A Short-Term High-Fat Diet Worsens Insulin Sensitivity with Changes in Metabolic Parameters in Non-Obese Japanese Men
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
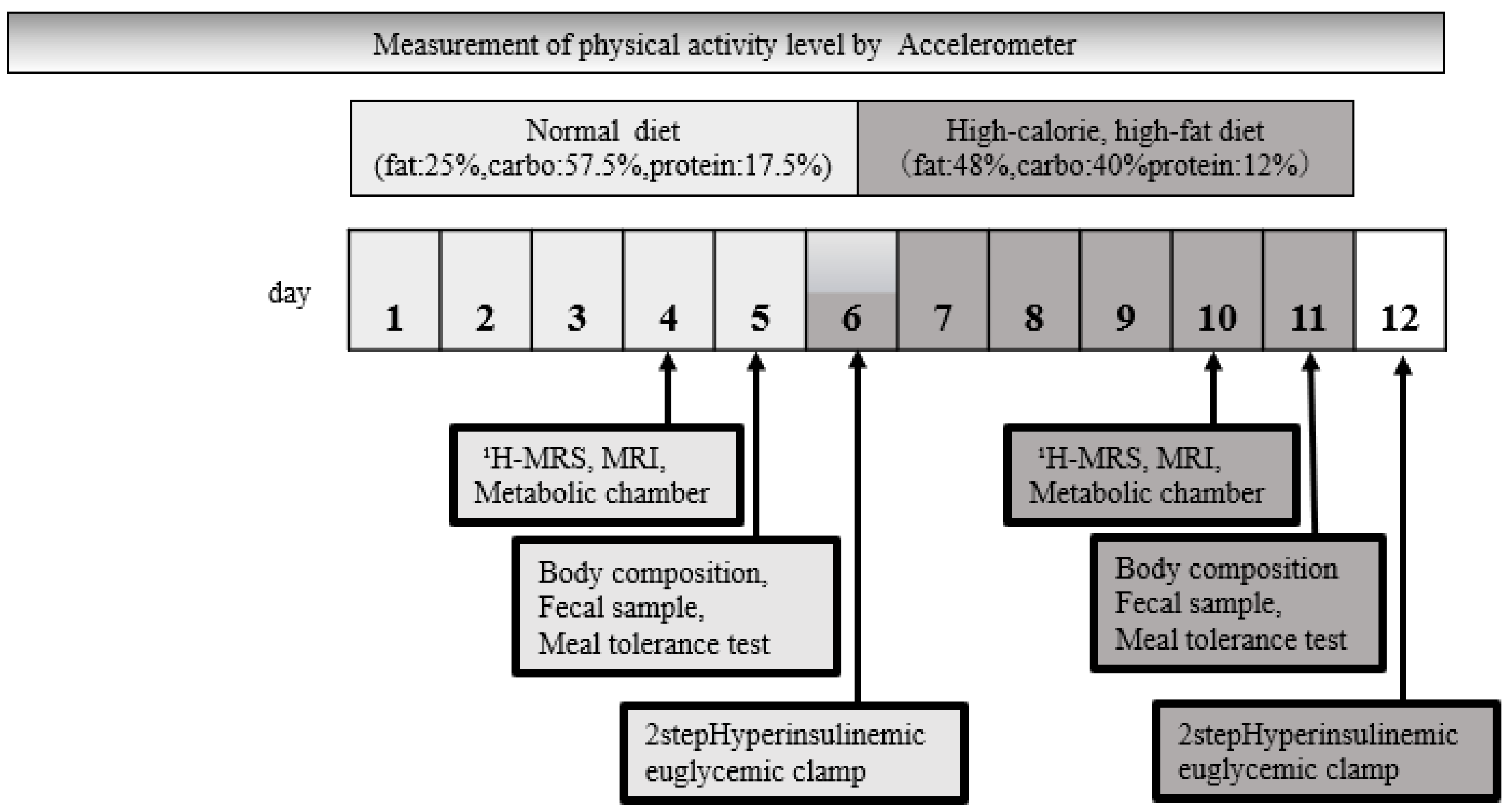
2.2. Study Design and Methods
2.3. Dietary and Physical Activity Manipulations
2.4. 1H-MRS and MRI
2.5. Two-Step Hyperinsulinemic Euglycemic Clamp Procedure
2.6. Determination of Tissue-Specific Insulin Sensitivity and MCRI with the Clamp Test
2.7. Biochemical Tests
2.8. Fecal Sample Evaluation
2.9. 16S Metagenomic Sequencing
2.10. Whole-Body Indirect Calorimetry
2.11. Meal Tolerance Test
2.12. Statistical Analysis
3. Results
3.1. Anthropometric and Metabolic Characteristics before and after Short-Term HCHFD
3.2. Change in Fat Distribution after Short-Term HCHFD
3.3. Insulin Sensitivity Evaluated with a Two-Step Hyperinsulinemic Euglycemic Clamp at Baseline and after Short-Term HCHFD
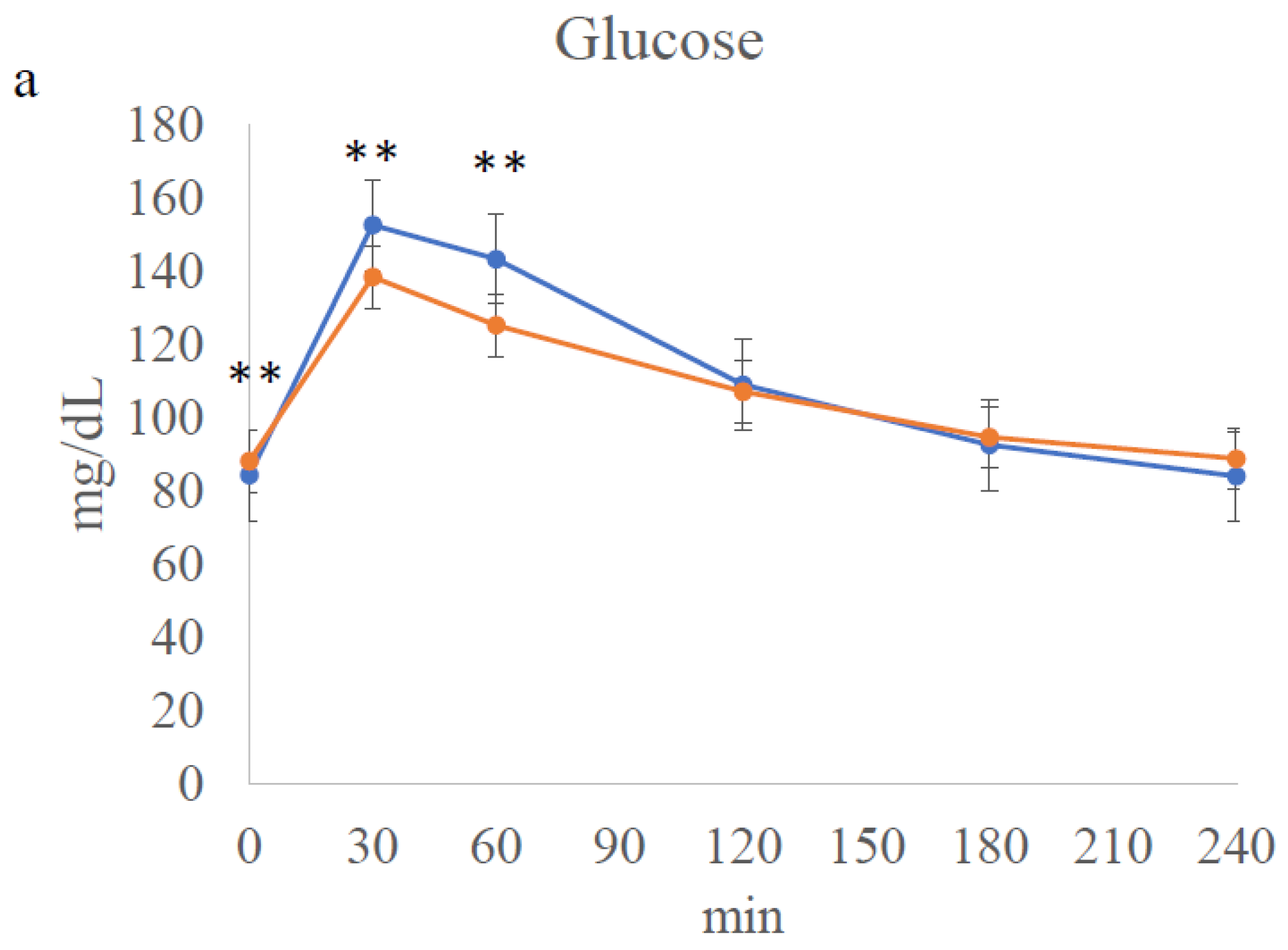
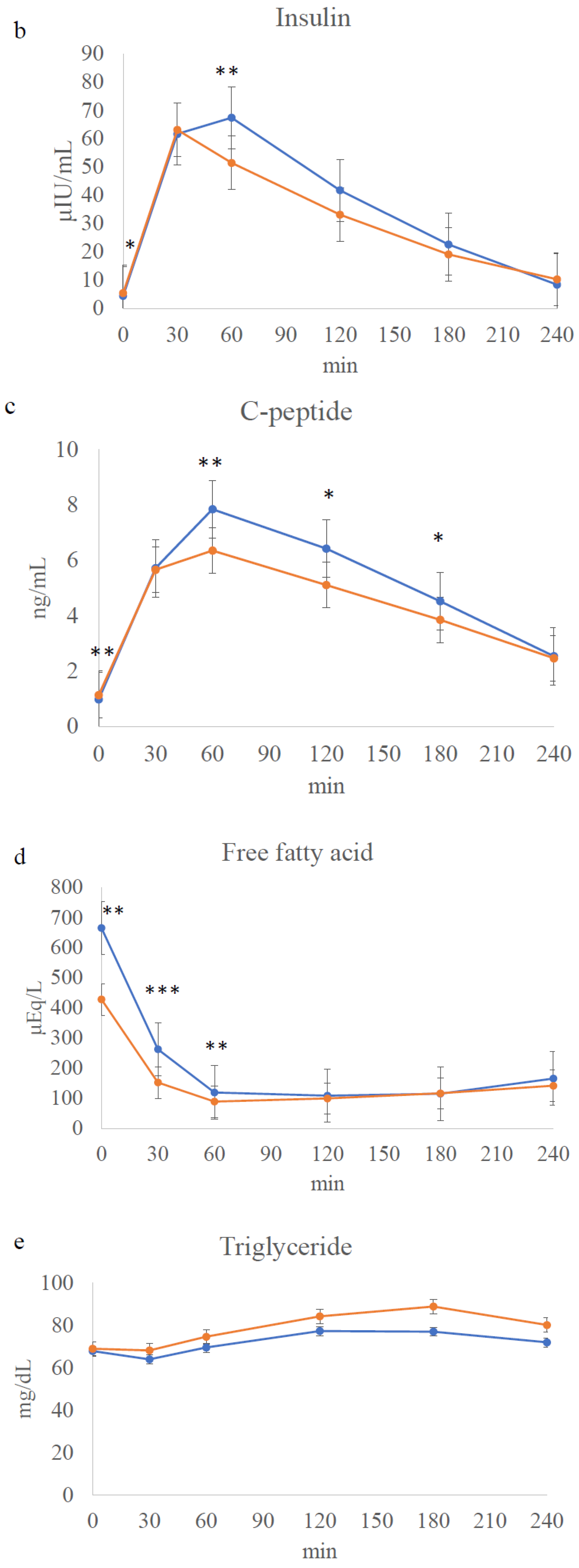
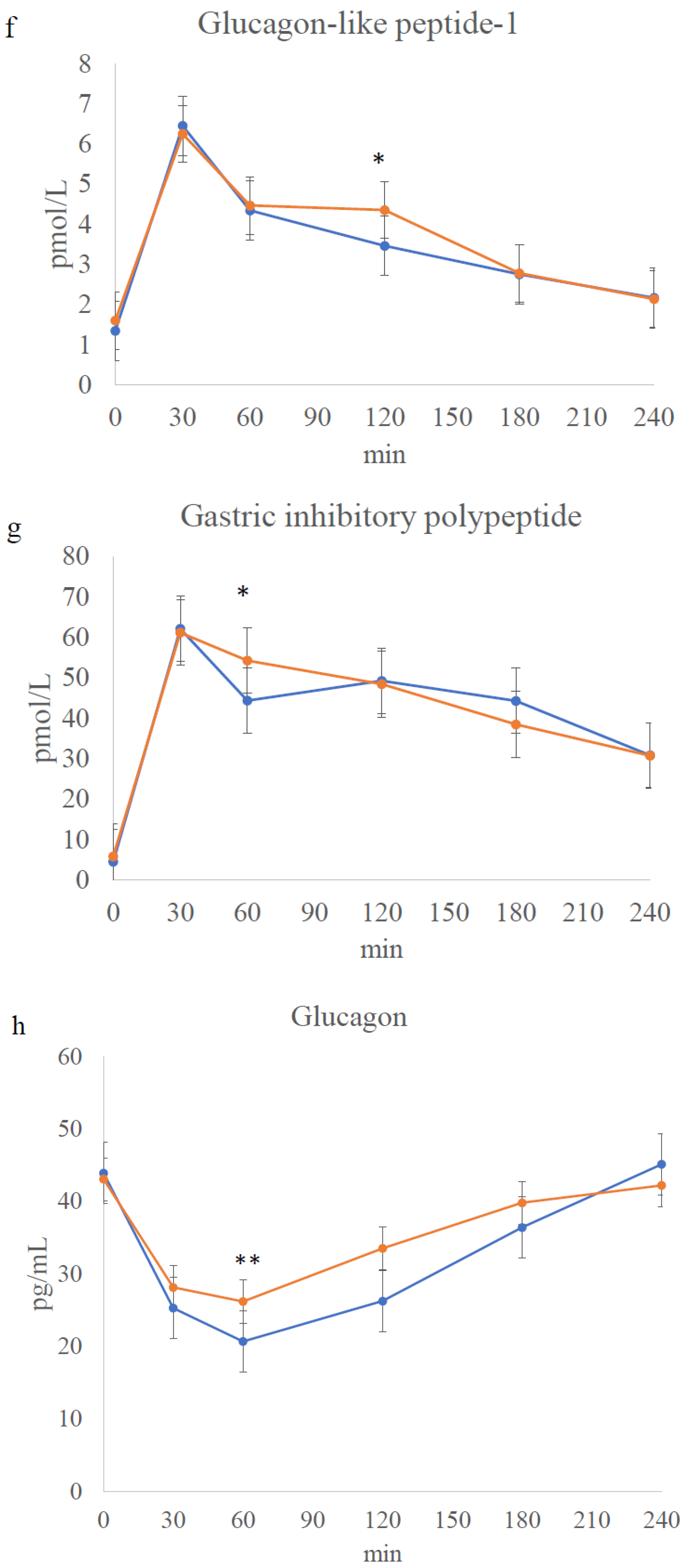
3.4. Meal Tolerance Test
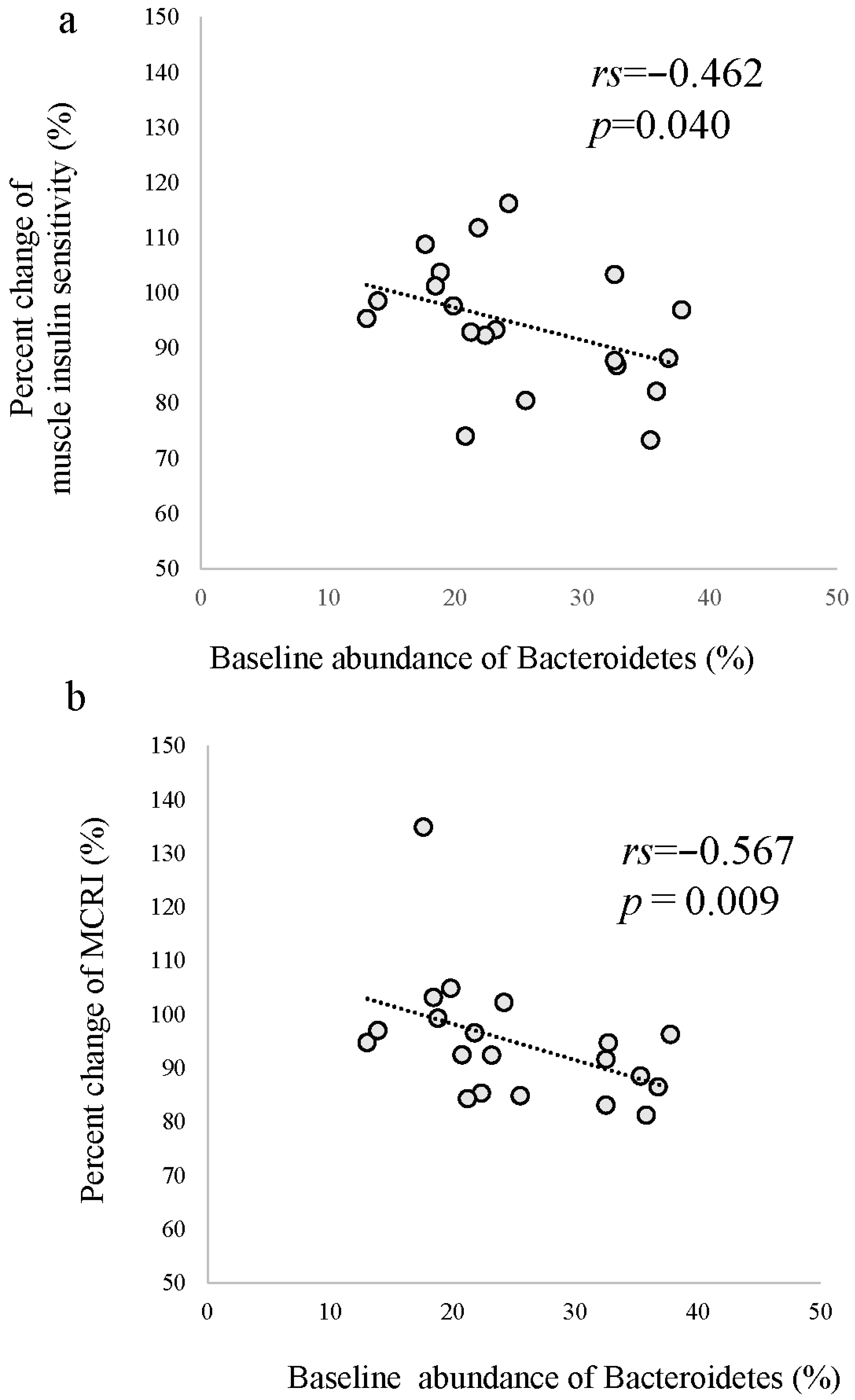
3.5. Effects of HCHFD on Gut Microbiota
3.6. Energy Metabolism Evaluated Using the Metabolic Chamber at Baseline and after HCHFD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, J.C.; Malik, V.; Jia, W.; Kadowaki, T.; Yajnik, C.S.; Yoon, K.H.; Hu, F.B. Diabetes in Asia: Epidemiology, risk factors, and pathophysiology. JAMA 2009, 301, 2129–2140. [Google Scholar] [CrossRef] [PubMed]
- Reaven, G.M. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988, 37, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Katsuki, A.; Sumida, Y.; Urakawa, H.; Gabazza, E.C.; Murashima, S.; Maruyama, N.; Morioka, K.; Nakatani, K.; Yano, Y.; Adachi, Y. Increased visceral fat and serum levels of triglyceride are associated with insulin resistance in Japanese metabolically obese, normal weight subjects with normal glucose tolerance. Diabetes Care 2003, 26, 2341–2344. [Google Scholar] [CrossRef]
- Takeno, K.; Tamura, Y.; Kawaguchi, M.; Kakehi, S.; Watanabe, T.; Funayama, T.; Furukawa, Y.; Kaga, H.; Yamamoto, R.; Kim, M.; et al. Relation between Insulin Sensitivity and Metabolic Abnormalities in Japanese Men with BMI of 23–25 kg/m2. J. Clin. Endocrinol. Metab. 2016, 101, 3676–3684. [Google Scholar] [CrossRef] [PubMed]
- Chooi, Y.C.; Ding, C.; Chan, Z.; Choo, J.; Sadananthan, S.A.; Michael, N.; Lee, Y.; Velan, S.S.; Magkos, F. Moderate Weight Loss Improves Body Composition and Metabolic Function in Metabolically Unhealthy Lean Subjects. Obesity 2018, 26, 1000–1007. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Chan, Z.; Chooi, Y.C.; Choo, J.; Sadananthan, S.A.; Chang, A.; Sasikala, S.; Michael, N.; Velan, S.S.; Magkos, F. Regulation of glucose metabolism in nondiabetic, metabolically obese normal-weight Asians. Am. J. Physiol.-Endocrinol. Metab. 2018, 314, E494–E502. [Google Scholar] [CrossRef]
- Micha, R.; Mozaffarian, D. Saturated fat and cardiometabolic risk factors, coronary heart disease, stroke, and diabetes: A fresh look at the evidence. Lipids 2010, 45, 893–905. [Google Scholar] [CrossRef]
- de Souza, R.J.; Mente, A.; Maroleanu, A.; Cozma, A.I.; Ha, V.; Kishibe, T.; Uleryk, E.; Budylowski, P.; Schünemann, H.; Beyene, J.; et al. Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: Systematic review and meta-analysis of observational studies. BMJ 2015, 351, h3978. [Google Scholar] [CrossRef]
- Funaki, M. Saturated fatty acids and insulin resistance. J. Med. Investig. 2009, 56, 88–92. [Google Scholar] [CrossRef]
- Bakker, L.E.; van Schinkel, L.D.; Guigas, B.; Streefland, T.C.; Jonker, J.T.; van Klinken, J.B.; van der Zon, G.C.; Lamb, H.J.; Smit, J.W.; Pijl, H.; et al. A 5-day high-fat, high-calorie diet impairs insulin sensitivity in healthy, young South Asian men but not in Caucasian men. Diabetes 2014, 63, 248–258. [Google Scholar] [CrossRef]
- Brons, C.; Jensen, C.B.; Storgaard, H.; Hiscock, N.J.; White, A.; Appel, J.S.; Jacobsen, S.; Nilsson, E.; Larsen, C.M.; Astrup, A.; et al. Impact of short-term high-fat feeding on glucose and insulin metabolism in young healthy men. J. Physiol. 2009, 587, 2387–2397. [Google Scholar] [CrossRef] [PubMed]
- Ott, B.; Skurk, T.; Lagkouvardos, L.; Fischer, S.; Buttner, J.; Lichtenegger, M.; Clavel, T.; Lechner, A.; Rychlik, M.; Haller, D.; et al. Short-Term Overfeeding with Dairy Cream Does Not Modify Gut Permeability, the Fecal Microbiota, or Glucose Metabolism in Young Healthy Men. J. Nutr. 2018, 148, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Adochio, R.L.; Leitner, J.W.; Gray, K.; Draznin, B.; Cornier, M.A. Early responses of insulin signaling to high-carbohydrate and high-fat overfeeding. Nutr. Metab. 2009, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Tai, E.S.; Lim, S.C.; Chew, S.K.; Tan, B.Y.; Tan, C.E. Homeostasis model assessment in a population with mixed ethnicity: The 1992 Singapore National Health Survey. Diabetes Res. Clin. Pract. 2000, 49, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Khoo, C.M.; Leow, M.K.; Sadananthan, S.A.; Lim, R.; Venkataraman, K.; Khoo, E.Y.; Velan, S.S.; Ong, Y.T.; Kambadur, R.; McFarlane, C.; et al. Body fat partitioning does not explain the interethnic variation in insulin sensitivity among Asian ethnicity: The Singapore adults metabolism study. Diabetes 2014, 63, 1093–1102. [Google Scholar] [CrossRef]
- Kanaya, A.M.; Herrington, D.; Vittinghoff, E.; Ewing, S.K.; Liu, K.; Blaha, M.J.; Dave, S.S.; Qureshi, F.; Kandula, N.R. Understanding the high prevalence of diabetes in U.S. south Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care 2014, 37, 1621–1628. [Google Scholar] [CrossRef]
- Ling, C.; Ronn, T. Epigenetics in Human Obesity and Type 2 Diabetes. Cell Metab. 2019, 29, 1028–1044. [Google Scholar] [CrossRef]
- Saad, M.J.; Santos, A.; Prada, P.O. Linking Gut Microbiota and Inflammation to Obesity and Insulin Resistance. Physiology 2016, 31, 283–293. [Google Scholar] [CrossRef]
- Kawano, Y.; Nakae, J.; Watanabe, N.; Kikuchi, T.; Tateya, S.; Tamori, Y.; Kaneko, M.; Abe, T.; Onodera, M.; Itoh, H. Colonic Pro-inflammatory Macrophages Cause Insulin Resistance in an Intestinal Ccl2/Ccr2-Dependent Manner. Cell Metab. 2016, 24, 295–310. [Google Scholar] [CrossRef]
- André, P.; Laugerette, F.; Féart, C. Metabolic Endotoxemia: A Potential Underlying Mechanism of the Relationship between Dietary Fat Intake and Risk for Cognitive Impairments in Humans? Nutrients 2019, 11, 1887. [Google Scholar] [CrossRef]
- Kakehi, S.; Tamura, Y.; Takeno, K.; Sakurai, Y.; Kawaguchi, M.; Watanabe, T.; Funayama, T.; Sato, F.; Ikeda, S.; Kanazawa, A.; et al. Increased intramyocellular lipid/impaired insulin sensitivity is associated with altered lipid metabolic genes in muscle of high responders to a high-fat diet. Am. J. Physiol.-Endocrinol. Metab. 2016, 310, E32–E40. [Google Scholar] [CrossRef]
- Kawaguchi, M.; Tamura, Y.; Kakehi, S.; Takeno, K.; Sakurai, Y.; Watanabe, T.; Funayama, T.; Sato, F.; Ikeda, S.; Ogura, Y.; et al. Association Between Expression of FABPpm in Skeletal Muscle and Insulin Sensitivity in Intramyocellular Lipid-Accumulated Nonobese Men. J. Clin. Endocrinol. Metab. 2014, 99, 3343–3352. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Campbell, L.V.; Xu, A.; Samocha-Bonet, D. Metabolically protective cytokines adiponectin and fibroblast growth factor-21 are increased by acute overfeeding in healthy humans. PLoS ONE 2013, 8, e78864. [Google Scholar] [CrossRef] [PubMed]
- Cahill, F.; Amini, P.; Wadden, D.; Khalili, S.; Randell, E.; Vasdev, S.; Gulliver, W.; Sun, G. Short-term overfeeding increases circulating adiponectin independent of obesity status. PLoS ONE 2013, 8, e74215. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ge, H.; Weiszmann, J.; Hecht, R.; Li, Y.S.; Véniant, M.M.; Xu, J.; Wu, X.; Lindberg, R.; Li, Y. Inhibition of lipolysis may contribute to the acute regulation of plasma FFA and glucose by FGF21 in ob/ob mice. FEBS Lett. 2009, 583, 3230–3234. [Google Scholar] [CrossRef] [PubMed]
- She, Q.Y.; Bao, J.F.; Wang, H.Z.; Liang, H.; Huang, W.; Wu, J.; Zhong, Y.; Ling, H.; Li, A.; Qin, S.L. Fibroblast growth factor 21: A “rheostat” for metabolic regulation? Metabolism 2022, 130, 155166. [Google Scholar] [CrossRef]
- Szczepanska, E.; Gietka-Czernel, M. FGF21: A Novel Regulator of Glucose and Lipid Metabolism and Whole-Body Energy Balance. Horm. Metab. Res. 2022, 54, 203–211. [Google Scholar] [CrossRef]
- McNamara, E.A.; Kilim, H.P.; Malabanan, A.O.; Whittaker, L.G.; Rosen, H.N. Enhanced Precision of the New Hologic Horizon Model Compared With the Old Discovery Model Is Less Evident When Fewer Vertebrae Are Included in the Analysis. J. Clin. Densitom. 2018, 21, 125–129. [Google Scholar] [CrossRef]
- Tamura, Y.; Tanaka, Y.; Sato, F.; Choi, J.B.; Watada, H.; Niwa, M.; Kinoshita, J.; Ooka, A.; Kumashiro, N.; Igarashi, Y.; et al. Effects of diet and exercise on muscle and liver intracellular lipid contents and insulin sensitivity in type 2 diabetic patients. J. Clin. Endocrinol. Metab. 2005, 90, 3191–3196. [Google Scholar] [CrossRef]
- Sato, F.; Tamura, Y.; Watada, H.; Kumashiro, N.; Igarashi, Y.; Uchino, H.; Maehara, T.; Kyogoku, S.; Sunayama, S.; Sato, H.; et al. Effects of diet-induced moderate weight reduction on intrahepatic and intramyocellular triglycerides and glucose metabolism in obese subjects. J. Clin. Endocrinol. Metab. 2007, 92, 3326–3329. [Google Scholar] [CrossRef]
- Kelley, D.E.; McKolanis, T.M.; Hegazi, R.A.; Kuller, L.H.; Kalhan, S.C. Fatty liver in type 2 diabetes mellitus: Relation to regional adiposity, fatty acids, and insulin resistance. Am. J. Physiol.-Endocrinol. Metab. 2003, 285, E906–E916. [Google Scholar] [CrossRef]
- Kotronen, A.; Seppälä-Lindroos, A.; Bergholm, R.; Yki-Järvinen, H. Tissue specificity of insulin resistance in humans: Fat in the liver rather than muscle is associated with features of the metabolic syndrome. Diabetologia 2008, 51, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Basu, R.; Barosa, C.; Jones, J.; Dube, S.; Carter, R.; Basu, A.; Rizza, R.A. Pathogenesis of prediabetes: Role of the liver in isolated fasting hyperglycemia and combined fasting and postprandial hyperglycemia. J. Clin. Endocrinol. Metab. 2013, 98, E409–E417. [Google Scholar] [CrossRef] [PubMed]
- Lindegaard, B.; Frosig, C.; Petersen, A.M.; Plomgaard, P.; Ditlevsen, S.; Mittendorfer, B.; Van Hall, G.; Wojtaszewski, J.F.; Pedersen, B.K. Inhibition of lipolysis stimulates peripheral glucose uptake but has no effect on endogenous glucose production in HIV lipodystrophy. Diabetes 2007, 56, 2070–2077. [Google Scholar] [CrossRef] [PubMed]
- Maggs, D.G.; Buchanan, T.A.; Burant, C.F.; Cline, G.; Gumbiner, B.; Hsueh, W.A.; Inzucchi, S.; Kelley, D.; Nolan, J.; Olefsky, J.M.; et al. Metabolic effects of troglitazone monotherapy in type 2 diabetes mellitus. A randomized, double-blind, placebo-controlled trial. Ann. Intern. Med. 1998, 128, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Tamura, Y.; Kakehi, S.; Funayama, T.; Gastaldelli, A.; Takeno, K.; Kawaguchi, M.; Yamamoto, R.; Sato, F.; Ikeda, S.; et al. Effects of sitagliptin on ectopic fat contents and glucose metabolism in type 2 diabetic patients with fatty liver: A pilot study. J. Diabetes Investig. 2015, 6, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Steele, R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann. N. Y. Acad. Sci. 1959, 82, 420–430. [Google Scholar] [CrossRef]
- Groop, L.C.; Bonadonna, R.C.; DelPrato, S.; Ratheiser, K.; Zyck, K.; Ferrannini, E.; DeFronzo, R.A. Glucose and free fatty acid metabolism in non-insulin-dependent diabetes mellitus. Evidence for multiple sites of insulin resistance. J. Clin. Investig. 1989, 84, 205–213. [Google Scholar] [CrossRef]
- Kotronen, A.; Juurinen, L.; Tiikkainen, M.; Vehkavaara, S.; Yki-Järvinen, H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology 2008, 135, 122–130. [Google Scholar] [CrossRef]
- Abdul-Ghani, M.; DeFronzo, R.A. Fasting hyperglycemia impairs glucose- but not insulin-mediated suppression of glucagon secretion. J. Clin. Endocrinol. Metab. 2007, 92, 1778–1784. [Google Scholar] [CrossRef]
- Kaga, H.; Tamura, Y.; Takeno, K.; Kakehi, S.; Funayama, T.; Furukawa, Y.; Nishitani-Yokoyama, M.; Shimada, K.; Daida, H.; Aoki, S.; et al. Correlates of insulin clearance in apparently healthy non-obese Japanese men. Sci. Rep. 2017, 7, 1462. [Google Scholar] [CrossRef] [PubMed]
- Takagi, T.; Naito, Y.; Inoue, R.; Kashiwagi, S.; Uchiyama, K.; Mizushima, K.; Tsuchiya, S.; Dohi, O.; Yoshida, N.; Kamada, K.; et al. Differences in gut microbiota associated with age, sex, and stool consistency in healthy Japanese subjects. J. Gastroenterol. 2019, 54, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Uchida, F.; Oh, S.; Shida, T.; Suzuki, H.; Yamagata, K.; Mizokami, Y.; Bukawa, H.; Tanaka, K.; Shoda, J. Effects of Exercise on the Oral Microbiota and Saliva of Patients with Non-Alcoholic Fatty Liver Disease. Int. J. Environ. Res. Public Health 2021, 18, 3470. [Google Scholar] [CrossRef]
- Katayose, Y.; Tasaki, M.; Ogata, H.; Nakata, Y.; Tokuyama, K.; Satoh, M. Metabolic rate and fuel utilization during sleep assessed by whole-body indirect calorimetry. Metabolism 2009, 58, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Tokuyama, K.; Ogata, H.; Katayose, Y.; Satoh, M. Algorithm for transient response of whole body indirect calorimeter: Deconvolution with a regularization parameter. J. Appl. Physiol. 2009, 106, 640–650. [Google Scholar] [CrossRef]
- Kobayashi, F.; Ogata, H.; Omi, N.; Nagasaka, S.; Yamaguchi, S.; Hibi, M.; Tokuyama, K. Effect of breakfast skipping on diurnal variation of energy metabolism and blood glucose. Obes. Res. Clin. Pract. 2014, 8, e249–e257. [Google Scholar] [CrossRef]
- Sato, M.; Nakamura, K.; Ogata, H.; Miyashita, A.; Nagasaka, S.; Omi, N.; Yamaguchi, S.; Hibi, M.; Umeda, T.; Nakaji, S.; et al. Acute effect of late evening meal on diurnal variation of blood glucose and energy metabolism. Obes. Res. Clin. Pract. 2011, 5, e220–e228. [Google Scholar] [CrossRef]
- Ferrannini, E. The theoretical bases of indirect calorimetry: A review. Metabolism 1988, 37, 287–301. [Google Scholar] [CrossRef]
- Yajima, K.; Iwayama, K.; Ogata, H.; Park, I.; Tokuyama, K. Meal rich in rapeseed oil increases 24-h fat oxidation more than meal rich in palm oil. PLoS ONE 2018, 13, e0198858. [Google Scholar] [CrossRef]
- Matsuda, M.; DeFronzo, R.A. Insulin sensitivity indices obtained from oral glucose tolerance testing: Comparison with the euglycemic insulin clamp. Diabetes Care 1999, 22, 1462–1470. [Google Scholar] [CrossRef]
- Rijkelijkhuizen, J.M.; Girman, C.J.; Mari, A.; Alssema, M.; Rhodes, T.; Nijpels, G.; Kostense, P.J.; Stein, P.P.; Eekhoff, E.M.; Heine, R.J.; et al. Classical and model-based estimates of beta-cell function during a mixed meal vs. an OGTT in a population-based cohort. Diabetes Res. Clin. Pract. 2009, 83, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Brodovicz, K.G.; Girman, C.J.; Simonis-Bik, A.M.; Rijkelijkhuizen, J.M.; Zelis, M.; Bunck, M.C.; Mari, A.; Nijpels, G.; Eekhoff, E.M.; Dekker, J.M. Postprandial metabolic responses to mixed versus liquid meal tests in healthy men and men with type 2 diabetes. Diabetes Res. Clin. Pract. 2011, 94, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, D.; Tamura, Y.; Takeno, K.; Kaga, H.; Someya, Y.; Kakehi, S.; Funayama, T.; Furukawa, Y.; Suzuki, R.; Kadowaki, S.; et al. Clinical Features of Nonobese, Apparently Healthy, Japanese Men With Reduced Adipose Tissue Insulin Sensitivity. J. Clin. Endocrinol. Metab. 2019, 104, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.P.B.; Texeira, T.F.S.; Ferreira, A.B.; Peluzio, M.D.C.G.; Alfenas, R.D.C.G. Influence of a high-fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br. J. Nutr. 2012, 108, 801–809. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Li, S.; Gan, R.Y.; Zhou, T.; Xu, D.P.; Li, H.B. Impacts of gut bacteria on human health and diseases. Int. J. Mol. Sci. 2015, 16, 7493–7519. [Google Scholar] [CrossRef]
- Kaga, H.; Tamura, Y.; Takeno, K.; Kakehi, S.; Someya, Y.; Funayama, T.; Furukawa, Y.; Suzuki, R.; Sugimoto, D.; Kadowaki, S.; et al. Higher C-Peptide Level During Glucose Clamp Is Associated With Muscle Insulin Resistance in Nonobese Japanese Men. J. Endocr. Soc. 2019, 3, 1847–1857. [Google Scholar] [CrossRef]
- Foley, K.P.; Zlitni, S.; Duggan, B.M.; Barra, N.G.; Anhê, F.F.; Cavallari, J.F.; Henriksbo, B.D.; Chen, C.Y.; Huang, M.; Lau, T.C.; et al. Gut microbiota impairs insulin clearance in obese mice. Mol. Metab. 2020, 42, 101067. [Google Scholar] [CrossRef]
- Huang, J.; Ledford, K.J.; Pitkin, W.B.; Russo, L.; Najjar, S.M.; Siragy, H.M. Targeted deletion of murine CEACAM 1 activates PI3K-Akt signaling and contributes to the expression of (Pro)renin receptor via CREB family and NF-κB transcription factors. Hypertension 2013, 62, 317–323. [Google Scholar] [CrossRef]
- Uchino, H.; Niwa, M.; Shimizu, T.; Nishiyama, K.; Kawamori, R. Impairment of early insulin response after glucose load, rather than insulin resistance, is responsible for postprandial hyperglycemia seen in obese type 2 diabetes: Assessment using nateglinide, a new insulin secretagogue. Endocr. J. 2000, 47, 639–641. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kadowaki, T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int. J. Obes. 2008, 32 (Suppl. 7), S13–S18. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and adiponectin receptors in insulin resistance, diabetes, and the metabolic syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef] [PubMed]
- Flippo, K.H.; Potthoff, M.J. Metabolic Messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Ukropcova, B.; Sereda, O.; de Jonge, L.; Bogacka, I.; Nguyen, T.; Xie, H.; Bray, G.A.; Smith, S.R. Family history of diabetes links impaired substrate switching and reduced mitochondrial content in skeletal muscle. Diabetes 2007, 56, 720–727. [Google Scholar] [CrossRef]
- Valentine, J.M.; Ahmadian, M.; Keinan, O.; Abu-Odeh, M.; Zhao, P.; Zhou, X.; Keller, M.P.; Gao, H.; Yu, R.T.; Liddle, C.; et al. β3-Adrenergic receptor downregulation leads to adipocyte catecholamine resistance in obesity. J. Clin. Investig. 2022, 132, e153357. [Google Scholar] [CrossRef]
- Rattarasarn, C. Dysregulated lipid storage and its relationship with insulin resistance and cardiovascular risk factors in non-obese Asian patients with type 2 diabetes. Adipocyte 2018, 7, 71–80. [Google Scholar] [CrossRef]
- Arai, H.; Yamamoto, A.; Matsuzawa, Y.; Saito, Y.; Yamada, N.; Oikawa, S.; Mabuchi, H.; Teramoto, T.; Sasaki, J.; Nakaya, N.; et al. Prevalence of metabolic syndrome in the general Japanese population in 2000. J. Atheroscler. Thromb. 2006, 13, 202–208. [Google Scholar] [CrossRef]
- Nazare, J.A.; Smith, J.D.; Borel, A.L.; Haffner, S.M.; Balkau, B.; Ross, R.; Massien, C.; Alméras, N.; Després, J.P. Ethnic influences on the relations between abdominal subcutaneous and visceral adiposity, liver fat, and cardiometabolic risk profile: The International Study of Prediction of Intra-Abdominal Adiposity and Its Relationship With Cardiometabolic Risk/Intra-Abdominal Adiposity. Am. J. Clin. Nutr. 2012, 96, 714–726. [Google Scholar] [CrossRef]
- Azuma, K.; Kadowaki, T.; Cetinel, C.; Kadota, A.; El-Saed, A.; Kadowaki, S.; Edmundowicz, D.; Nishio, Y.; Sutton-Tyrrell, K.; Okamura, T.; et al. Higher liver fat content among Japanese in Japan compared with non-Hispanic whites in the United States. Metabolism 2009, 58, 1200–1207. [Google Scholar] [CrossRef]

| Baseline | After HCHFD | p Value | |
|---|---|---|---|
| Body weight (kg) | 61.6 ± 6.1 | 62.0 ± 6.1 | 0.021 |
| Fat free mass (kg) | 52.8 ± 5.3 | 53.4 ± 5.4 | 0.015 |
| Body mass index (kg/m2) | 20.6 ± 1.8 | 20.7 ± 1.7 | 0.019 |
| Fat mass (kg) | 6.7 ± 1.4 | 6.6 ± 1.5 | 0.727 |
| Percent body fat (%) | 13.1 ± 3.1 | 12.7 ± 3.1 | 0.116 |
| Serum insulin (μIU/mL) | 4.7 ± 1.2 | 5.5 ± 2.4 | 0.274 |
| C-peptide (ng/mL) | 1.1 ± 0.2 | 1.2 ± 0.3 | 0.099 |
| Plasma glucose (mg/dL) | 86.6 ± 5.6 | 90.5 ± 5.4 | 0.012 |
| Free fatty acids (μEq/L) | 665.0 ± 229.4 | 377.3 ± 180.6 | <0.001 |
| Total cholesterol (mg/dL) | 152.8 ± 19.7 | 161.0 ± 23.2 | 0.022 |
| High-density lipoprotein cholesterol (mg/dL) | 52.6 ± 11.0 | 59.3 ± 11.0 | 0.001 |
| Low-density lipoprotein cholesterol (mg/dL) | 88.2 ± 19.6 | 92.0 ± 21.6 | 0.163 |
| Triglycerides (mg/dL) | 66.5 ± 14.8 | 70.5 ± 28.4 | 0.412 |
| Aspartate aminotransferase (U/L) | 18.7 ± 5.2 | 18.4 ± 5.2 | 0.689 |
| Alanine aminotransferase (U/L) | 15.6 ± 6.5 | 17.4 ± 6.9 | 0.095 |
| Alkaline phosphatase (U/L) | 189.5 ± 44.4 | 203.6 ± 47.0 | 0.073 |
| γ-Glutamyl transpeptidase (U/L) | 15.9 ± 3.9 | 14.6 ± 3.2 | 0.007 |
| Total bile acid (μmol/L) | 5.4 ± 5.0 | 7.7 ± 8.5 | 0.040 |
| Acetoacetic acid (μmol/L) | 29.5 ± 23.3 | 19.1 ± 11.5 | 0.021 |
| 3-hydroxyacetic acid (μmol/L) | 82.6 ± 72.4 | 33.2 ± 23.4 | <0.001 |
| Total ketone bodies (μmol/L) | 112.1 ± 95.3 | 52.3 ± 34.2 | 0.002 |
| Total adiponectin (μg/mL) | 5.37 ± 2.15 | 5.80 ± 2.20 | 0.004 |
| High-molecular-weight adiponectin (μg/mL) | 1.93 ± 1.30 | 2.14 ± 1.42 | 0.021 |
| FGF-21 (pg/mL) | 107.5 ± 60.0 | 169.7 ± 107.4 | 0.001 |
| Interleukin 6 (pg/mL) | 1.2 ± 2.3 | 1.2 ± 1.7 | 0.984 |
| Leptin (ng/mL) | 4.5 ± 1.6 | 5.0 ± 1.7 | 0.048 |
| Monocyte chemoattractant protein-1 (pg/mL) | 200.1 ± 46.7 | 200.3 ± 32.8 | 0.768 |
| LBP (μg/mL) | 4.9 ± 0.7 | 5.6 ± 0.7 | 0.006 |
| High-sensitivity CRP (ng/mL) | 157.0 ± 308.8 | 224.3 ± 228.6 | 0.073 |
| Tumor necrosis factor alpha (pg/mL) | 0.70 ± 0.18 | 0.68 ± 0.16 | 0.365 |
| Basal metabolic rate (kcal/min) | 0.94 ± 0.12 | 0.95 ± 0.12 | 0.509 |
| Basal RQ | 0.89 ± 0.08 | 0.92 ± 0.05 | 0.103 |
| Sleep energy expenditure (kcal/min) | 0.87 ± 0.09 | 0.88 ± 0.09 | 0.407 |
| Sleep RQ | 0.83 ± 0.03 | 0.87 ± 0.04 | <0.001 |
| Baseline | After HCHFD | p Value | |
|---|---|---|---|
| Intrahepatic lipid (%) | 0.5 ± 0.4 | 1.5 ± 1.0 | <0.001 |
| Intramyocellular lipid-TA (S-fat/Cre) | 1.7 ± 1.2 | 2.5 ± 1.3 | 0.009 |
| Intramyocellular lipid-SOL (S-fat/Cre) | 5.2 ± 2.7 | 6.8 ± 2.7 | 0.005 |
| Abdominal visceral fat area (cm2) | 46.0 ± 11.2 | 46.6 ± 10.2 | 0.476 |
| Abdominal subcutaneous fat area (cm2) | 71.7 ± 26.4 | 73.7 ± 26.8 | 0.217 |
| SSSI during the first step (μU/mL) | 17.1 ± 2.1 | 18.4 ± 2.7 | 0.019 |
| SSsc during the first step (ng/mL) | 0.72 ± 0.25 | 0.82 ± 0.32 | 0.019 |
| SSSI during the second step (μU/mL) | 31.5 ± 4.1 | 34.4 ± 4.9 | 0.002 |
| SSsc during the second step (ng/mL) | 0.60 ± 0.27 | 0.73 ± 0.28 | 0.006 |
| MCRI during the second step (mL/min per m2) | 692.4 ± 94.1 | 653.1 ± 99.7 | 0.004 |
| Basal EGP (mg/m2/min) | 76.9 ± 3.4 | 79.4 ± 4.7 | 0.023 |
| % reduction in EGP during the first step (%/μU) | 75.2 ± 11.6 | 73.5 ± 13.6 | 0.419 |
| % reduction in EGP/SSSI during the first step (%/μU·mL−1) | 4.46 ± 0.85 | 4.09 ± 0.97 | 0.044 |
| Rd during the second step (mg/kg FFM/min−1) | 7.49 ± 1.20 | 7.67 ± 1.29 | 0.268 |
| Rd/SSSI during the second step (mg/kg FFM·min−1/μU·mL−1) | 0.24 ± 0.06 | 0.23 ± 0.05 | 0.042 |
| Adipose tissue insulin resistance index | 3209.5 ± 1519.6 | 2345.1 ± 2012.5 | 0.023 |
| Baseline | After HCHFD | p Value | |
|---|---|---|---|
| AUC-glucose (mg/dL × min × 103) | 26.9 ± 27.0 | 25.9 ± 2.7 | 0.131 |
| AUC-FFA (μEq/L × min × 103) | 41.6 ± 8.2 | 32.2 ± 6.3 | <0.001 |
| AUC-insulin (IU/mL × min × 103) | 9.0 ± 3.8 | 7.7 ± 2.7 | 0.039 |
| AUC-CPR (ng/mL × min × 103) | 1.3 ± 0.3 | 1.1 ± 0.3 | 0.007 |
| AUC-glucagon (pg/mL × min × 103) | 7.4 ± 2.8 | 8.3 ± 3.1 | 0.140 |
| AUC-GIP (pmol/L × min × 103) | 10.5 ± 2.8 | 10.5 ± 3.0 | 0.986 |
| AUC-GLP-1 (pmol/L × min × 103) | 0.85 ± 0.38 | 0.90 ± 0.40 | 0.339 |
| Insulin sensitivity index (comp) | 10.0 ± 5.5 | 9.5 ± 4.6 | 0.794 |
| Insulinogenic index | 0.9 ± 0.3 | 1.3 ± 0.6 | <0.001 |
| Insulinogenic index (c-peptide) | 0.072 ± 0.022 | 0.101 ± 0.049 | <0.001 |
| Baseline | After HCHFD | p Value | |
|---|---|---|---|
| Actinobacteria (%) | 6.89 ± 4.86 | 9.73 ± 5.66 | 0.005 |
| Bacteroidetes (%) | 25.19 ± 7.88 | 25.79 ± 9.00 | 0.970 |
| Firmicutes (%) | 63.05 ± 9.72 | 60.91 ± 11.14 | 0.232 |
| Fusobacteria (%) | 1.75 ± 2.76 | 1.41 ± 2.59 | 0.156 |
| Proteobacteria (%) | 2.82 ± 3.33 | 2.16 ± 1.04 | 0.970 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kadowaki, S.; Tamura, Y.; Sugimoto, D.; Kaga, H.; Suzuki, R.; Someya, Y.; Yamasaki, N.; Sato, M.; Kakehi, S.; Kanazawa, A.; et al. A Short-Term High-Fat Diet Worsens Insulin Sensitivity with Changes in Metabolic Parameters in Non-Obese Japanese Men. J. Clin. Med. 2023, 12, 4084. https://doi.org/10.3390/jcm12124084
Kadowaki S, Tamura Y, Sugimoto D, Kaga H, Suzuki R, Someya Y, Yamasaki N, Sato M, Kakehi S, Kanazawa A, et al. A Short-Term High-Fat Diet Worsens Insulin Sensitivity with Changes in Metabolic Parameters in Non-Obese Japanese Men. Journal of Clinical Medicine. 2023; 12(12):4084. https://doi.org/10.3390/jcm12124084
Chicago/Turabian StyleKadowaki, Satoshi, Yoshifumi Tamura, Daisuke Sugimoto, Hideyoshi Kaga, Ruriko Suzuki, Yuki Someya, Nozomu Yamasaki, Motonori Sato, Saori Kakehi, Akio Kanazawa, and et al. 2023. "A Short-Term High-Fat Diet Worsens Insulin Sensitivity with Changes in Metabolic Parameters in Non-Obese Japanese Men" Journal of Clinical Medicine 12, no. 12: 4084. https://doi.org/10.3390/jcm12124084
APA StyleKadowaki, S., Tamura, Y., Sugimoto, D., Kaga, H., Suzuki, R., Someya, Y., Yamasaki, N., Sato, M., Kakehi, S., Kanazawa, A., Kawamori, R., & Watada, H. (2023). A Short-Term High-Fat Diet Worsens Insulin Sensitivity with Changes in Metabolic Parameters in Non-Obese Japanese Men. Journal of Clinical Medicine, 12(12), 4084. https://doi.org/10.3390/jcm12124084






