR osteosynth*[tiab] OR plate*[tiab] OR print*[tiab]) AND (“Wrist Injuries”[Mesh] OR “Wrist Joint”[Mesh] OR “Wrist”[Mesh] OR “Radius Fractures”[Mesh] OR “Ulna Fractures”[Mesh] OR “Jaw Fractures”[Mesh] OR “Mandibular Injuries”[Mesh] OR “Orbital Fractures”[Mesh] OR “Zygomatic Fractures”[Mesh] OR “Ankle Fractures”[Mesh] OR “Ankle Joint”[Mesh] OR “Ankle”[Mesh] OR fibula*[tiab] OR malleol*[tiab] OR zygo*[tiab] OR mandib*[tiab] OR orbit*[tiab] OR maxil*[tiab] OR ankl*[tiab] OR wrist*[tiab] OR radius*[tiab] OR ulna*[tiab]) AND (preform*[tiab] OR pre-form*[tiab] OR precontour*[tiab] OR contour*[tiab] OR anatomical*[tiab] OR lock*[tiab] OR 3D*[tiab] OR dimensi* [tiab]) AND (“Postoperative Complications”[Mesh] OR “Patient Satisfaction”[Mesh] OR “Length of Stay”[Mesh] OR “Operative Time”[Mesh] OR “Reoperation”[Mesh] OR reduc*[tiab] OR reposit*[tiab] OR stabil*[tiab] OR stabl*[tiab] OR complication*[tiab] OR satisfaction[tiab] OR displac*[tiab] OR reconstru* [tiab]) AND (“Controlled Clinical Trial” [Publication Type] OR “Cohort Studies”[Mesh] OR “Evaluation Study” [Publication Type] OR “Comparative Study” [Publication Type] OR controlled-study[tiab] OR controlled-trial[tiab] OR clinical-study[tiab] OR clinical-trial*[tiab] OR random*[tiab] OR prospectiv*[tiab] OR follow-up[tiab] OR cohort[tiab] OR groups[tiab] OR trial[ti] OR compar*[ti] OR evaluat*[ti] OR vs[ti] OR versus[ti]) NOT (“Review” [Publication Type] OR (“Animals”[Mesh] NOT “Humans”[Mesh])) |
| EMBASE | (‘fracture’/exp OR ‘fracture fixation’/exp OR fractur*:ab,ti,kw) AND (‘osteosynthesis’/exp OR ‘bone plate’/exp OR (fixat* OR osteosynth* OR plate*):ab,ti,kw) AND (‘wrist injury’/exp OR ‘wrist’/exp OR ‘radius fracture’/exp OR ‘ulna fracture’/exp OR ‘jaw fracture’/exp OR ‘orbit fracture’/exp OR ‘ankle fracture’/exp OR ‘ankle’/exp OR (fibula* OR malleol* OR zygo* OR mandib* OR orbit* OR maxil* OR ankl* OR wrist* OR radius* OR ulna*):ab,ti,kw) AND (preform* OR pre-form* OR precontour* OR contour* OR anatomical* OR lock*):ab,ti,kw AND (‘postoperative complication’/exp OR ‘complication’/de OR ‘patient satisfaction’/exp OR ‘length of stay’/exp OR ‘operation duration’/exp OR ‘reoperation’/exp OR (reduc* OR reposit* OR stabil* OR stabl* OR complication* OR satisf* OR displac*):ab,ti,kw) AND (‘controlled clinical trial’/exp OR ‘multicenter study’/exp OR ‘cohort analysis’/exp OR ‘follow up’/exp OR ‘evaluation study’/exp OR ‘comparative study’/exp OR ‘controlled study’/de OR ‘controlled-study’ OR (‘controlled-trial’ OR ‘clinical-study’ OR ‘clinical-trial*’ OR random* OR prospectiv* OR follow-up OR cohort OR groups):ab,ti OR (trial OR compar* OR evaluat* OR vs OR versus):ti) NOT (‘review’/de OR ‘conference abstract’/it OR (‘animal’/exp NOT ‘human’/exp)) |
| COCHRANE | ([mh “Fractures, Bone”] OR [mh “Fracture Fixation”] OR fractur*:ti,ab) AND ([mh “Fracture Fixation, Internal”] OR [mh “Bone Plates”] OR fixat*:ti,ab OR osteosynth*:ti,ab OR plate*:ti,ab) AND ([mh “Wrist Injuries”] OR [mh “Wrist Joint”] OR [mh Wrist] OR [mh “Radius Fractures”] OR [mh “Ulna Fractures”] OR [mh “Jaw Fractures”] OR [mh “Mandibular Injuries”] OR [mh “Orbital Fractures”] OR [mh “Zygomatic Fractures”] OR [mh “Ankle Fractures”] OR [mh “Ankle Joint”] OR [mh Ankle] OR fibula*:ti,ab OR malleol*:ti,ab OR zygo*:ti,ab OR mandib*:ti,ab OR orbit*:ti,ab OR maxil*:ti,ab OR ankl*:ti,ab OR wrist*:ti,ab OR radius*:ti,ab OR ulna*:ti,ab) AND (preform*:ti,ab OR pre-form*:ti,ab OR precontour*:ti,ab OR contour*:ti,ab OR anatomical*:ti,ab OR lock*:ti,ab) |
| Author | Year of Publication | Follow-Up (Months) | Radiographic Assessment/Imaging Technique | Conventional Plates | Material | Precontoured Plates | Material |
|---|---|---|---|---|---|---|---|
| Orbital Fractures | |||||||
| Wilmowsky et al. [40] | (2020) | 3 and 6 months | CT and CBCT: pre-operative and immediate, 3 and 6 months post-operatively | Non-preformed orbital floor plates | Titanium | Implant customised in size and shape based on the individual 3-dimensional template | Titanium |
| Sigron et al. [41] | (2020) | N/A | CT and CBCT: pre-operatively and post-operatively | Orbital floor mesh plate (MatrixMIDFACE, DePuy Synthes, Solothurn, Switzerland) | Titanium | Pre-bent plates (MatrixMIDFACE, DePuy Synthes, Solothurn, Switzerland or MODUS Midface OPS 1.5, Medartis, Basel, Switzerland) | Titanium |
| Zieliński et al. [42] | (2017) | 1 and 6 months | CT: pre-operatively, 1 week post-operatively | Standard, intraoperatively bent mesh | Titanium | Individual implants (23 patients) or pre-bent mesh on a 3D model (16 patients) | UHMW-PE (18 patients), ZrO2 (5 patients), and titanium (16 patients) |
| Fan et al. [36] | (2017) | N/A | CT: pre-operatively and post-operatively | Medpor- mesh | Titanium | Medpor-Titanium mesh trimmed according to the contour of the simulated bone template | Titanium |
| Scolozzi [37] | (2011) | Patients were followed for at least 6 weeks | CT: pre-operatively and post-operatively | Non-preformed radial orbital mesh plate (0.3 to 0.4 mm | Titanium | 3-dimensional preformed MatrixORBITAL orbital mesh (Synthes-CH 4436, Oberdorf, Switzerland) | Titanium |
| Momjian et al. [39] | (2011) | Patients were followed for at least 6 months | CT: pre-operatively and post-operatively | Non-pre-shaped mesh plates 0.3 mm (Synthes, CH 4436 Oberdorf Switzerland) | Titanium | Three-dimensionally preformed mesh plates 0.4 mm (Synthes, CH 4436 Oberdorf Switzerland) | Titanium |
| Scolozzi et al. [38] | (2010) | Patients were followed for at least 6 weeks | CT: pre-operatively and post-operatively | Non-preformed orbital mesh plates | Titanium | 3D preformed orbital mesh plates | Titanium |
| Upper limb fractures | |||||||
| Kong et al. [49] | (2020) | 1-, 2-, 3-, and 6 months | CT-scan: pre-operatively X-ray: intraoperatively, 1-, 2-, 3-, and 6 months post-operatively | Volar plate and K-wire fixation | N/A | Pre-bending of the volar plate 3D physical fracture model | N/A |
| Ellwein et al. [43] | (2019) | A minimum follow-up time of 24 months was required | Radiographs: pre-operatively, intraoperatively and post-operatively | Aptus olecranon low-profile double-plate (Fa. Medartis, Basel, Switzerland) | N/A | 3.5 mm olecranon locking compression plate (LCP) (Fa. DePuySynthes, Umkirchen, Germany) | N/A |
| Chen et al. [48] | (2019) | 3, 6, and 12 months | Radiographs and CT: preoperatively Anterior-posterior and lateral X-ray: 3, 6, and 12 months postoperatively Postoperatively CT: surgeon preference | Conventional volar locking plate (FA-LCP) | N/A | 2.4 mm VA-LCP low profile plating system | N/A |
| DelSole et al. [47] | (2016) | 2 week, 6 week, 3 months and 6 months | Radiographs: pre-operatively, 2-weeks, 6-weeks, 3 months, and 6 months postoperatively | 6- or 7-hole one-third tubular plate | N/A | Pre-contoured LP (Acumed (Hillsboro, OR, USA), Stryker (Kalamazoo, MI, USA), Zimmer (Warsaw, IN, USA), or Dupuy- Synthes (Paoli, PA, USA)) | N/A |
| Shuang et al. [46] | (2016) | 6 months | CT: pre-operative X-rays and 3D reconstructed CT: and post-operatively | Conventional | N/A | Osteosynthesis plates with proper sized and number of holes fabricated using a 3D printer | N/A |
| You et al. [45] | (2016) | A minimum follow-up time of 12 months was required | CT-scan and anteroposterior position: pre-operatively Double radiograph: 1-day post-operatively (3D group only) | Plate and screws | Steel | Preselected and prefabricated proximal humeral locking plate and screws determined by a 3D-print model simulation | Steel |
| Tang et al. [44] | (2012) | Immediately, 3 months and 1 year | Anteroposterior radiograph: Immediately, 3 months and 1 year postoperatively | Locking plate (Synthes, Bettlach, Switzerland) | N/A | Anatomical plate (Weigao Orthopaedic Device Co Ltd, Weihai City, China) | N/A |
| Lower limb fractures | |||||||
| Zyskowski et al. [52] | (2021) | 6 and 12 weeks, 6 months and 1 year | Radiographs: pre-operatively, 6 and 12 weeks, 6 months and 1 year postoperatively | DePuy Synthes® one-third semitubular plate | N/A | NEWCLIP TECHNICS, Active Ankle® polyaxial locking plate | N/A |
| Park et al. [50] | (2020) | 2 and 6 weeks, 3 and 12 months | Anteroposterior and lateral radiographs: preoperatively and postoperatively | 2.7 mm fixed-angle LCP (Depuy Synthes, Oberdorf, Switzerland) | N/A | 2.7mm variable-angle LCP (Depuy Synthes, West Chester, PA, USA) | N/A |
| Bilgetekin et al. [51] | (2019) | 15th day and monthly after surgery | Radiographs of the antero-posterior, lateral and mortise view: preoperatively, postoperatively and at every follow-up | Locking tubular (1/3 Tubular Locking Compression Plates ©Xrbest Jiangsu. China) | N/A | Locking anatomical plate (Distal Fibula Locking Compression Plates ©Xrbest Jiangsu. China) | N/A |
| Zhang et al. [56] | (2019) | Patients were followed-up for more than 12 months | CT-scan, anteroposterior X-ray or lateral X-ray: preoperatively and postoperatively | 3.5-mm locking compression plate (LCP) | N/A | Plate preselected and prefabricated on the 3D-printed log-splitter injury physical model | N/A |
| Zheng et al. [53] | (2018) | Patients were followed-up for at least 12 months | CT: preoperative Anteroposterior and lateral X-ray: immediately after the operation, 3, 6 and 18 months postoperatively | Plates and screws | N/A | Preselected and prefabricated plate and screws determined by the 3D-printed model | N/A |
| Zheng et al. [54] | (2017) | Patients were followed-up for at least 12 months | CT: preoperatively Anteroposterior and oblique X-ray: immediately after the operation, 1, 3, 6, 12 and 15 months postoperatively | Plates and screws | N/A | Preselected and prefabricated plate and screws determined by the 3D-printed model | Steel |
| Tsukada et al. [55] | (2013) | 3, 6 and 12 months | X-ray: intra-operatively, 3, 6 and 12 months postoperatively | Straight plate (LCP Metaphysical plate, Synthes Japan, Tokyo, Japan) | Titanium | Pre-shaped plate (distal fibula plate, Stryker Japan, Tokyo, Japan) | Titanium |
| Parameter I: Stability and Reduction. | Anatomical 3D Placement (N/Total Population) |
|---|---|
| Orbital Fractures | |
| Scolozzi (2011) [37] | 2D: 10/10; 100% 3D: 10/10; 100% |
| Scolozzi (2010) [38] | 2D: 10/10; 100% 3D: 10/10; 100% |
| Parameter II: Clinical Outcome | Operation Time (Mean min ± SD, Total Population) | Placement Time (Mean min ± SD, Total Population) | Hospitalization Period (Mean Days ± SD, Total Population) |
|---|---|---|---|
| Orbital Fractures | |||
| Sigron (2020) [41] | 2D: 99.8 ± 28.9 (12) 3D: 57.3 ± 23.4 (10) * p = 0.001 | 2D: 3.8 ± 3.0 (12) 3D: 4.6 ± 3.9 (10) | |
| Wilmowsky (2020) [40] | 2D: 11.1 ± 7.7 (11) 3D: 5.5 ± 5.4 (25) * p = 0.001 | ||
| Fan (2017) [36] | 2D: 95.37 ± 22.19 (27) 3D: 75.34 ± 15.68 (29) * p < 0.05 | ||
| Zielinkski (2017) [42] | 2D: Median: 100; range: 20–420 (54) 3D: Median: 80; range: 20–410 (39) | 2D: Median: 4.5; range: 2–11 (54) 3D: Median: 5; range: 1–20 (39) | |
| Parameter III: Specific for the Fractured Site | Orbital Volume (Mean mL ± SD, Total Population) | Absolute Volume Difference (Mean mL ± SD, Total Population) | Maximum Fracture Collapse (Mean mm2 ± SD, Total Population) | Congruence of Infraorbital Rim (Complete) (N/Total Population) | Congruence of Infraorbital Rim (Good) (N/Total Population) | Congruence of Infraorbital Rim (Acceptable) (N/Total Population) | Fracture Area (Mean mm2 ± SD, Total Population) | Loss of Binocular Single Vision (N/Total Population) | Enopthalmos (N/Total Population) | Limitation of the Inferior Oblique Muscle (N/Total Population) |
|---|---|---|---|---|---|---|---|---|---|---|
| Orbital Fractures | ||||||||||
| Sigron (2020) [41] | 2D: 30.1 ± 4.2 (12) 3D: 25.7 ± 3.0 (10) * p = 0.010 | 2D: 1.6 ± 1.2 (12) 3D: 1.0 ± 0.7 (10) | 2D: 6.9 ± 2.3 (12) 3D: 8.6 ± 5.4 (10) | 2D: 408.5 ± 137.5 (12) 3D: 389.4 ± 135.1 (10) | ||||||
| Wilmowsky (2020) [40] | 2D: 6/11;54% 3D: 15/25;60% | 2D: 6/11;54% 3D: 6/25;24% | 2D: 1/11;9% 3D: 4/25;16% | |||||||
| Zielinski (2017) [42] | 2D: 9/54; 16% 3D: 5/39; 13% | |||||||||
| Scolozzi (2011) [37] | 2D: 0/10;0% 3D: 0/10;0% | 2D: 0/10;0% 3D: 0/10;0% | ||||||||
| Momjian et al. (2011) [39] | 2D: 21.76 (15) 3D: 20.28 (15) | 2D: 0.004 (15) 3D: 0.345 (15) | 2D: 0/15;0% 3D: 0/15;0% | |||||||
| Scolozzi (2010) [38] | 2D:19.215 (10) 3D: 21.791 (10) | 2D: 0.26 (10) 3D: 0.081 (10) | ||||||||
| Parameter III: Specific for the fractured site | Maximum width difference between fracture zone and implant (mean mm ± SD, total population) | Maximum depth difference between fracture zone and implant (mean mm ± SD, total population) | Area difference between fracture site and implant (mean mm2 ± SD, total population) | Angle difference in medial and inferior wall corner (mean ◦ ± SD, total population) | Enophthalmos (mean mm ± SD, total population) | Superior sulcus deformity (N/total population) | Diplopia (N/total population) | |||
| Orbital fractures | ||||||||||
| Fan (2017) [36] | 2D: 5.60 ± 0.90 (27) 3D: 2.51 ± 0.53 (29) * p < 0.05 | 2D: 4.61 ± 0.89 (27) 3D: 2.58 ± 0.46 (29) * p < 0.05 | 2D: 84.05 ± 20.89 (27) 3D: 43.59 ± 9.53 (29) * p < 0.05 | 2D: 12.58 ± 5.04 (27) 3D: 2.82 ± 0.44 (29) * p < 0.05 | 2D: 2.5 ± 1.0 (27) 3D: 1.0 ± 0.5 (29) * p < 0.05 | 2D: 5/27; 18.5% 3D: 2/29;6.9% * p < 0.05 | ||||
| Momjian et al. (2011) [39] | 2D: 3/15; 20% 3D: 1/15; 6.6% | |||||||||
| Parameter IV: Specific for the Fractured Site | DASH-Score (Mean ± SD, Total Population) | Pain (Mean VAS-Score ± SD, Total Population) | MEPS (mean ± SD, Total Population) | MMWS (mean ± SD, Total Population) | Range of Motion: Extension/Flexion (mean ± SD, Total Population) | Range of Motion: Pronation/Supination (mean ± SD, Total Population) |
|---|---|---|---|---|---|---|
| Upper Limb Fractures | ||||||
| Kong (2020) [49] | 2D: 24.5 ± 7.0 (16) 3D: 23.8 ± 8.1 (16) | 2D: 0.9 ± 0.3 (16) 3D: 0.9 ± 0.2 (16) | ||||
| Ellwein (2019) [43] | 2D: 94 ± 10 (range: 65–100) (25) 3D: 96 ± 11 (range: 60–100) (22) | 2D: 127 ± 15 (80–145) (25) 3D: 130 ± 21 (range: 40–150) (25) | 2D: 170 (range: 30–180) (25) 3D: 174 (range: 95–180) (25) | |||
| Chen (2019) [48] | 2D: 12.8 (range: 6–18) (28) 3D: 9.2 (range: 2–12) (19) * p = 0.02 | 2D: 83.5 (range: 75–90) (28) 3D: 93.8 (range: 85–100) (19) * p < 0.01 | 2D: 82.8 % 1 3D: 94.8 % 1 * p < 0.01 | 2D: 84.5 % 1 3D: 93.8 % 1 * p < 0.01 | ||
| Shuang (2016) [46] | 2D: 79 ± 13 (7) 3D: 85 ± 9 (6) | 2D: 93 ± 24 (7) 3D: 98 ± 27 (6) | 2D: 167 ± 21 (7) 3D: 160 ± 17 (6) | |||
| Tang (2012) [44] | 2D: 5.96 ± 3.48 (17) 3D: 7.04 ± 4.40 (16) | |||||
| Parameter IV: specific for the fractured site | Radial height (mean mm, total population) | Radial inclination (mean, total population) | Volar tilt (mean, total population) | |||
| Upper limb fractures | ||||||
| Chen (2019) [48] | 2D: 20.2 (28) 3D: 19.9 (19) | 2D: 10.2 (28) 3D: 10.2 (19) | 2D: 8.37 (28) 3D: 7.11 (19) | |||
| Parameter II: Complications | Screw Loosening (N/Total Population) | Necrosis (N/Total Population) | Infection (N/Total Population) | Nerve Injury (N/Total Population) |
|---|---|---|---|---|
| Upper Limb Fractures | ||||
| Kong (2020) [49] | 2D: 1/16; 6.25% 3D: 0/16; 0% | 2D: 0/16; 0% 3D: 0/16; 0% | ||
| Ellwein (2019) [43] | 2D: 1/25; 4% 3D: 0/22; 0% | |||
| Chen (2019) [48] | 2D:0/28; 0% 3D: 0/19; 0% | 2D: 0/28; 0% 3D: 0/19; 0% | ||
| DelSole (2016) [47] | 2D: 1/14; 7.14% 3D; 0/8; 0% | |||
| Shuang (2016) [46] | 2D: 0/7; 0% 3D: 0/6; 0% | |||
| Parameter III: Clinical Outcome | Revision Surgery (N/Total Population) | Hardware Failure (N/Total Population) | Cost of the Plate (Mean Dollars, Total Population) | Operation Time (Mean in min ± SD, Total Population) | Intra-Operative Blood Loss (Mean mL ± SD, Total Population) | Intraoperative Fluoroscopy (Mean Fluoroscopy Number ± SD, Total Population) | Patients Satisfaction (N/Total Population) |
|---|---|---|---|---|---|---|---|
| Upper Limb Fractures | |||||||
| Kong (2020) [49] | 2D: 63.5±5.9 (16) 3D: 51.4 ± 6.8 (16) * p < 0.001 | 2D: 74.2±10.3 (16) 3D: 52.3±9.9 (16) * p < 0.001 | 2D: 5.6±1.1 (16) 3D: 4.2 ± 1.3 (16) * p = 0.002 | ||||
| Ellwein (2019) [43] | 2D: 7/25; 28% 3D: 8/22; 36.36% | 2D: 0/25; 0% 3D: 0/22; 0% | 2D: 80 ± 29 (range: 29–150) (25) 3D: 86 ± 26 (range: 41–141) (22) | 2D: 24/25; 96% 3D: 20/22; 91% | |||
| Chen (2019) [48] | 2D: 2/28; 7.14% 3D: 0/19; 0% | ||||||
| DelSole (2016) [47] | 2D: 1/14; 7.14% 3D: 1/8; 12.5% | 2D: 0/14; 0% 3D: 0/8; 0% | 2D: 157.50 (14) 3D: 2071.00 (8) | ||||
| You (2016) [45] | 2D: 92.03 ± 10.31 (32) 3D: 77.65 ± 8.09 (34) * p < 0.05 | 2D: 281.25 ± 57.85 (32) 3D: 235.29 ± 63.40 (34) * p < 0.05 | 2D: 10.59 ± 1.36 (32) 3D: 7.12 ± 1.57 (34) * p < 0.05 | ||||
| Shuang (2016) [46] | 2D: 92.3 ± 17.4 (7) 3D: 70.6 ± 12.1 (6) * p = 0.026 | ||||||
| Tang (2012) [44] | 2D: 0/17; 0% 3D: 2/16; 0% | 2D: 75.2 (range, 45–120) (17) 3D: 78.2 (range, 50–140) (16) | 2D: 158.3 (range, 100–350) (17) 3D: 176.1 (range, 100–400) (17) | ||||
| Parameter I: Stability and Reduction | Nonunion (N/Total Population) | Delayed Union (N/Total Population) | Malunion (N/Total Population) | Bone Union (N/Total Population) | Rate of Anatomic Reduction (N/Total Population) |
|---|---|---|---|---|---|
| Lower Limb Fractures | |||||
| Zyskowski (2021) [52] | 2D: 0/25; 0% 3D: 0/20; 0% | ||||
| Park (2020) [50] | 2D: 0/22; 0% 3D: 0/23; 0% | 2D: 0/22; 0% 3D: 1/23; 4.3% | 2D: 1/22; 4.5% 3D: 1/23; 4.3% | ||
| Bilgetekin (2019) [51] | 2D: 0/37; 0% 3D: 0/25; 0% | ||||
| Zhang (2019) [56] | 2D: 0/13; 0% 3D: 0/16; 0% | 2D: 1/13;10.5% 3D: 2/16;12.5% | 2D: 1/13; 10.5% 3D: 0/16; 0% | 2D: 9/13; 69.2% 3D: 13/16; 81.3% | |
| Zheng (2018) [53] | 2D: 0/48; 0% 3D: 0/45; 0% | 2D: 3/48; 6.3% 3D: 2/45;4.4% | 2D: 1/48; 2.1% 3D: 1/45; 2.2% | 2D: 36/48; 75% 3D: 41/45; 91.1% * p = 0.04 | |
| Zheng (2017) [54] | 2D: 0/40; 0% 3D: 0/35; 0% | ||||
| Tsukada (2013) [55] | 2D: 19/21; 90.5% 3D: 23/24; 95.8% | ||||
| Parameter II: Complications | Plate Palpable (N/Total Population) | Swelling (N/Total Population) | Deep Vein Thrombosis (N/Total Population) | Infection (N/Total Population) | Screw Loosening (N/Total Population) |
|---|---|---|---|---|---|
| Lower Limb Fractures | |||||
| Zyskowski (2021) [52] | 2D: 0/25; 0% 3D: 2/20; 10% | 2D: 1/25; 4% 3D: 0/20; 0% | 2D: 1/25; 4% 3D: 0/20; 0% | 2D: 3/25; 12% 3D: 1/20; 5% | 2D: 0/25; 0% 3D: 1/20; 5% |
| Park (2020) [50] | 2D: 0/22; 0% 3D: 1/23; 4.3% | ||||
| Bilgetekin (2019) [51] | 2D: 0/37; 0% 3D: 1/25; 4% | ||||
| Zhang (2019) [56] | 2D: 1/13; 10.5% 3D: 1/16; 8.3% | ||||
| Zheng (2018) [53] | 2D: 4/48; 8.3% 3D: 3/45; 6.7% | ||||
| Zheng (2017) [54] | 2D: 1/40; 2.5% 3D: 2/35; 5.7% | ||||
| Tsukada (2013) [55] | 2D: 0/21; 0% 3D: 0/23; 0% | 2D: 0/21; 0% 3D: 2/23; 8.7% | |||
| Parameter III: Clinical Outcome | Removed Plates (N/Total Population) | Fracture to Union Time (Mean Months ± SD, Total Population) | Pain (Mean VAS-Score ± SD, Total Population) | Operation Time (Mean min ± SD, Total Population) | Intraoperative Fluoroscopy (Mean Fluoroscopy Number ± SD, Total Population) | Intraoperative Blood Loss (Mean mL ± SD, Total Population) |
|---|---|---|---|---|---|---|
| Lower Limb Fractures | ||||||
| Zyskowski (2021) [52] | 2D: 10/25; 40% 3D: 13/20; 65% | |||||
| Park (2020) [50] | 2D: 83.5 ± 31.2 min (22) 3D: 65.8 ± 23.9 min (23) * p = 0.04 | |||||
| Bilgetekin (2019) [51] | 2D: 2/37; 5.4% 3D: 0/25; 0% | |||||
| Zhang (2019) [56] | 2D: 5.2 ± 1.3 (13) 3D: 5.1 ± 1.2 (16) | 2D: 124.5 ± 11.5 (13) 3D: 107.8 ± 10.2 (16) * p < 0.001 | 2D: 11.7 ± 2.4 (13) 3D: 7.3 ± 2.7 (16) * p < 0.001 | 2D: 133.7 ± 26.2 (13) 3D: 99.6 ± 19.3 (13) * p < 0.001 | ||
| Zheng (2018) [53] | 2D: 5.3 ± 1.2 (48) 3D: 5.0 ± 1.1 (45) | 2D: 2.9 ± 1.2 (48) 3D: 2.6 ± 0.9 (45) | 2D: 90.2 ± 10.9 (48) 3D: 74.1 ± 8.2 (45) * p < 0.001 | 2D: 11.0 ± 2.9 (48) 3D: 7.6 ± 2.2 (45) * p < 0.001 | 2D: 159.8 ± 26.5 (48) 3D: 117.1 ± 20.7 (45) * p < 0.001 | |
| Zheng (2017) [54] | 2D: 3.2 ± 0.4 (40) 3D: 3.0 ± 0.3 (35) | 2D: 2.8 ± 1.2 (40) 3D: 2.6 ± 0.9 (35) | 2D: 91.3 ± 11.2 (40) 3D: 71.4 ± 6.8 (35) * p < 0.0001 | 2D: 8.6 ± 2.7 (40) 3D: 5.6 ± 1.96 (35) * p < 0.0001 | 2D: 288.7 ± 34.8 (40) 3D: 226.1 ± 22.6 (35) * p < 0.0001 | |
| Parameter IV: Specific for the Fractured Site | Range of Motion: Plantarflexion (Mean ± SD, Total Population) | Range of Motion: Dorsiflexion (Mean ± SD, Total Population) | AOFAS (Mean ± SD, Total Population) | Sagittal Motion: Normal (N/Total Population) | Hindfoot Motion: Normal (N/Total Population) |
|---|---|---|---|---|---|
| Lower Limb Fractures | |||||
| Zyskowski (2021) [52] | 2D: 38 ± 3 (25) 3D: 39 ± 2 (25) | 2D: 22 ± 2 (25) 3D: 22 ± 3 (25) | |||
| Zhang (2019) [56] | 2D: 26.7 ± 3.4 (13) 3D: 27.9 ± 2.8 (16) | 2D: 23.5 ± 3.8 (13) 3D: 24.3 ± 3.9 (16) | 2D: 74.8 ± 9.3 (13) 3D: 75.5 ± 8.5 (16) | ||
| Bilgetekin (2019) [51] | 2D: Median (min-max): 87.0 (73–100) (37) 3D: Median (min-max): 85.0 (71–100) (25) | 2D: 30/37; 81.1% 3D: 17/25; 68.0% | 2D: 35/37; 94.6% 3D: 25/25; 100% | ||
| Zheng (2018) [53] | 2D: 25.9 ± 8.7 (48) 3D: 27.4 ± 8.5 (45) | 2D: 14.2 ± 5.0 (48) 3D: 15.1 ± 4.8 (45) | 2D: 84.7 ± 9.0 (48) 3D: 87.4 ± 8.7 (45) | ||
| Zheng (2017) [54] | 2D: 85.8 ± 9.0 (40) 3D: 87.6 ± 7.6 (35) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raghoebar, I.I.; Dubois, L.; de Lange, J.; Schepers, T.; Don Griot, P.; Essig, H.; Rozema, F. The Effectiveness of Three-Dimensional Osteosynthesis Plates versus Conventional Plates for the Treatment of Skeletal Fractures: A Systematic Review and Meta-Analysis. J. Clin. Med. 2023, 12, 4661. https://doi.org/10.3390/jcm12144661
Raghoebar II, Dubois L, de Lange J, Schepers T, Don Griot P, Essig H, Rozema F. The Effectiveness of Three-Dimensional Osteosynthesis Plates versus Conventional Plates for the Treatment of Skeletal Fractures: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2023; 12(14):4661. https://doi.org/10.3390/jcm12144661
Chicago/Turabian StyleRaghoebar, Iva Ilse, Leander Dubois, Jan de Lange, Tim Schepers, Peter Don Griot, Harald Essig, and Frederik Rozema. 2023. "The Effectiveness of Three-Dimensional Osteosynthesis Plates versus Conventional Plates for the Treatment of Skeletal Fractures: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 12, no. 14: 4661. https://doi.org/10.3390/jcm12144661
APA StyleRaghoebar, I. I., Dubois, L., de Lange, J., Schepers, T., Don Griot, P., Essig, H., & Rozema, F. (2023). The Effectiveness of Three-Dimensional Osteosynthesis Plates versus Conventional Plates for the Treatment of Skeletal Fractures: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 12(14), 4661. https://doi.org/10.3390/jcm12144661