Deep Vein Thrombosis Is Facilitated by Endothelial-Derived Extracellular Vesicles via the PDI–GRP94–GPIIb/IIIa Pathway in Mice
Abstract
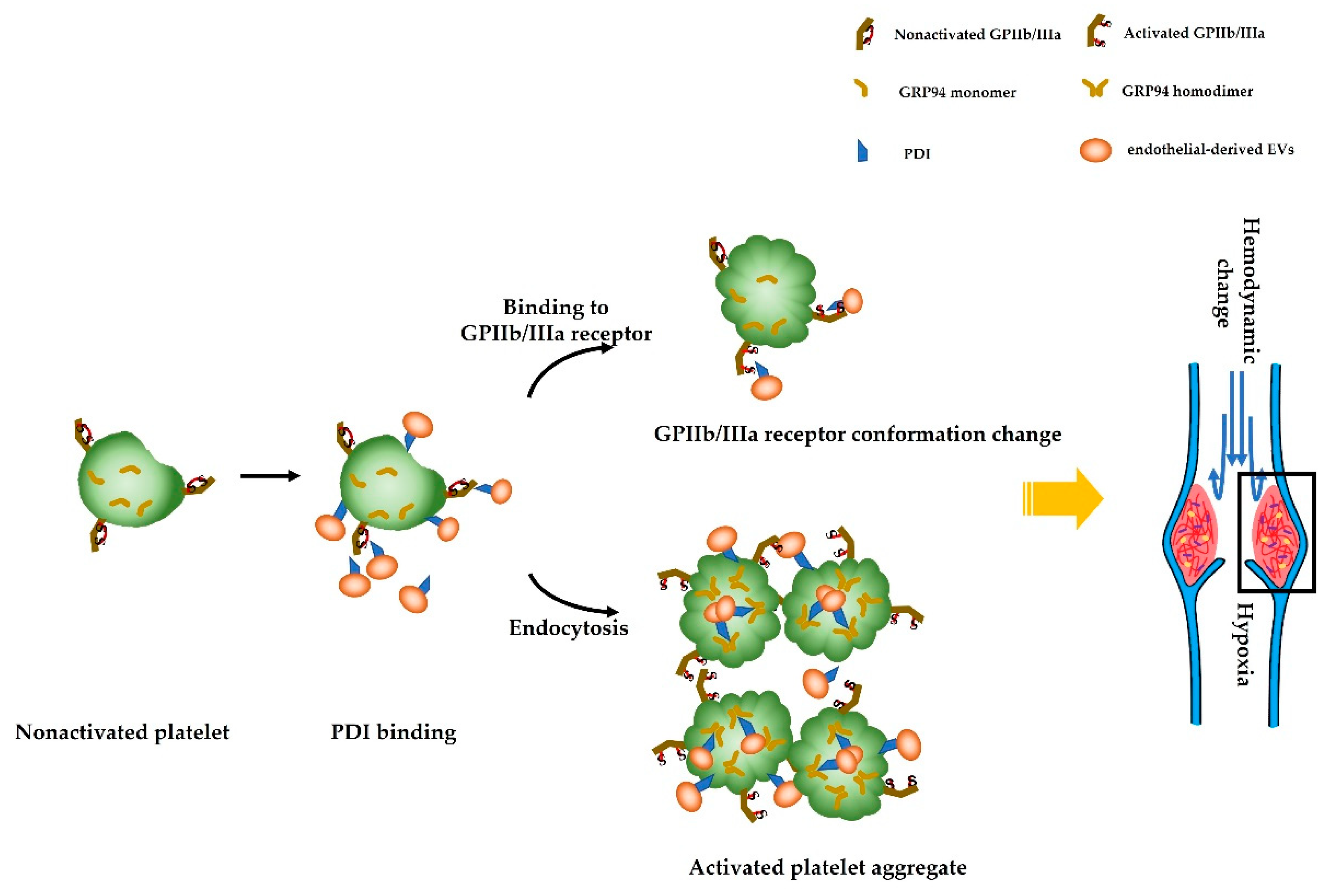
1. Introduction
2. Materials and Methods
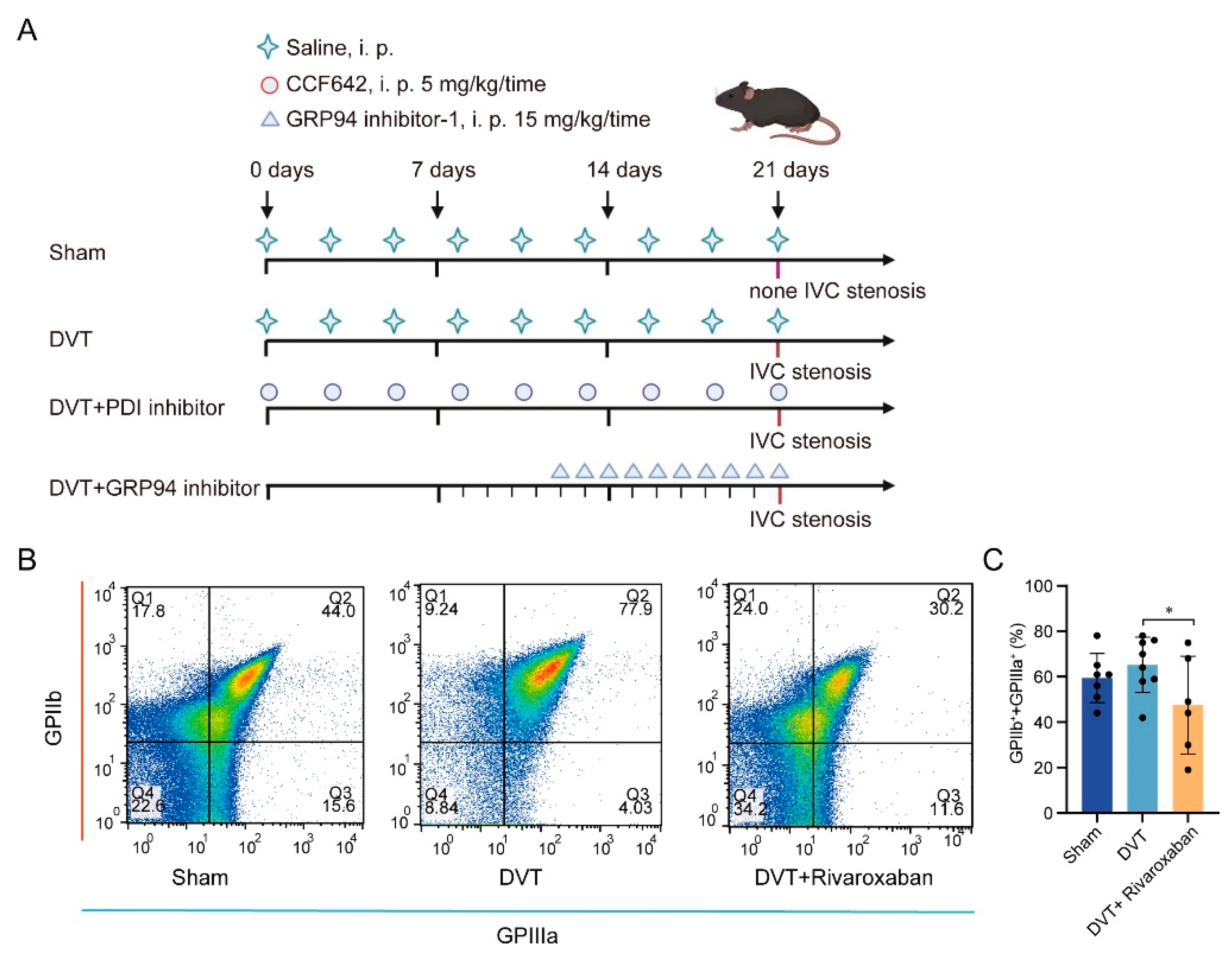
2.1. Animal Model
2.2. Isolation of Platelets and Plasma
2.3. Clot Retraction
2.4. Platelet Spreading on Immobilized Fibrinogen
2.5. Flow Cytometry (FCM)
2.6. Histological Examination
2.7. Cell Culture
2.8. Migration Assay
2.9. Extracellular Vesicles (EVs) Extraction
2.10. PKH26 Staining
2.11. Fluorescence Resonance Energy Transfer (FRET)
2.12. Platelet Aggregometry
2.13. Western Blot
2.14. Statistical Analysis
3. Results
3.1. DVT Mouse Model Establishment and Platelet Activation in Thrombus Formation
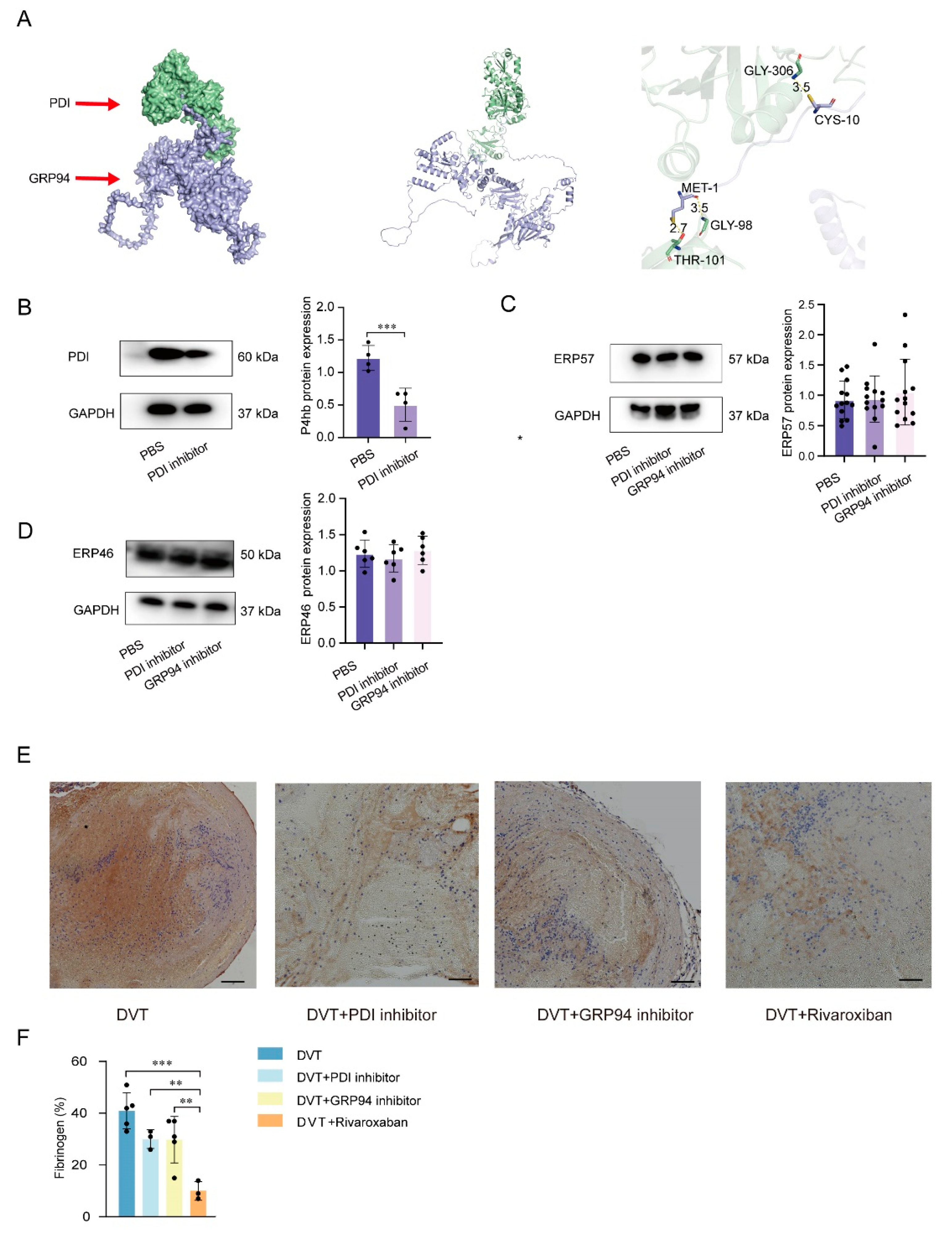
3.2. PDI and GRP94 Accelerated Thrombus Formation Mediated by Increased Platelet Activation Levels in Plasma
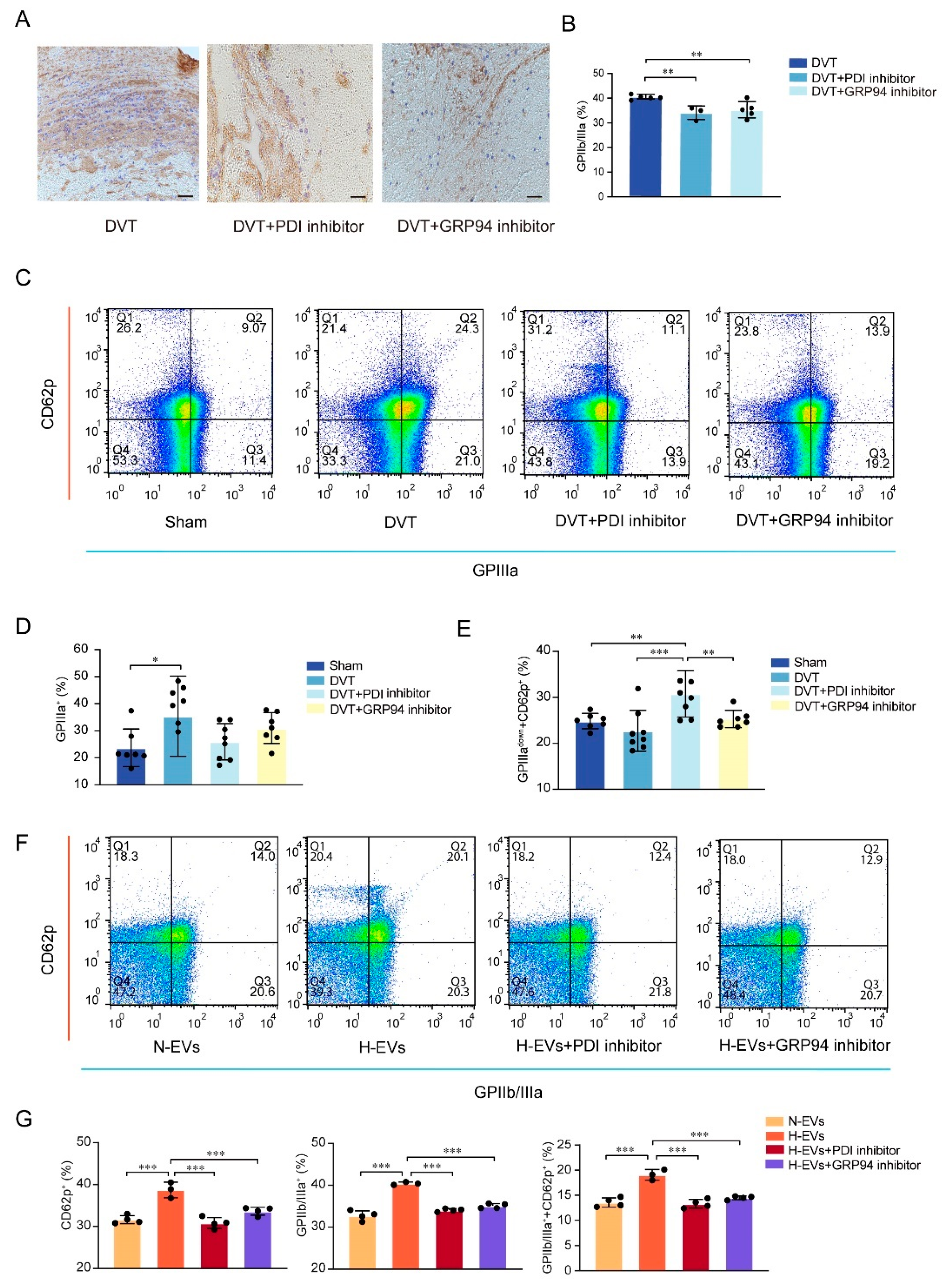
3.3. Inhibition of PDI and GRP94 Represses the GPIIb Submit, the GPIIIa Submit, and the GPIIb/IIIa Integrin Expression upon Platelet Activation
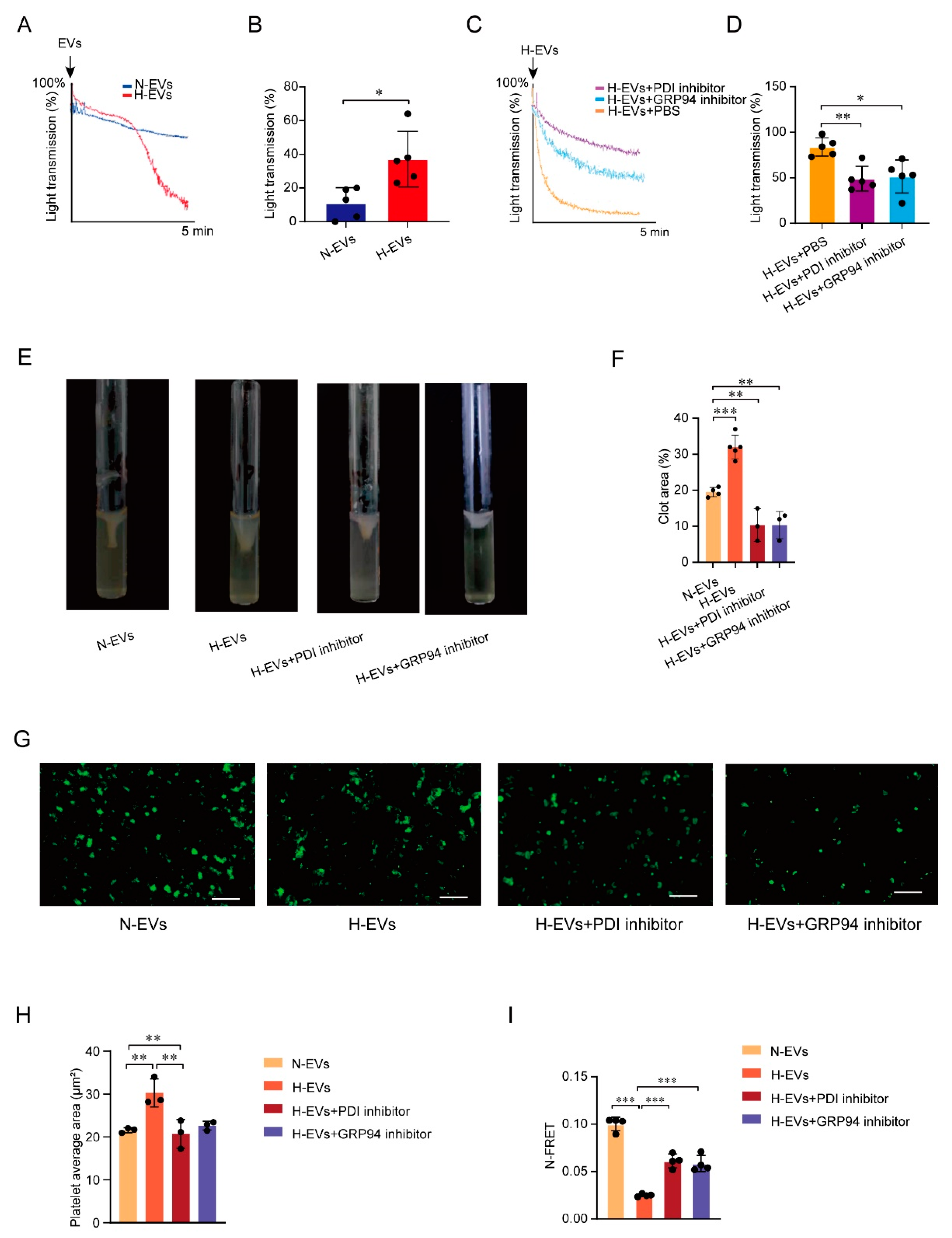
3.4. Hypoxia-Induced Endothelial-Derived EVs Carrying PDI Endocytosed by Platelets Induced GRP94 Monomers to Change into Dimers, Resulting in Platelet Activation
3.5. PDI Regulates Platelet Function and Hypoxia-Induced Endothelial-Derived EVs Induce GPIIb/IIIa Conformation Activation
4. Discussion
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Schulman, S.; Ageno, W.; Konstantinides, S.V. Venous thromboembolism: Past, present and future. Thromb. Haemost. 2017, 117, 1219–1229. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, J.; Shao, X.; Dong, F.; Wang, J.; Wang, D.; Wu, S.; Xie, W.; Wan, J.; Chen, H.; et al. Trends in Hospitalization and In-Hospital Mortality from VTE, 2007 to 2016, in China. Chest 2019, 155, 342–353. [Google Scholar] [CrossRef] [PubMed]
- Holbrook, L.-M.; Watkins, N.A.; Simmonds, A.D.; Jones, C.I.; Ouwehand, W.H.; Gibbins, J.M. Platelets release novel thiol isomerase enzymes which are recruited to the cell surface following activation. Br. J. Haematol. 2010, 148, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Snir, O.; Wilsgård, L.; Latysheva, N.; Wahlund, C.J.E.; Brækkan, S.K.; Hindberg, K.; Hansen, J. Plasma levels of platelet-derived microvesicles are associated with risk of future venous thromboembolism. J. Thromb. Haemost. 2022, 20, 899–908. [Google Scholar] [CrossRef]
- Adair, B.D.; Alonso, J.L.; van Agthoven, J.; Hayes, V.; Ahn, H.S.; Yu, I.-S.; Lin, S.-W.; Xiong, J.-P.; Poncz, M.; Arnaout, M.A. Structure-guided design of pure orthosteric inhibitors of αIIbβ3 that prevent thrombosis but preserve hemostasis. Nat. Commun. 2020, 11, 398. [Google Scholar] [CrossRef] [PubMed]
- Mackman, N.; Bergmeier, W.; Stouffer, G.A.; Weitz, J.I. Therapeutic strategies for thrombosis: New targets and approaches. Nat. Rev. Drug Discov. 2020, 19, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Cho, J. Protein disulfide isomerase in thrombosis and vascular inflammation. J. Thromb. Haemost. 2013, 11, 2084–2091. [Google Scholar] [CrossRef]
- Qin, R.-R.; Zhu, H.; Wang, F.; Song, M.; Lin, P.-L.; Xing, Y.-Q.; Zhang, W.; Zhong, M.; Wang, Z.-H. Platelet activation in diabetic mice models: The role of vascular endothelial cell-derived protein disulfide isomerase-mediated GP IIb/IIIa receptor activation. Aging 2019, 11, 6358–6370. [Google Scholar] [CrossRef]
- Cho, J.; Furie, B.C.; Coughlin, S.R.; Furie, B. A critical role for extracellular protein disulfide isomerase during thrombus formation in mice. J. Clin. Investig. 2008, 118, 1123–1131. [Google Scholar] [CrossRef]
- Kim, K.; Hahm, E.; Li, J.; Holbrook, L.-M.; Sasikumar, P.; Stanley, R.G.; Ushio-Fukai, M.; Gibbins, J.; Cho, J. Platelet protein disulfide isomerase is required for thrombus formation but not for hemostasis in mice. Blood 2013, 122, 1052–1061. [Google Scholar] [CrossRef]
- Jackson, J.W.; Rivera-Marquez, G.M.; Beebe, K.; Tran, A.D.; Trepel, J.B.; Gestwicki, J.E.; Blagg, B.S.; Ohkubo, S.; Neckers, L.M. Pharmacologic dissection of the overlapping impact of heat shock protein family members on platelet function. J. Thromb. Haemost. 2020, 18, 1197–1209. [Google Scholar] [CrossRef]
- Marzec, M.; Eletto, D.; Argon, Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2012, 1823, 774–787. [Google Scholar] [CrossRef]
- Kriss, C.L.; Pinilla-Ibarz, J.A.; Mailloux, A.W.; Powers, J.J.; Tang, C.-H.; Kang, C.W.; Zanesi, N.; Epling-Burnette, P.K.; Sotomayor, E.M.; Croce, C.M.; et al. Overexpression of TCL1 activates the endoplasmic reticulum stress response: A novel mechanism of leukemic progression in mice. Blood 2012, 120, 1027–1038. [Google Scholar] [CrossRef]
- Jiang, F.; Guo, A.-P.; Xu, J.-C.; You, Q.-D.; Xu, X.-L. Discovery of a Potent Grp94 Selective Inhibitor with Anti-Inflammatory Efficacy in a Mouse Model of Ulcerative Colitis. J. Med. Chem. 2018, 61, 9513–9533. [Google Scholar] [CrossRef] [PubMed]
- Vatolin, S.; Phillips, J.G.; Jha, B.K.; Govindgari, S.; Hu, J.; Grabowski, D.; Parker, Y.; Lindner, D.J.; Zhong, F.; Distelhorst, C.W.; et al. Novel Protein Disulfide Isomerase Inhibitor with Anticancer Activity in Multiple Myeloma. Cancer Res. 2016, 76, 3340–3350. [Google Scholar] [CrossRef]
- Dignat-George, F.; Boulanger, C.M. The Many Faces of Endothelial Microparticles. Arter. Thromb. Vasc. Biol. 2011, 31, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Han, D.; Awad, M.A.; Leibowitz, J.L.; Griffith, B.P.; Wu, Z.J. Role of thrombin to non-physiological shear stress induced platelet activation and function alternation. Thromb. Res. 2022, 219, 141–149. [Google Scholar] [CrossRef]
- Woulfe, D.; Yang, J.; Brass, L. ADP and platelets: The end of the beginning. J. Clin. Investig. 2001, 107, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lu, J.; Wei, G.; Tong, H.; Zhou, J.; Ding, Y.; Zhang, S.; Xu, X.; Lai, R.; Luo, Q.; et al. Tacrolimus ameliorates thrombocytopenia in an ITP mouse model. Ann. Hematol. 2020, 99, 2315–2322. [Google Scholar] [CrossRef]
- Hegazy, S.; Elsabaawy, M.; Eltabakh, M.; Hammad, R.; Bedair, H. CD62P (P-selectin) expression as a platelet activation marker in patients with liver cirrhosis with and without cholestasis. Clin. Exp. Hepatol. 2021, 7, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Chai, S.; Zhang, F.; Lu, L. Overexpressed microRNA-103a-3p inhibits acute lower-extremity deep venous thrombosis via inhibition of CXCL12. IUBMB Life 2019, 72, 492–504. [Google Scholar] [CrossRef]
- Chen, D.; Liu, Y.; Liu, P.; Zhou, Y.; Jiang, L.; Yuan, C.; Huang, M. Orally delivered rutin in lipid-based nano-formulation exerts strong antithrombotic effects by protein disulfide isomerase inhibition. Drug Deliv. 2022, 29, 1824–1835. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Y.; Lu, F.; Chen, X.; Yang, D.; Cao, Y.; Zhang, W.; Chen, J.; Zheng, L.; Wang, G.; et al. Extracellular vesicles derived from oesophageal cancer containing P4HB promote muscle wasting via regulating PHGDH/Bcl-2/caspase-3 pathway. J. Extracell. Vesicles 2021, 10, e12060. [Google Scholar] [CrossRef]
- Kamarehei, M.; Pejman, S.; Ardestani, S.K.; Zahednasab, H.; Firouzi, M.; Harirchian, M.H. Inhibition of protein disulfide isomerase has neuroprotective effects in a mouse model of experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2020, 82, 106286. [Google Scholar] [CrossRef]
- Palta, S.; Saroa, R.; Palta, A. Overview of the coagulation system. Indian J. Anaesth. 2014, 58, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.M.; Reyes, L.; Duncan, R.M.; Bian, H.; Strobel, E.D.; Hyman, S.L.; Reitz, A.B.; Dolloff, N.G. Tuning isoform selectivity and bortezomib sensitivity with a new class of alkenyl indene PDI inhibitor. Eur. J. Med. Chem. 2019, 186, 111906. [Google Scholar] [CrossRef] [PubMed]
- Robinson, R.M.; Reyes, L.; Duncan, R.M.; Bian, H.; Reitz, A.B.; Manevich, Y.; McClure, J.J.; Champion, M.M.; Chou, C.J.; Sharik, M.E.; et al. Inhibitors of the protein disulfide isomerase family for the treatment of multiple myeloma. Leukemia 2018, 33, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Klaihmon, P.; Phongpao, K.; Kheansaard, W.; Noulsri, E.; Khuhapinant, A.; Fucharoen, S.; Morales, N.P.; Svasti, S.; Pattanapanyasat, K.; Chaichompoo, P. Microparticles from splenectomized β-thalassemia/HbE patients play roles on procoagulant activities with thrombotic potential. Ann. Hematol. 2016, 96, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Huang, X.; Zhu, M.; Zhang, S.; Zhang, Y. High concentration of the plasma microparticles for venous thromboembolism associated with lung cancer. Clin. Respir. J. 2020, 14, 481–487. [Google Scholar] [CrossRef]
- Koganti, S.; Eleftheriou, D.; Gurung, R.; Hong, Y.; Brogan, P.; Rakhit, R. Persistent circulating platelet and endothelial derived microparticle signature may explain on-going pro-thrombogenicity after acute coronary syndrome. Thromb. Res. 2021, 206, 60–65. [Google Scholar] [CrossRef]
- Swiatkowska, M.; Ponamarczuk, H.; Popielarski, M.; Stasiak, M.; Bednarek, R.; Studzian, M.; Pulaski, L.; Babinska, A. Contribution of activated beta3 integrin in the PDI release from endothelial cells. Front. Biosci. 2018, 23, 1612–1627. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, Y.; Wang, L.; Rauova, L.; Hayes, V.M.; Poncz, M.; Essex, D.W. The C-terminal CGHC motif of protein disulfide isomerase supports thrombosis. J. Clin. Investig. 2015, 125, 4391–4406. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Gao, J.; Liang, Z.; Yang, D. PDI-Regulated Disulfide Bond Formation in Protein Folding and Biomolecular Assembly. Molecules 2020, 26, 171. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Jha, V.; Min, J.-K.; Cho, J. Protein disulfide isomerase in cardiovascular disease. Exp. Mol. Med. 2020, 52, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Schulman, S.; Bendapudi, P.; Sharda, A.; Chen, V.; Bellido-Martin, L.; Jasuja, R.; Furie, B.C.; Flaumenhaft, R.; Furie, B. Extracellular Thiol Isomerases and Their Role in Thrombus Formation. Antioxidants Redox Signal. 2016, 24, 1–15. [Google Scholar] [CrossRef]
- Roher, N.; Miró, F.; Boldyreff, B.; Llorens, F.; Plana, M.; Issinger, O.-G.; Itarte, E. The C-terminal domain of human grp94 protects the catalytic subunit of protein kinase CK2 (CK2α) against thermal aggregation. JBIC J. Biol. Inorg. Chem. 2001, 268, 429–436. [Google Scholar] [CrossRef] [PubMed]

| Group Name | Length of Thrombi (mm) | p |
|---|---|---|
| DVT | 3.7 ± 3.6 | |
| DVT and PDI inhibitor | 0.8 ± 1.2 | 0.009 |
| DVT and GRP94 inhibitor | 1.4 ± 1.8 | 0.035 |
| DVT and rivaroxaban | 2.5 ± 2.1 | 0.295 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, H.; Tong, Z.; Jiao, Y.; Han, H.; Ma, Y.; Li, Y.; Jia, X.; Hu, B.; Zhang, W.; Zhong, M.; et al. Deep Vein Thrombosis Is Facilitated by Endothelial-Derived Extracellular Vesicles via the PDI–GRP94–GPIIb/IIIa Pathway in Mice. J. Clin. Med. 2023, 12, 4265. https://doi.org/10.3390/jcm12134265
Lan H, Tong Z, Jiao Y, Han H, Ma Y, Li Y, Jia X, Hu B, Zhang W, Zhong M, et al. Deep Vein Thrombosis Is Facilitated by Endothelial-Derived Extracellular Vesicles via the PDI–GRP94–GPIIb/IIIa Pathway in Mice. Journal of Clinical Medicine. 2023; 12(13):4265. https://doi.org/10.3390/jcm12134265
Chicago/Turabian StyleLan, Hongtao, Zhoujie Tong, Yaqiong Jiao, Haitao Han, Ying Ma, Yulin Li, Xu Jia, Boang Hu, Wei Zhang, Ming Zhong, and et al. 2023. "Deep Vein Thrombosis Is Facilitated by Endothelial-Derived Extracellular Vesicles via the PDI–GRP94–GPIIb/IIIa Pathway in Mice" Journal of Clinical Medicine 12, no. 13: 4265. https://doi.org/10.3390/jcm12134265
APA StyleLan, H., Tong, Z., Jiao, Y., Han, H., Ma, Y., Li, Y., Jia, X., Hu, B., Zhang, W., Zhong, M., & Wang, Z. (2023). Deep Vein Thrombosis Is Facilitated by Endothelial-Derived Extracellular Vesicles via the PDI–GRP94–GPIIb/IIIa Pathway in Mice. Journal of Clinical Medicine, 12(13), 4265. https://doi.org/10.3390/jcm12134265






