Evidence for Impaired Renin Activity in Postural Orthostatic Tachycardia Syndrome
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Examination and Blood Sample Collection
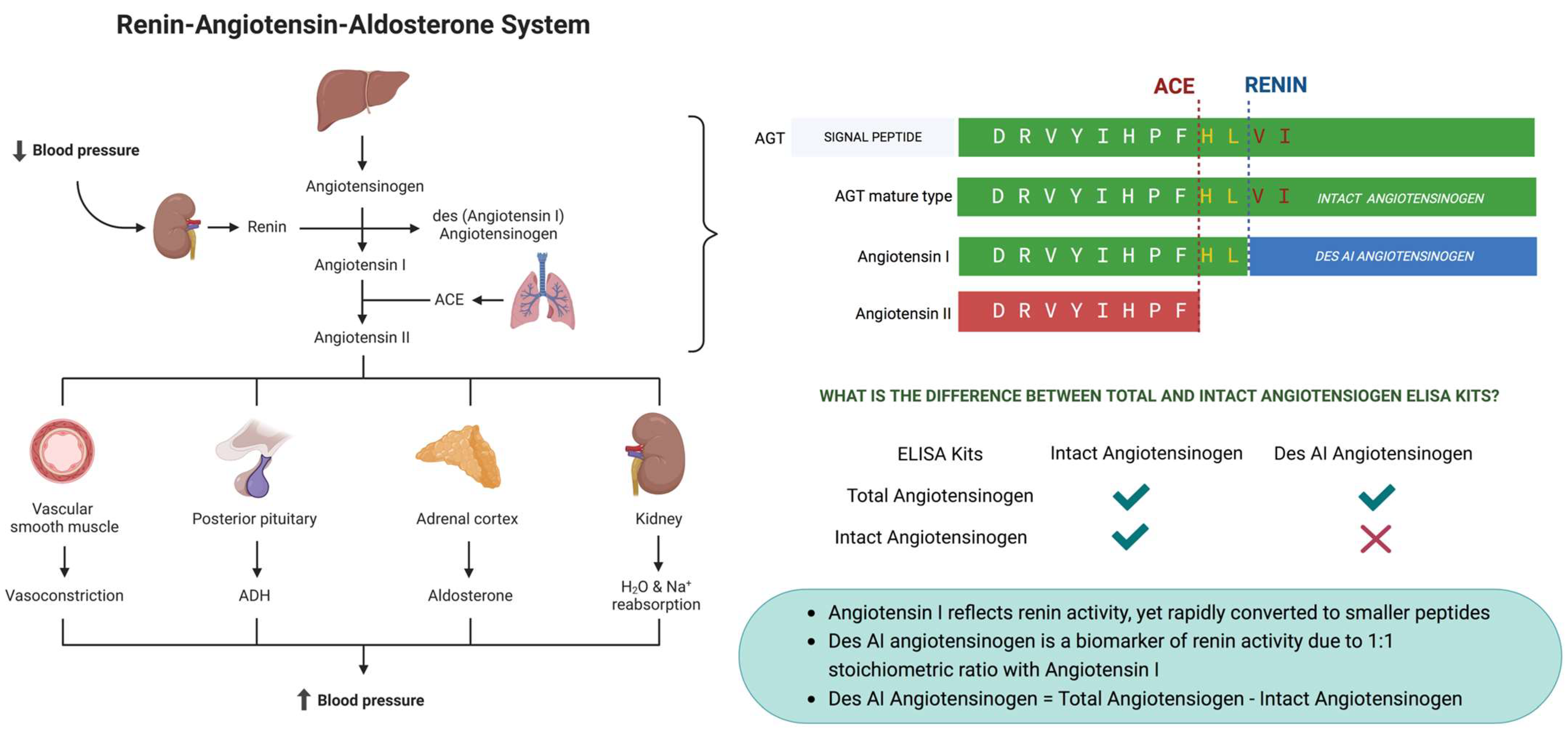
2.3. Determination of Renin Activity
2.4. Determination of Aldosterone Concentration
2.5. Statistical Analysis
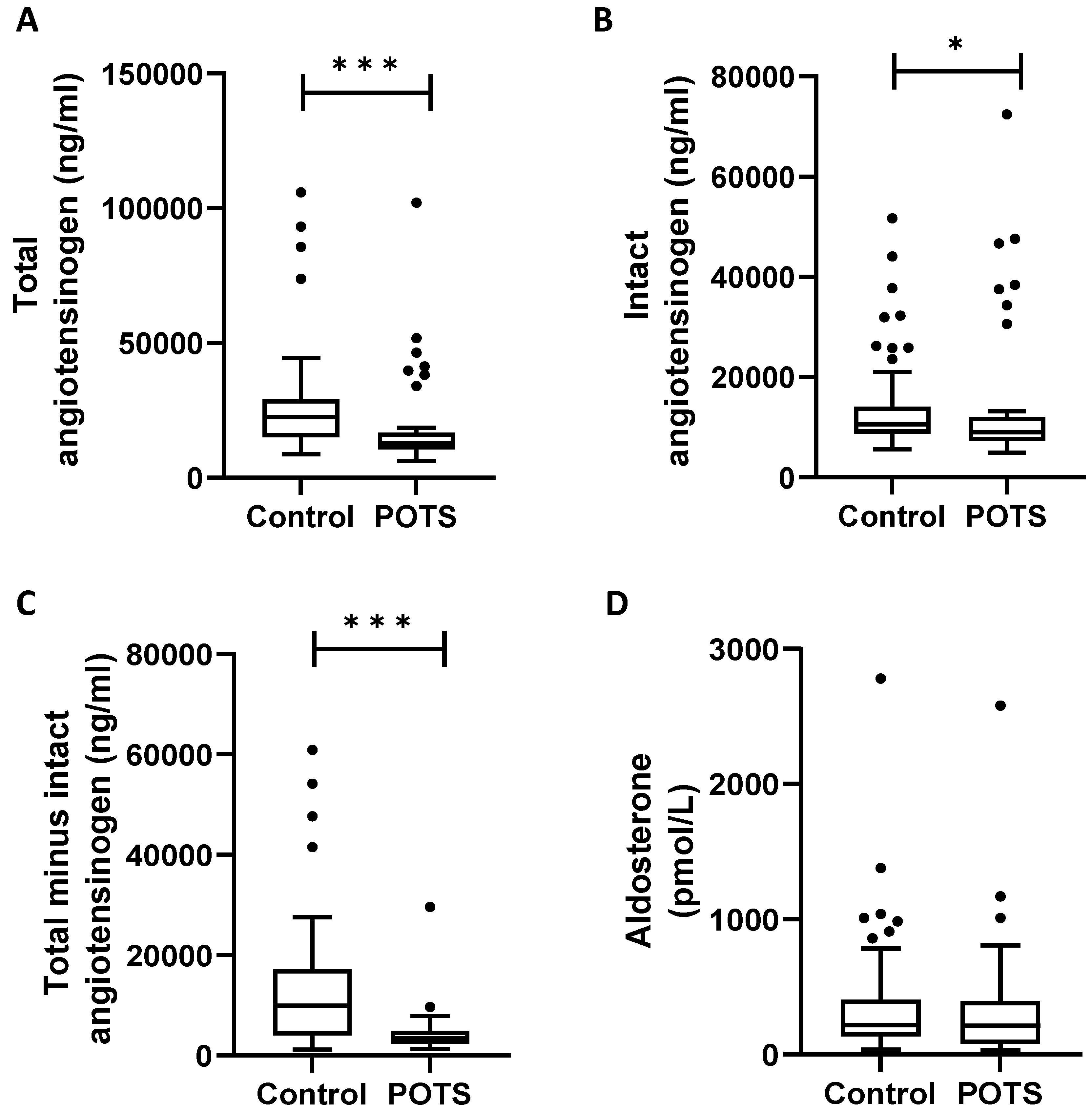
3. Results
4. Discussion
4.1. Renin–Angiotensin–Aldosterone System
4.2. Autonomic Nervous System and RAAS
4.3. Hemodynamic Alterations in POTS
4.4. Previous Reports on RAAS in POTS
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fedorowski, A. Postural orthostatic tachycardia syndrome: Clinical presentation, aetiology and management. J. Intern. Med. 2019, 285, 352–366. [Google Scholar] [CrossRef]
- Mar, P.L.; Raj, S.R. Postural Orthostatic Tachycardia Syndrome: Mechanisms and New Therapies. Annu. Rev. Med. 2020, 71, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Kharraziha, I.; Holm, H.; Bachus, E.; Melander, O.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Monitoring of cerebral oximetry in patients with postural orthostatic tachycardia syndrome. Europace 2019, 21, 1575–1583. [Google Scholar] [CrossRef]
- Bryarly, M.; Phillips, L.T.; Fu, Q.; Vernino, S.; Levine, B.D. Postural Orthostatic Tachycardia Syndrome: JACC Focus Seminar. J. Am. Coll. Cardiol. 2019, 73, 1207–1228. [Google Scholar] [CrossRef]
- Zhao, S.; Tran, V.H. Postural Orthostatic Tachycardia Syndrome. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Arnold, A.C.; Ng, J.; Raj, S.R. Postural tachycardia syndrome—Diagnosis, physiology, and prognosis. Auton. Neurosci. 2018, 215, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Vernino, S.; Bourne, K.M.; Stiles, L.E.; Grubb, B.P.; Fedorowski, A.; Stewart, J.M.; Arnold, A.C.; Pace, L.A.; Axelsson, J.; Boris, J.R.; et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting—Part 1. Auton. Neurosci. 2021, 235, 102828. [Google Scholar] [CrossRef]
- Raj, V.; Opie, M.; Arnold, A.C. Cognitive and psychological issues in postural tachycardia syndrome. Auton. Neurosci. 2018, 215, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Astudillo, L.; Laure, A.; Fabry, V.; Pugnet, G.; Maury, P.; Labrunée, M.; Sailler, L.; Pavy-Le Traon, A. [Postural tachycardia syndrome (PoTS): An up-to-date]. Rev. Med. Interne 2018, 39, 627–634. [Google Scholar] [CrossRef]
- Patel, S.; Rauf, A.; Khan, H.; Abu-Izneid, T. Renin-angiotensin-aldosterone (RAAS): The ubiquitous system for homeostasis and pathologies. Biomed. Pharmacother. 2017, 94, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Sparks, M.A.; Crowley, S.D.; Gurley, S.B.; Mirotsou, M.; Coffman, T.M. Classical Renin-Angiotensin system in kidney physiology. Compr. Physiol. 2014, 4, 1201–1228. [Google Scholar] [CrossRef]
- Miller, A.J.; Arnold, A.C. The renin-angiotensin system in cardiovascular autonomic control: Recent developments and clinical implications. Clin. Auton. Res. 2019, 29, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, H.I.; Garland, E.M.; Biaggioni, I.; Black, B.K.; Dupont, W.D.; Robertson, D.; Raj, S.R. Abnormalities of angiotensin regulation in postural tachycardia syndrome. Heart Rhythm. 2011, 8, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Mar, P.L.; Raj, S.R. Neuronal and hormonal perturbations in postural tachycardia syndrome. Front. Physiol. 2014, 5, 220. [Google Scholar] [CrossRef] [PubMed]
- Kharraziha, I.; Axelsson, J.; Ricci, F.; Di Martino, G.; Persson, M.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Serum Activity Against G Protein-Coupled Receptors and Severity of Orthostatic Symptoms in Postural Orthostatic Tachycardia Syndrome. J. Am. Heart Assoc. 2020, 9, e015989. [Google Scholar] [CrossRef] [PubMed]
- Fedorowski, A.; Li, H.; Yu, X.; Koelsch, K.A.; Harris, V.M.; Liles, C.; Murphy, T.A.; Quadri, S.M.S.; Scofield, R.H.; Sutton, R.; et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Europace 2017, 19, 1211–1219. [Google Scholar] [CrossRef]
- Yu, X.; Li, H.; Murphy, T.A.; Nuss, Z.; Liles, J.; Liles, C.; Aston, C.E.; Raj, S.R.; Fedorowski, A.; Kem, D.C. Angiotensin II Type 1 Receptor Autoantibodies in Postural Tachycardia Syndrome. J. Am. Heart Assoc. 2018, 7, e008351. [Google Scholar] [CrossRef]
- Gunning, W.T., 3rd; Stepkowski, S.M.; Kramer, P.M.; Karabin, B.L.; Grubb, B.P. Inflammatory Biomarkers in Postural Orthostatic Tachycardia Syndrome with Elevated G-Protein-Coupled Receptor Autoantibodies. J. Clin. Med. 2021, 10, 623. [Google Scholar] [CrossRef]
- Brinth, L.S.; Pors, K.; Mehlsen, J. Postural orthostatic tachycardia syndrome. Ugeskr. Laeger. 2018, 180, 853–856. [Google Scholar]
- Dahan, S.; Tomljenovic, L.; Shoenfeld, Y. Postural Orthostatic Tachycardia Syndrome (POTS)—A novel member of the autoimmune family. Lupus 2016, 25, 339–342. [Google Scholar] [CrossRef]
- Pizon, T.; Rajzer, M.; Wojciechowska, W.; Drozdz, T.; Drozdz, D.; Rojek, M.; Gruszka, K.; Czarnecka, D. Plasma renin activity, serum aldosterone concentration and selected organ damage indices in essential arterial hypertension. Arch. Med. Sci. 2021, 17, 9–18. [Google Scholar] [CrossRef]
- Favre, L.; Vallotton, M.B.; Muller, A.F. Relationship between plasma concentrations of angiotensin I, angiotensin II and plasma renin activity during cardio-pulmonary bypass in man. Eur. J. Clin. Investig. 1974, 4, 135–140. [Google Scholar] [CrossRef]
- Kurtz, A. Control of renin synthesis and secretion. Am. J. Hypertens. 2012, 25, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Erdös, E.G. The angiotensin I converting enzyme. Fed. Proc. 1977, 36, 1760–1765. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N. Hypertension and the bradykinin system. Curr. Hypertens. Rep. 2009, 11, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Oudart, N. The renin-angiotensin system: Current data. Ann. Pharm. Fr. 2005, 63, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Hellman, P.; Björklund, P.; Åkerström, T. Aldosterone-Producing Adenomas. Vitam. Horm. 2019, 109, 407–431. [Google Scholar] [CrossRef]
- Hall, J.; Bourne, K.M.; Vernino, S.; Hamrefors, V.; Kharraziha, I.; Nilsson, J.; Sheldon, R.S.; Fedorowski, A.; Raj, S.R. Detection of G Protein-Coupled Receptor Autoantibodies in Postural Orthostatic Tachycardia Syndrome Using Standard Methodology. Circulation 2022, 146, 613–622. [Google Scholar] [CrossRef]
- Raj, S.R.; Biaggioni, I.; Yamhure, P.C.; Black, B.K.; Paranjape, S.Y.; Byrne, D.W.; Robertson, D. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation 2005, 111, 1574–1582. [Google Scholar] [CrossRef]
- Stewart, J.M.; Glover, J.L.; Medow, M.S. Increased plasma angiotensin II in postural tachycardia syndrome (POTS) is related to reduced blood flow and blood volume. Clin. Sci. 2006, 110, 255–263. [Google Scholar] [CrossRef]
- Garland, E.M.; Raj, S.R.; Black, B.K.; Harris, P.A.; Robertson, D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology 2007, 69, 790–798. [Google Scholar] [CrossRef]
| Covariates | POTS + (n = 46) | POTS − (n = 48) | p-Value |
|---|---|---|---|
| Age, years | 27 ± 9 | 30 ± 9 | 0.053 |
| Female sex, n (%) | 39 (85) | 36 (75) | 0.238 |
| SBP, supine (mmHg) | 117 ± 13 | 115 ± 11 | 0.221 |
| DBP, supine (mmHg) | 73 ± 9 | 69 ± 9 | 0.008 |
| HR, supine (bpm) | 69 ± 11 | 63 ± 11 | 0.006 |
| SBP, standing 1 min (mmHg) | 121 ± 18 | 114 ± 11 | 0.13 |
| DBP, standing 1 min (mmHg) | 84 ± 13 | 75 ± 8 | <0.001 |
| HR, standing 1 min (bpm) | 93 ± 19 | 79 ± 13 | <0.001 |
| SBP, standing 3 min (mmHg) | 117 ± 16 | 112 ± 14 | 0.080 |
| DBP, standing 3 min (mmHg) | 81 ± 13 | 72 ± 10 | 0.001 |
| HR, standing 3 min (bpm) | 96 ± 18 | 83 ± 12 | <0.001 |
| SBP, standing 5 min (mmHg) | 117 ± 18 | 112 ± 13 | 0.08 |
| DBP, standing 5 min (mmHg) | 80 ± 15 | 74 ± 11 | 0.015 |
| HR, standing 5 min (bpm) | 93 ± 18 | 84 ± 13 | 0.005 |
| Spearman Correlation Coefficient | Systolic BP Supine | Systolic BP Standing 1 min | Systolic BP Standing 3 min | Systolic BP Standing 5 min | |
|---|---|---|---|---|---|
| (mmHg) | (mmHg) | (mmHg) | (mmHg) | ||
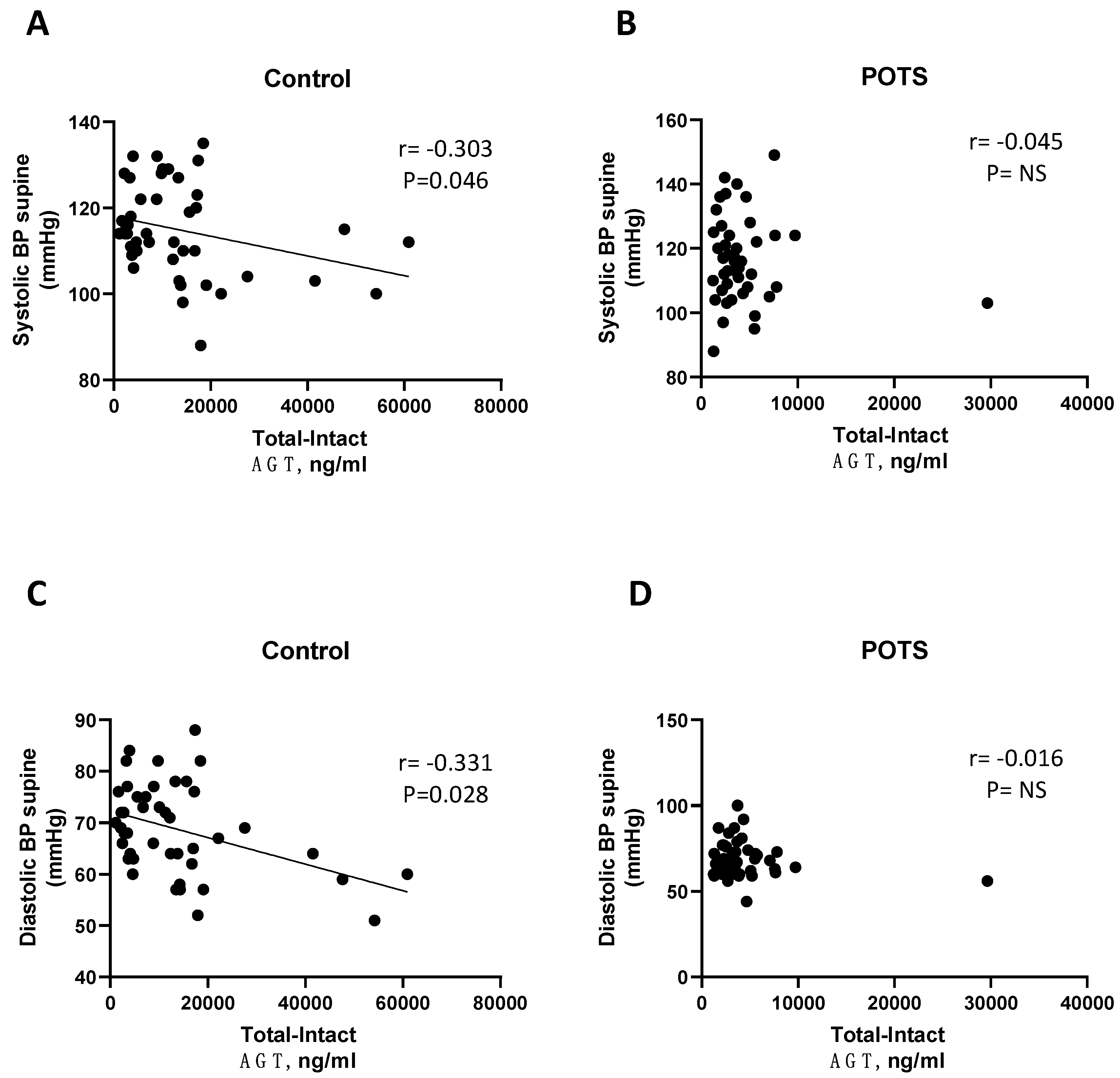
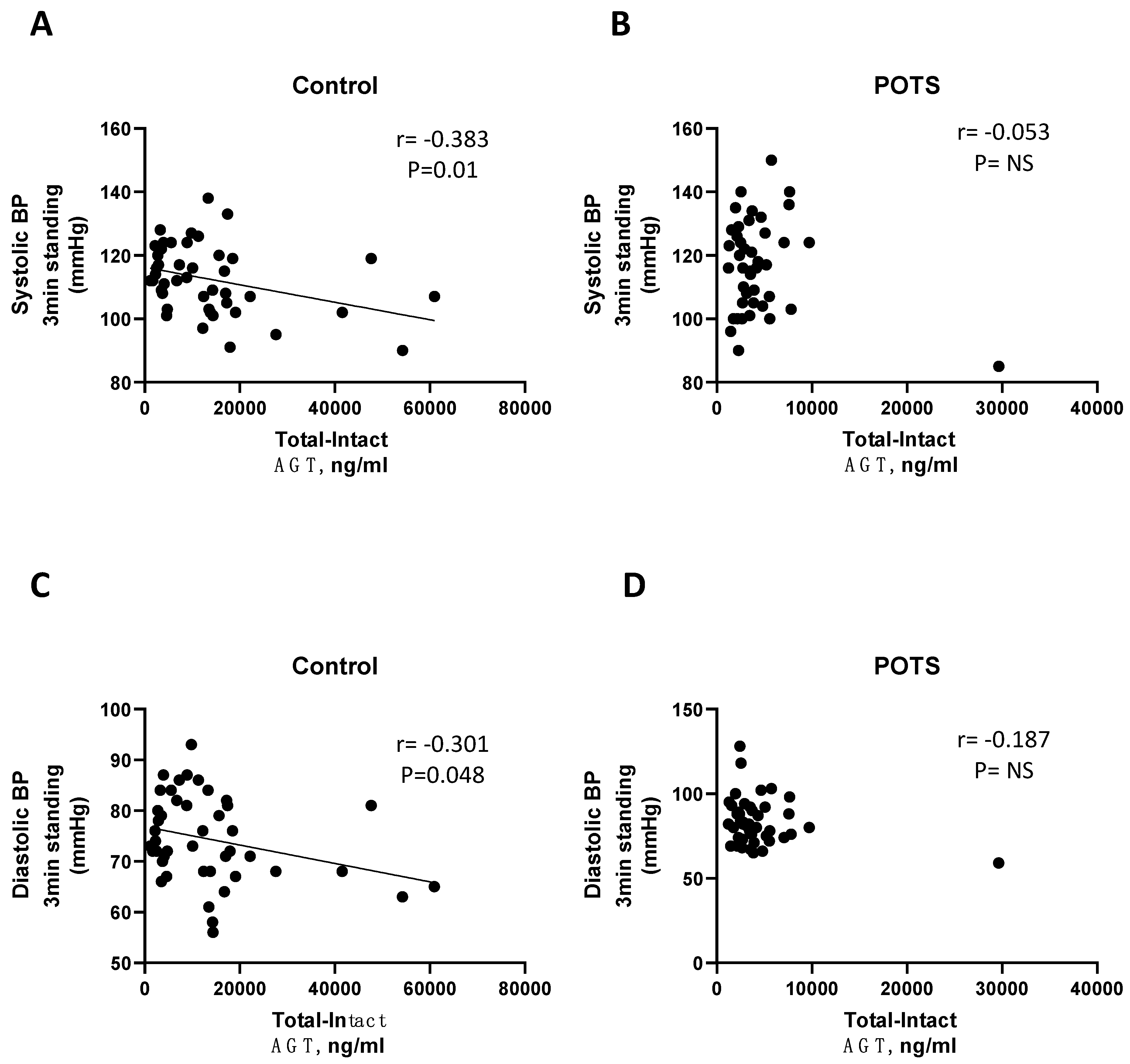
| Control | Angiotensin I (ng/mL) | r = −0.303 | r = −0.507 | r = −0.383 | r = −0.320 |
| p-value | 0.046 | 0.001 | 0.01 | 0.039 | |
| Aldosterone (pmol/L) | r = −0.71 | r = −0.143 | r = −0.062 | r = −0.046 | |
| p-value | 0.648 | 0.361 | 0.69 | 0.775 | |
| POTS | Angiotensin I (ng/mL) | r = 0.061 | r = −0.157 | r = 0.015 | r = −0.073 |
| p-value | 0.687 | 0.321 | 0.925 | 0.649 | |
| Aldosterone (pmol/L) | r = −0.128 | r = −0.222 | r = −0.276 | r = −0.244 | |
| p-value | 0.407 | 0.163 | 0.073 | 0.129 | |
| Spearman Correlation Coefficient | Diastolic BP Supine | Diastolic BP Standing 1 min | Diastolic BP Standing 3 min | Diastolic BP Standing 5 min | |
|---|---|---|---|---|---|
| (mmHg) | (mmHg) | (mmHg) | (mmHg) | ||
| Control | Angiotensin I (ng/mL) | r = −0.331 | r = −0.308 | r = −0.300 | r = −0.276 |
| p-value | 0.028 | 0.044 | 0.048 | 0.077 | |
| Aldosterone (pmol/L) | r = −0.090 | r = −0.200 | r = −0.142 | r = −0.046 | |
| p-value | 0.059 | 0.2 | 0.358 | 0.772 | |
| POTS | Angiotensin I (ng/mL) | r = −0.245 | r = −0.051 | r = −0.201 | r = −0.278 |
| p-value | 0.101 | 0.748 | 0.191 | 0.079 | |
| Aldosterone (pmol/L) | r = −0.106 | r = −0.244 | r = −0.137 | r = −0.175 | |
| p-value | 0.492 | 0.125 | 0.382 | 0.28 | |
| Spearman Correlation Coefficient | HR Supine | HR Standing 1 min | HR Standing 3 min | HR Standing 5 min | |
|---|---|---|---|---|---|
| (bpm) | (bpm) | (bpm) | (bpm) | ||
| Control | Angiotensin I (ng/mL) | r = −0.269 | r = 0.054 | r = 0.052 | r = −0.248 |
| p-value | 0.078 | 0.73 | 0.739 | 0.114 | |
| Aldosterone (pmol/L) | r = 0.209 | r = 0.035 | r = 0.184 | r = 0.233 | |
| p-value | 0.174 | 0.824 | 0.232 | 0.138 | |
| POTS | Angiotensin I (ng/mL) | r = −0.068 | r = −0.106 | r = −0.017 | r = −0.195 |
| p-value | 0.654 | 0.504 | 0.914 | 0.227 | |
| Aldosterone (pmol/L) | r = 0.299 | r = 0.082 | r = 0.282 | r = 0.150 | |
| p-value | 0.049 | 0.611 | 0.067 | 0.363 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spahic, J.M.; Mattisson, I.Y.; Hamrefors, V.; Johansson, M.; Ricci, F.; Nilsson, J.; Melander, O.; Sutton, R.; Fedorowski, A. Evidence for Impaired Renin Activity in Postural Orthostatic Tachycardia Syndrome. J. Clin. Med. 2023, 12, 4660. https://doi.org/10.3390/jcm12144660
Spahic JM, Mattisson IY, Hamrefors V, Johansson M, Ricci F, Nilsson J, Melander O, Sutton R, Fedorowski A. Evidence for Impaired Renin Activity in Postural Orthostatic Tachycardia Syndrome. Journal of Clinical Medicine. 2023; 12(14):4660. https://doi.org/10.3390/jcm12144660
Chicago/Turabian StyleSpahic, Jasmina Medic, Ingrid Yao Mattisson, Viktor Hamrefors, Madeleine Johansson, Fabrizio Ricci, Jan Nilsson, Olle Melander, Richard Sutton, and Artur Fedorowski. 2023. "Evidence for Impaired Renin Activity in Postural Orthostatic Tachycardia Syndrome" Journal of Clinical Medicine 12, no. 14: 4660. https://doi.org/10.3390/jcm12144660
APA StyleSpahic, J. M., Mattisson, I. Y., Hamrefors, V., Johansson, M., Ricci, F., Nilsson, J., Melander, O., Sutton, R., & Fedorowski, A. (2023). Evidence for Impaired Renin Activity in Postural Orthostatic Tachycardia Syndrome. Journal of Clinical Medicine, 12(14), 4660. https://doi.org/10.3390/jcm12144660









