Effect of Bifidobacterium bifidum G9-1 on the Intestinal Environment and Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-like Symptoms in Patients with Quiescent Crohn’s Disease: A Prospective Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
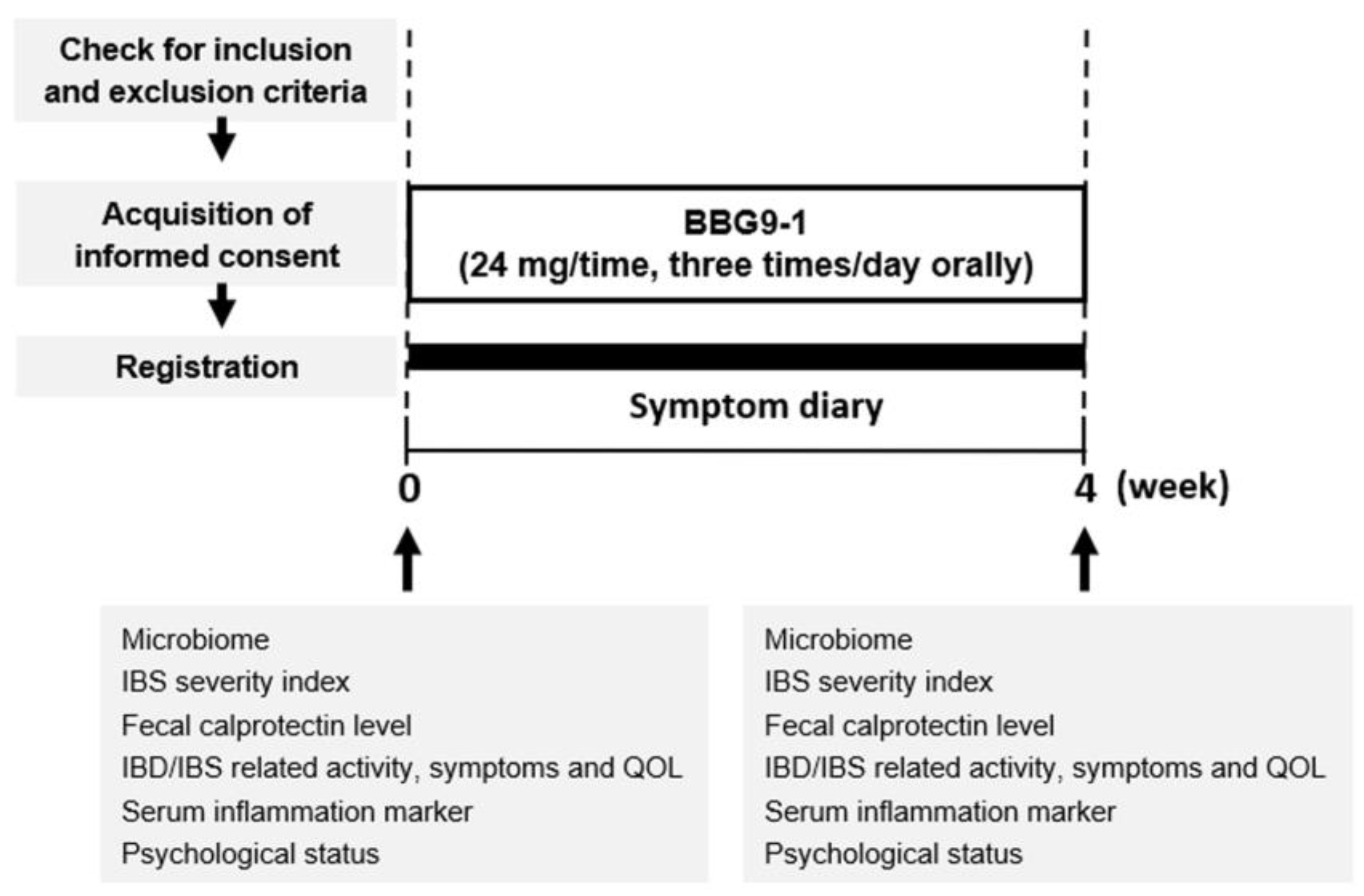
2.2. Study Design
2.3. Questionnaires
2.4. Measurement of the Fecal Calprotectin Level
2.5. Analysis of the Gut Microbiome
2.6. Statistical Analyses
3. Results
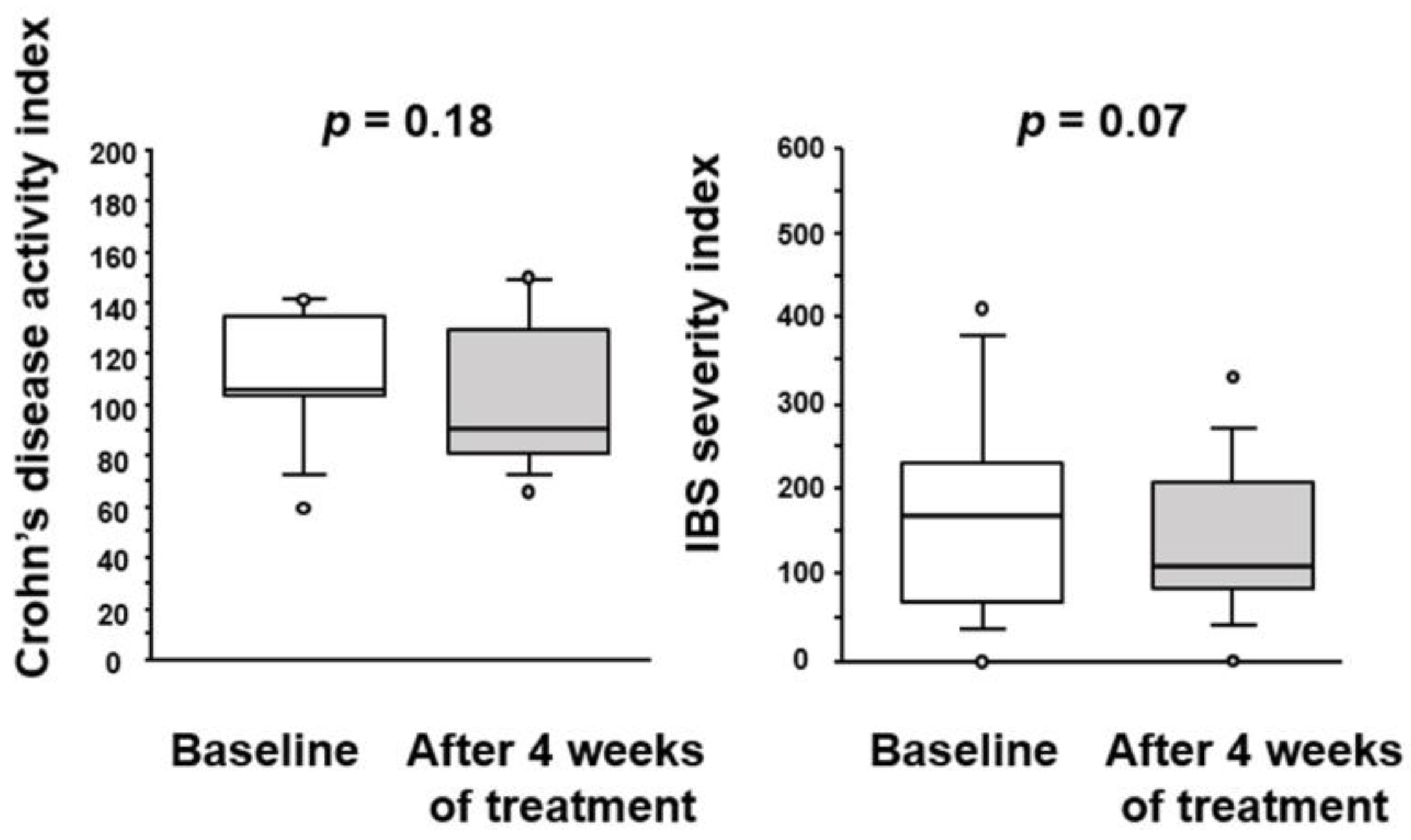
3.1. Effect of BBG9-1 on CD Activity Index and IBS Severity Index in Patients with Quiescent CD Patients and IBS-D-like Symptoms
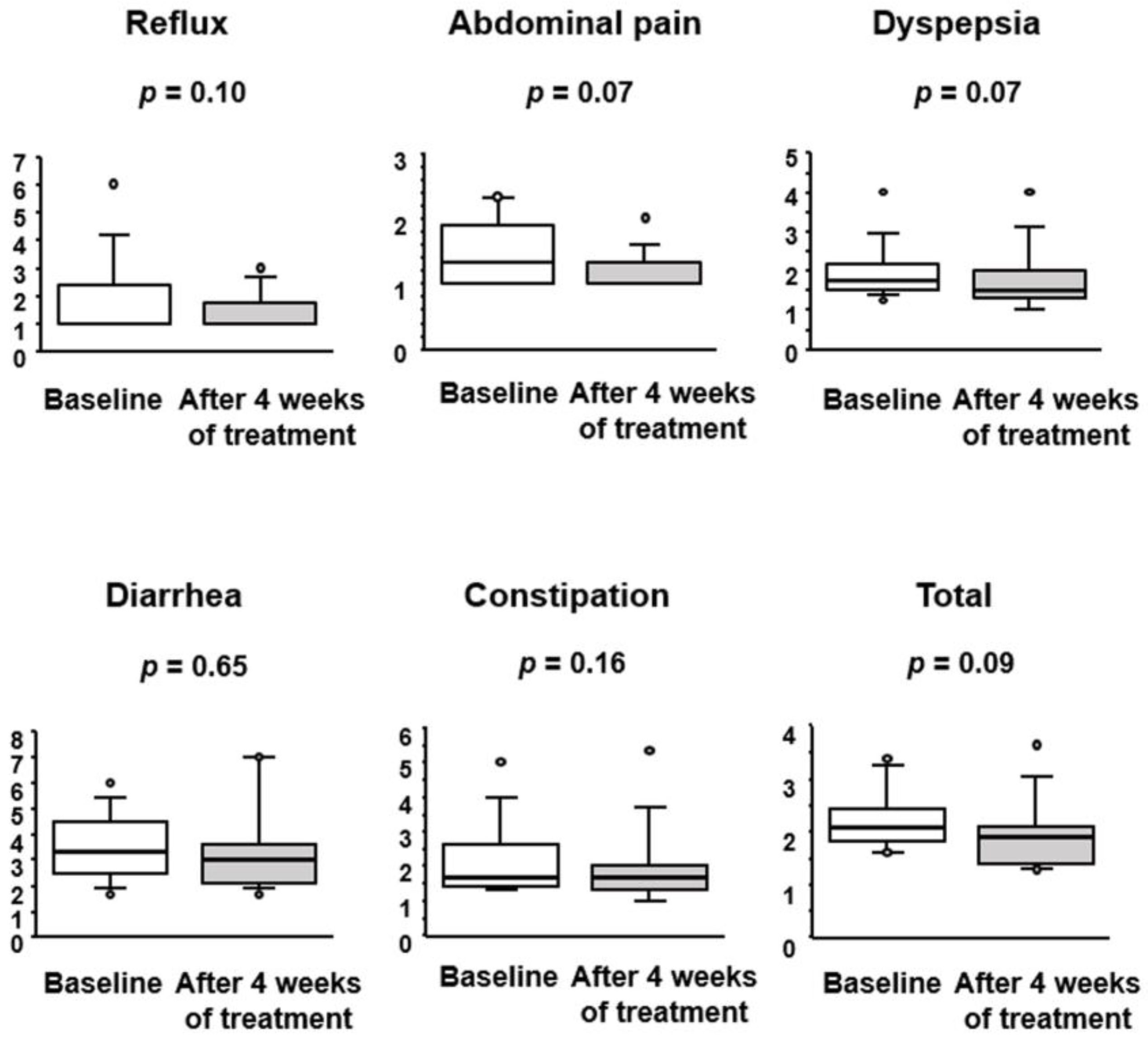
3.2. Effect of BBG9-1 on Gastrointestinal Symptoms in Patients with Quiescent CD and IBS-D-like Symptoms
3.3. Effect of BBG9-1 on Disease-Specific and Health-Related Quality of Life in Patients with Quiescent CD and IBS-D-like Symptoms
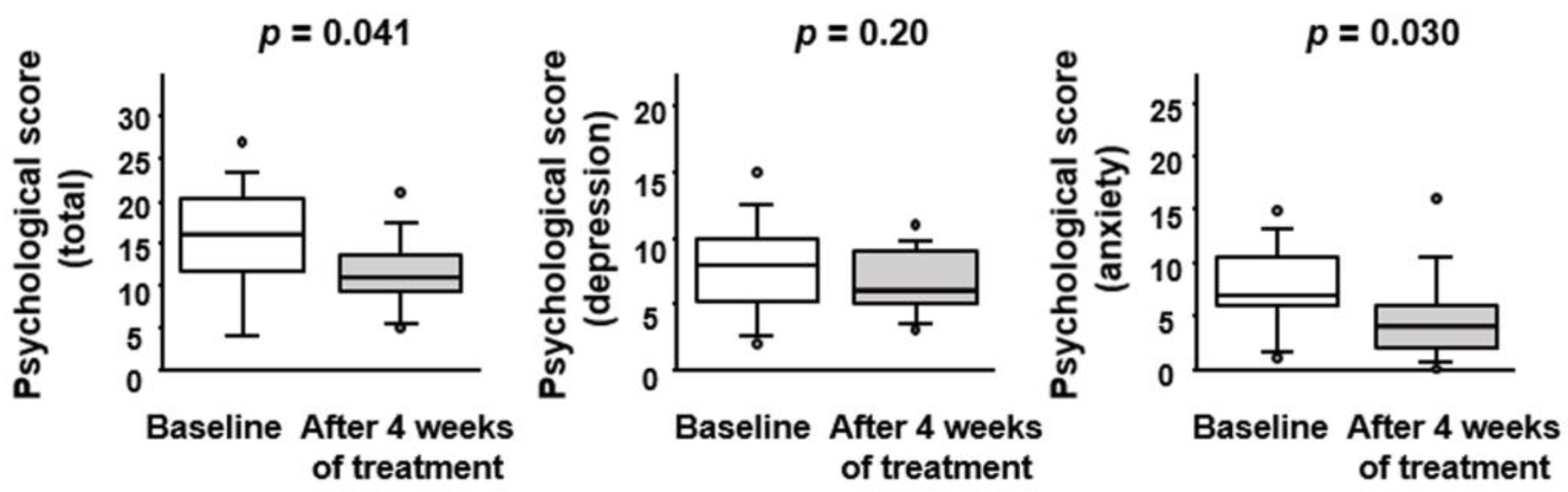
3.4. Effect of BBG9-1 on Depression and Anxiety Status in Patients with Quiescent CD and IBS-D-like Symptoms
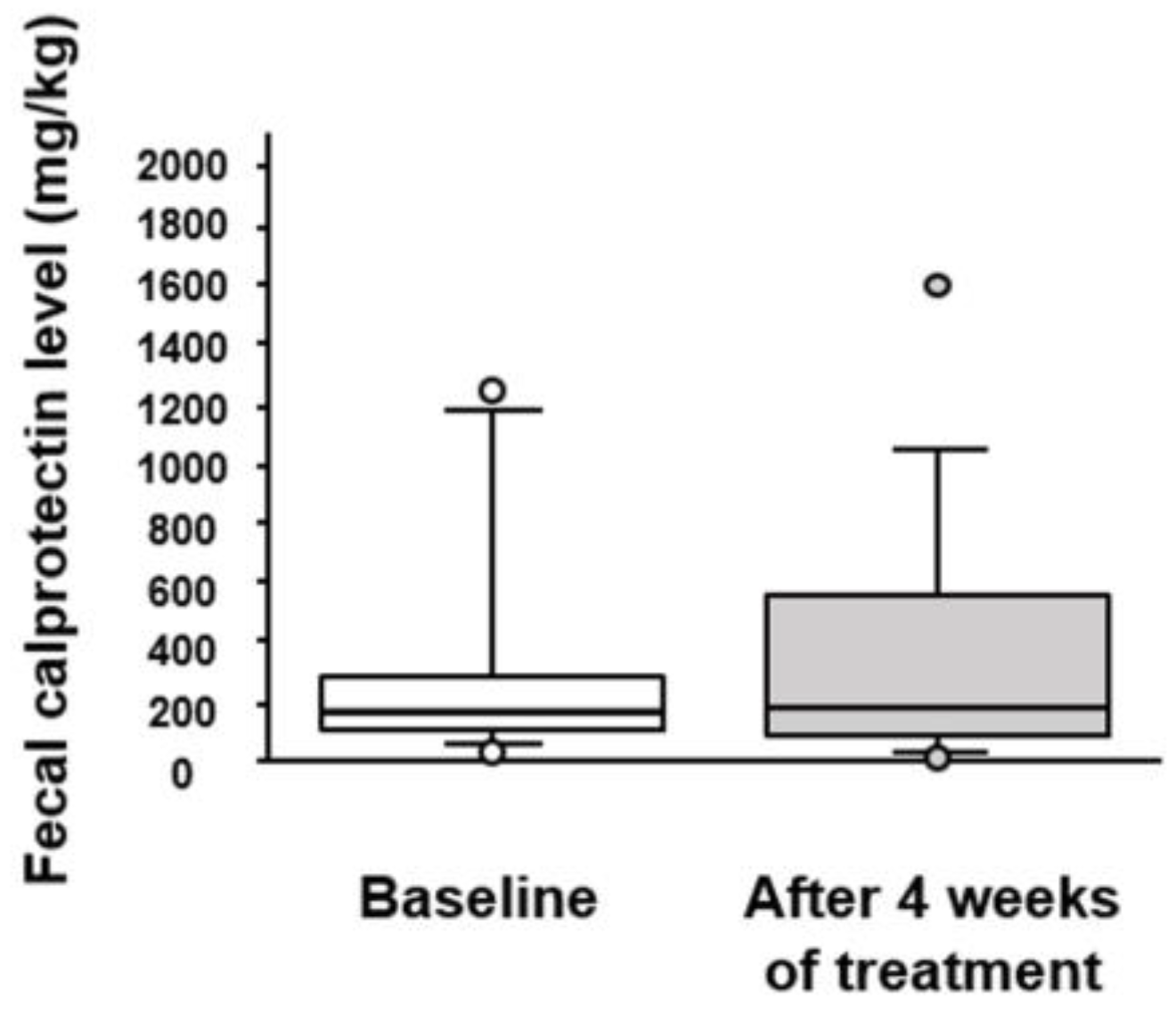
3.5. Effect of BBG9-1 on Fecal Calprotectin and Serum Cytokines Levels in Patients with Quiescent CD and IBS-D-like Symptoms
3.6. Effect of BBG9-1 on the Fecal Microbiome in Patients with qCD and IBS-D-like Symptoms
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, D.H.; Cheon, J.H. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017, 17, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Simrén, M.; Axelsson, J.; Gillberg, R.; Abrahamsson, H.; Svedlund, J.; Björnsson, E.S. Quality of life in inflammatory bowel disease in remission: The impact of IBS-like symptoms and associated psychological factors. Am. J. Gastroenterol. 2002, 97, 389–396. [Google Scholar] [CrossRef] [PubMed]
- Tomita, T.; Kato, Y.; Takimoto, M.; Yamasaki, T.; Kondo, T.; Kono, T.; Tozawa, K.; Yokoyama, Y.; Ikehara, H.; Ohda, Y.; et al. Prevalence of irritable bowel syndrome-like symptoms in Japanese patients with inactive inflammatory bowel disease. J. Neurogastroenterol. Motil. 2016, 22, 661–669. [Google Scholar] [CrossRef] [PubMed]
- Gracie, D.J.; Williams, C.J.; Sood, R.; Mumtaz, S.; Bholah, M.H.; Hamlin, P.J.; Ford, A.C. Negative effects on psychological health and quality of life of genuine irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease. Clin. Gastroenterol. Hepatol. 2017, 15, 376–384.e5. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef]
- Vivinus-Nébot, M.; Frin-Mathy, G.; Bzioueche, H.; Dainese, R.; Bernard, G.; Anty, R.; Filippi, J.; Saint-Paul, M.C.; Tulic, M.K.; Verhasselt, V.; et al. Functional bowel symptoms in quiescent inflammatory bowel diseases: Role of epithelial barrier disruption and low-grade inflammation. Gut 2014, 63, 744–752. [Google Scholar] [CrossRef]
- Keohane, J.; O’Mahony, C.; O’Mahony, L.; O’Mahony, S.; Quigley, E.M.; Shanahan, F. Irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease: A real association or reflection of occult inflammation? Am. J. Gastroenterol. 2010, 105, 1789–1794. [Google Scholar] [CrossRef]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Quigley, E.M. Overlapping irritable bowel syndrome and inflammatory bowel disease: Less to this than meets the eye? Ther. Adv. Gastroenterol. 2016, 9, 199–212. [Google Scholar] [CrossRef]
- Tomita, T.; Fukui, H.; Morishita, D.; Maeda, A.; Makizaki, Y.; Tanaka, Y.; Ohno, H.; Oshima, T.; Miwa, H. Diarrhea-predominant irritable bowel syndrome-like symptoms in patients with quiescent Crohn’s disease: Comprehensive analysis of clinical features and intestinal environment including the gut microbiome, organic acids, and intestinal permeability. J. Neurogastroenterol. Motil. 2023, 29, 102–112. [Google Scholar] [CrossRef]
- Perera, L.P.; Radigan, M.; Guilday, C.; Banerjee, I.; Eastwood, D.; Babygirija, R.; Massey, B.T. Presence of irritable bowel syndrome symptoms in quiescent inflammatory bowel disease is associated with high rate of anxiety and depression. Dig. Dis. Sci. 2019, 64, 1923–1928. [Google Scholar] [CrossRef] [PubMed]
- Fairbrass, K.M.; Costantino, S.J.; Gracie, D.J.; Ford, A.C. Prevalence of irritable bowel syndrome-type symptoms in patients with inflammatory bowel disease in remission: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Walmsley, R.S.; Ayres, R.C.; Pounder, R.E.; Allan, R.N. A simple clinical colitis activity index. Gut 1998, 43, 29–32. [Google Scholar] [CrossRef]
- Tomita, T.; Fukui, H.; Morishita, D.; Mori, S.; Oshima, T.; Shinzaki, S.; Miwa, H. Efficacy of serotonin type 3 receptor antagonist ramosetron on diarrhea-predominant irritable bowel syndrome (IBS-D)-like symptoms in patients with quiescent inflammatory bowel disease: A randomized, double-blind, placebo-controlled trial. J. Clin. Med. 2022, 11, 6882. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, M.; Nakajima, S.; Oshima, T.; Whitehead, W.E.; Sperber, A.D.; Palsson, O.S.; Drossman, D.A.; Miwa, H.; Fukudo, S. Validity and reliability of the Japanese version of the Rome III diagnostic questionnaire for irritable bowel syndrome and functional dyspepsia. J. Neurogastroenterol. Motil. 2015, 21, 537–544. [Google Scholar] [CrossRef]
- Atia, O.; Goren, I.; Fischler, T.S.; Weisband, Y.L.; Greenfeld, S.; Kariv, R.; Ledderman, N.; Matz, E.; Rimon, R.M.; Dotan, I.; et al. 5-aminosalicylate maintenance is not superior to no maintenance in patients with newly diagnosed Crohn’s disease-A nationwide cohort study. Aliment. Pharm. 2023, 57, 1004–1013. [Google Scholar] [CrossRef]
- Shinozaki, M.; Kanazawa, M.; Sagami, Y.; Endo, Y.; Hongo, M.; Drossman, D.A.; Whitehead, W.E.; Fukudo, S. Validation of the Japanese version of the Rome II modular questionnaire and irritable bowel syndrome severity index. J. Gastroenterol. 2006, 41, 491–494. [Google Scholar] [CrossRef]
- Kanazawa, M.; Drossman, D.A.; Shinozaki, M.; Sagami, Y.; Endo, Y.; Palsson, O.S.; Hongo, M.; Whitehead, W.E.; Fukudo, S. Translation and validation of a Japanese version of the irritable bowel syndrome-quality of life measure (IBS-QOL-J). Biopsychosoc. Med. 2007, 1, 6. [Google Scholar] [CrossRef]
- Dimenäs, E.; Glise, H.; Hallerbäck, B.; Hernqvist, H.; Svedlund, J.; Wiklund, I. Quality of life in patients with upper gastrointestinal symptoms. An improved evaluation of treatment regimens? Scand. J. Gastroenterol. 1993, 28, 681–687. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Dewey, J.E.; Gandek, B. How to Score and Interpret Single-Item Health Status Measures: A Manual for Users of the SF-8TM Health Survey; QualityMetric Incorporated: Johnston, RI, USA, 2001. [Google Scholar]
- Guyatt, G.; Mitchell, A.; Irvine, E.J.; Singer, J.; Williams, N.; Goodacre, R.; Tompkins, C. A new measure of health status for clinical trials in inflammatory bowel disease. Gastroenterology 1989, 96, 804–810. [Google Scholar] [CrossRef]
- Russel, M.G.; Pastoor, C.J.; Brandon, S.; Rijken, J.; Engels, L.G.; van der Heijde, D.M.; Stockbrügger, R.W. Validation of the Dutch translation of the Inflammatory Bowel Disease Questionnaire (IBDQ): A health-related quality of life questionnaire in inflammatory bowel disease. Digestion 1997, 58, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Snaith, R.P. The hospital anxiety and depression scale. Health Qual. Life Outcomes 2003, 1, 29. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Nishida, A.; Matsuda, S.; Kira, F.; Watanabe, S.; Kuriyama, M.; Kawakami, K.; Aikawa, Y.; Oda, N.; Arai, K.; et al. Usefulness of machine learning-based gut microbiome analysis for identifying patients with irritable bowels syndrome. J. Clin. Med. 2020, 9, 2403. [Google Scholar] [CrossRef]
- Ozer, M.; Bengi, G.; Colak, R.; Cengiz, O.; Akpinar, H. Prevalence of irritable bowel syndrome-like symptoms using Rome IV criteria in patients with inactive inflammatory bowel disease and relation with quality of life. Medicine 2020, 99, e20067. [Google Scholar] [CrossRef]
- Fukui, H.; Xu, X.; Miwa, H. Role of gut microbiota-gut hormone axis in the pathophysiology of functional gastrointestinal disorders. J. Neurogastroenterol. Motil. 2018, 24, 367–386. [Google Scholar] [CrossRef]
- Lynch, S.V.; Pedersen, O. The human intestinal microbiome in health and disease. N. Engl. J. Med. 2016, 375, 2369–2379. [Google Scholar] [CrossRef]
- Sood, A.; Midha, V.; Makharia, G.K.; Ahuja, V.; Singal, D.; Goswami, P.; Tandon, R.K. The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin. Gastroenterol. Hepatol. 2009, 7, 1202–1209. [Google Scholar]
- Sisson, G.; Ayis, S.; Sherwood, R.A.; Bjarnason, I. Randomised clinical trial: A liquid multi-strain probiotic vs. placebo in the irritable bowel syndrome—A 12 week double-blind study. Aliment. Pharm. 2014, 40, 51–62. [Google Scholar] [CrossRef]
- Ford, A.C.; Harris, L.A.; Lacy, B.E.; Quigley, E.M.M.; Moayyedi, P. Systematic review with meta-analysis: The efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment. Pharm. 2018, 48, 1044–1060. [Google Scholar] [CrossRef]
- Kruis, W.; Fric, P.; Pokrotnieks, J.; Lukás, M.; Fixa, B.; Kascák, M.; Kamm, M.A.; Weismueller, J.; Beglinger, C.; Stolte, M.; et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut 2004, 53, 1617–1623. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, L.; McCarthy, J.; Kelly, P.; Hurley, G.; Luo, F.; Chen, K.; O’Sullivan, G.C.; Kiely, B.; Collins, J.K.; Shanahan, F.; et al. Lactobacillus and bifidobacterium in irritable bowel syndrome: Symptom responses and relationship to cytokine profiles. Gastroenterology 2005, 128, 541–551. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.M.; Stason, W.B.; Legedza, A.; Ock, S.M.; Kaptchuk, T.J.; Conboy, L.; Canenguez, K.; Park, J.K.; Kelly, E.; Jacobson, E.; et al. The placebo effect in irritable bowel syndrome trials: A meta-analysis. Neurogastroenterol. Motil. 2005, 17, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Fukui, H.; Oshima, T.; Tanaka, Y.; Oikawa, Y.; Makizaki, Y.; Ohno, H.; Tomita, T.; Watari, J.; Miwa, H. Effect of probiotic Bifidobacterium bifidum G9-1 on the relationship between gut microbiota profile and stress sensitivity in maternally separated rats. Sci. Rep. 2018, 8, 12384. [Google Scholar] [CrossRef]
- Wang, X.; Fukui, H.; Ran, Y.; Xu, X.; Ebisutani, N.; Nakanishi, T.; Tanaka, Y.; Maeda, A.; Makizaki, Y.; Tomita, T.; et al. Probiotic Bifidobacterium bifidum G9-1 has a preventive effect on the acceleration of colonic permeability and M1 macrophage population in maternally separated rats. Biomedicines 2021, 9, 641. [Google Scholar] [CrossRef]
- Makizaki, Y.; Maeda, A.; Oikawa, Y.; Tamura, S.; Tanaka, Y.; Nakajima, S.; Ohno, H.; Yamamura, H. Probiotic Bifidobacterium bifidum G9-1 ameliorates phytohemagglutinin-induced diarrhea caused by intestinal dysbiosis. Microbiol. Immunol. 2019, 63, 481–486. [Google Scholar] [CrossRef]
- Chang, L. The role of stress on physiologic responses and clinical symptoms in irritable bowel syndrome. Gastroenterology 2011, 140, 761–765. [Google Scholar] [CrossRef]
- Margolis, K.G.; Cryan, J.F.; Mayer, E.A. The microbiota-gut-brain axis: From motility to mood. Gastroenterology 2021, 160, 1486–1501. [Google Scholar] [CrossRef]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; Donald, D.M.; Dietrich, D.; Ramadhar, T.T.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Joyce, S.A.; MacSharry, J.; Casey, P.G.; Kinsella, M.; Murphy, E.F.; Shanahan, F.; Hill, C.; Gahan, C.G. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc. Natl. Acad. Sci. USA 2014, 111, 7421–7426. [Google Scholar] [CrossRef]
- Ridlon, J.M.; Harris, S.C.; Bhowmik, S.; Kang, D.J.; Hylemon, P.B. Consequences of bile salt biotransformations by intestinal bacteria. Gut Microbes 2016, 7, 22–39. [Google Scholar] [CrossRef] [PubMed]
- Pavlidis, P.; Powell, N.; Vincent, R.P.; Ehrlich, D.; Bjarnason, I.; Hayee, B. Systematic review: Bile acids and intestinal inflammation-luminal aggressors or regulators of mucosal defence? Aliment. Pharm. 2015, 42, 802–817. [Google Scholar] [CrossRef] [PubMed]
- Di Vincenzo, F.; Puca, P.; Lopetuso, L.R.; Petito, V.; Masi, L.; Bartocci, B.; Murgiano, M.; De Felice, M.; Petronio, L.; Gasbarrini, A.; et al. Bile acid-related regulation of mucosal inflammation and intestinal motility: From pathogenesis to therapeutic application in IBD and microscopic colitis. Nutrients 2022, 14, 2664. [Google Scholar] [CrossRef]
- Jia, B.; Zou, Y.; Han, X.; Bae, J.W.; Jeon, C.O. Gut microbiome-mediated mechanisms for reducing cholesterol levels: Implications for ameliorating cardiovascular disease. Trends Microbiol. 2023, 31, 76–91. [Google Scholar] [CrossRef] [PubMed]
- Horáčková, Š.; Plocková, M.; Demnerová, K. Importance of microbial defence systems to bile salts and mechanisms of serum cholesterol reduction. Biotechnol. Adv. 2018, 36, 682–690. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1907272. [Google Scholar] [CrossRef]
- Li, L.; Li, T.; Gu, Y.; Wang, X.; Xie, R.; Sun, Y.; Wang, B.; Cao, H. Regulation of gut microbiota-bile acids axis by probiotics in inflammatory bowel disease. Front. Immunol. 2022, 13, 974305. [Google Scholar] [CrossRef]
- De Nardo, D.; Labzin, L.I.; Kono, H.; Seki, R.; Schmidt, S.V.; Beyer, M.; Xu, D.; Zimmer, S.; Lahrmann, C.; Schildberg, F.A.; et al. High-density lipoprotein mediates anti-inflammatory reprogramming of macrophages via the transcriptional regulator ATF3. Nat. Immunol. 2014, 15, 152–160. [Google Scholar] [CrossRef]
- Wurfel, M.M.; Kunitake, S.T.; Lichenstein, H.; Kane, J.P.; Wright, S.D. Lipopolysaccharide (LPS)-binding protein is carried on lipoproteins and acts as a cofactor in the neutralization of LPS. J. Exp. Med. 1994, 180, 1025–1035. [Google Scholar] [CrossRef]
- Corsetti, M.; Tack, J. FDA and EMA end points: Which outcome end points should we use in clinical trials in patients with irritable bowel syndrome? Neurogastroenterol. Motil. 2013, 25, 453–457. [Google Scholar] [CrossRef]


| Age (years) | 47.3 ± 10.5 (31–71) |
| Sex (male/female) | 8/3 |
| BMI (kg/m2) | 21.3 ± 4.0 (15.4–29.8) |
| Duration of disease (years) | 19.6 ± 7.7 (8–33) |
| Current medications (%) | |
| 5-ASA | 6 (54.5) |
| Steroids | 0 (0.0) |
| Immunomodulator | 0 (0.0) |
| Biologics therapy | 1 (9.1) |
| Elemental diet | 10 (90.9) |
| Prior surgery (%) | 8 (72.7) |
| C-reactive protein (mg/dL) | 0.08 ± 0.10 (0.00–0.24) |
| Crohn’s disease activity index | 110.4 ± 25.4 (59.2–141.1) |
| Cytokines (pg/mL) | Baseline | After 4-Weeks-Treatment | p Value |
|---|---|---|---|
| IL-1b | 0.01 ± 0.04 | 0.02 ± 0.07 | 0.75 |
| IL-2 | 0.08 ± 0.27 | 0.00 ± 0.00 | 0.34 |
| IL-4 | 0.00 ± 0.00 | 0.01 ± 0.02 | 0.34 |
| IL-5 | 0.00 ± 0.00 | 0.00 ± 0.00 | – |
| IL-6 | 0.58 ± 0.71 | 0.40 ± 0.81 | 0.53 |
| IL-7 | 0.39 ± 0.66 | 0.64 ± 1.31 | 0.51 |
| IL-8 | 8.63 ± 6.59 | 4.64 ± 2.95 | 0.08 |
| IL-10 | 0.00 ± 0.00 | 0.07 ± 0.22 | 0.35 |
| IL-12 | 0.06 ± 0.21 | 0.25 ± 0.83 | 0.50 |
| IL-13 | 0.00 ± 0.00 | 0.00 ± 0.00 | – |
| IL-17 | 0.07 ± 0.21 | 0.01 ± 0.02 | 0.35 |
| G-CSF | 0.31 ± 0.89 | 0.31 ± 0.89 | 1.00 |
| GM-CSF | 0.00 ± 0.00 | 0.00 ± 0.00 | – |
| IFN-γ | 0.35 ± 0.33 | 0.26 ± 0.21 | 0.13 |
| MCP-1 | 7.47 ± 6.71 | 4.46 ± 3.73 | 0.048 |
| MIP-1b | 16.84 ± 9.91 | 11.68 ± 5.70 | 0.08 |
| TNF-α | 3.22 ± 4.10 | 2.74 ± 2.31 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tomita, T.; Fukui, H.; Okugawa, T.; Nakanishi, T.; Mieno, M.; Nakai, K.; Eda, H.; Kitayama, Y.; Oshima, T.; Shinzaki, S.; et al. Effect of Bifidobacterium bifidum G9-1 on the Intestinal Environment and Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-like Symptoms in Patients with Quiescent Crohn’s Disease: A Prospective Pilot Study. J. Clin. Med. 2023, 12, 3368. https://doi.org/10.3390/jcm12103368
Tomita T, Fukui H, Okugawa T, Nakanishi T, Mieno M, Nakai K, Eda H, Kitayama Y, Oshima T, Shinzaki S, et al. Effect of Bifidobacterium bifidum G9-1 on the Intestinal Environment and Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-like Symptoms in Patients with Quiescent Crohn’s Disease: A Prospective Pilot Study. Journal of Clinical Medicine. 2023; 12(10):3368. https://doi.org/10.3390/jcm12103368
Chicago/Turabian StyleTomita, Toshihiko, Hirokazu Fukui, Takuya Okugawa, Takashi Nakanishi, Masatoshi Mieno, Keisuke Nakai, Hirotsugu Eda, Yoshitaka Kitayama, Tadayuki Oshima, Shinichiro Shinzaki, and et al. 2023. "Effect of Bifidobacterium bifidum G9-1 on the Intestinal Environment and Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-like Symptoms in Patients with Quiescent Crohn’s Disease: A Prospective Pilot Study" Journal of Clinical Medicine 12, no. 10: 3368. https://doi.org/10.3390/jcm12103368
APA StyleTomita, T., Fukui, H., Okugawa, T., Nakanishi, T., Mieno, M., Nakai, K., Eda, H., Kitayama, Y., Oshima, T., Shinzaki, S., & Miwa, H. (2023). Effect of Bifidobacterium bifidum G9-1 on the Intestinal Environment and Diarrhea-Predominant Irritable Bowel Syndrome (IBS-D)-like Symptoms in Patients with Quiescent Crohn’s Disease: A Prospective Pilot Study. Journal of Clinical Medicine, 12(10), 3368. https://doi.org/10.3390/jcm12103368










