The Prevalence of Irritable Bowel Syndrome after Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Their Association: A Systematic Review and Meta-Analysis of Observational Studies
Abstract
1. Introduction
2. Methods
2.1. Eligibility Criteria
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Synthesis and Statistical Analysis
3. Results
3.1. Study Selection
3.2. Study Characteristics
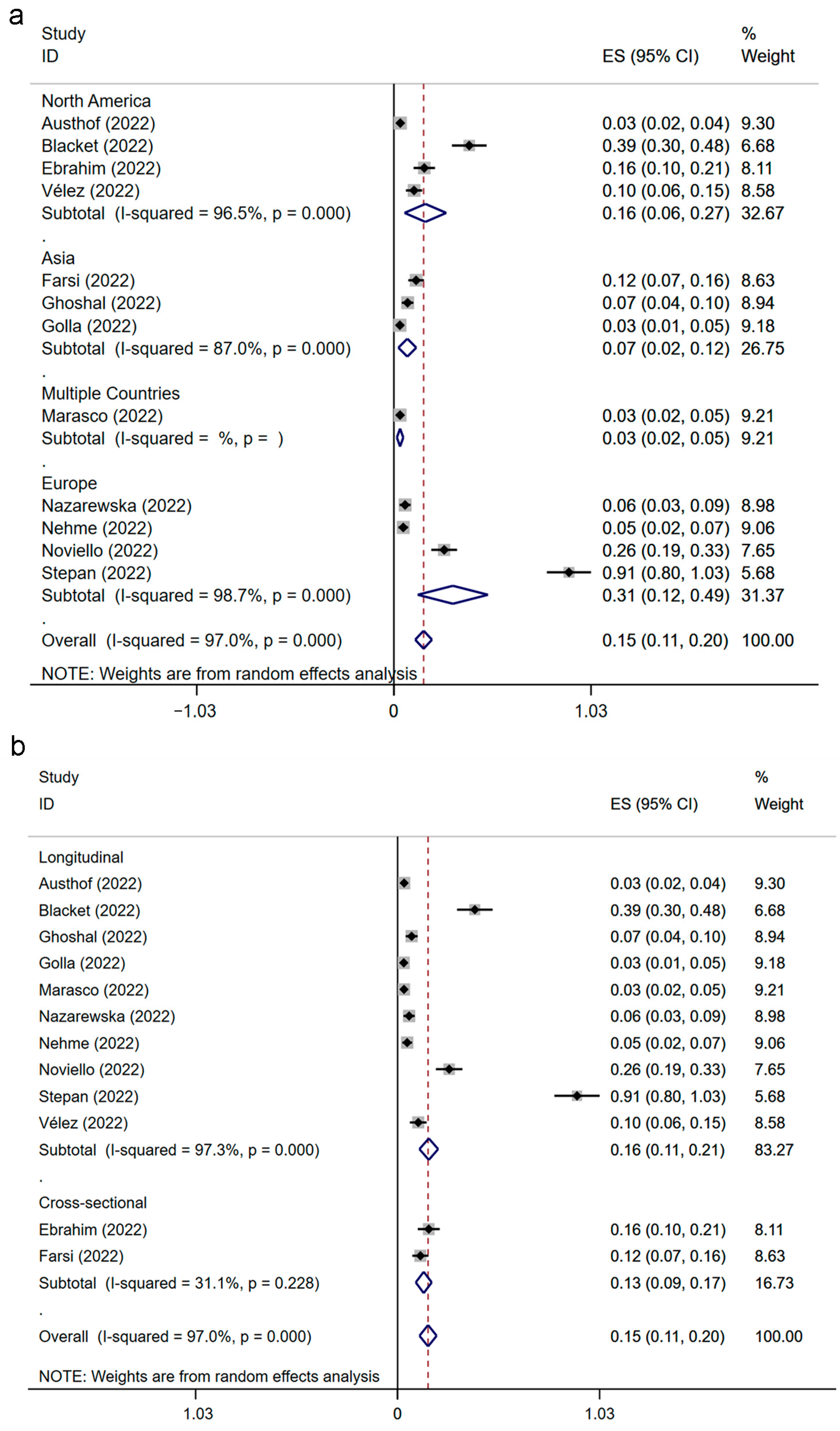
3.3. Prevalence of IBS Following SARS-CoV-2 Infection
3.4. Prevalence of IBS following SARS-CoV-2 Infection Classified by Region and Study Design
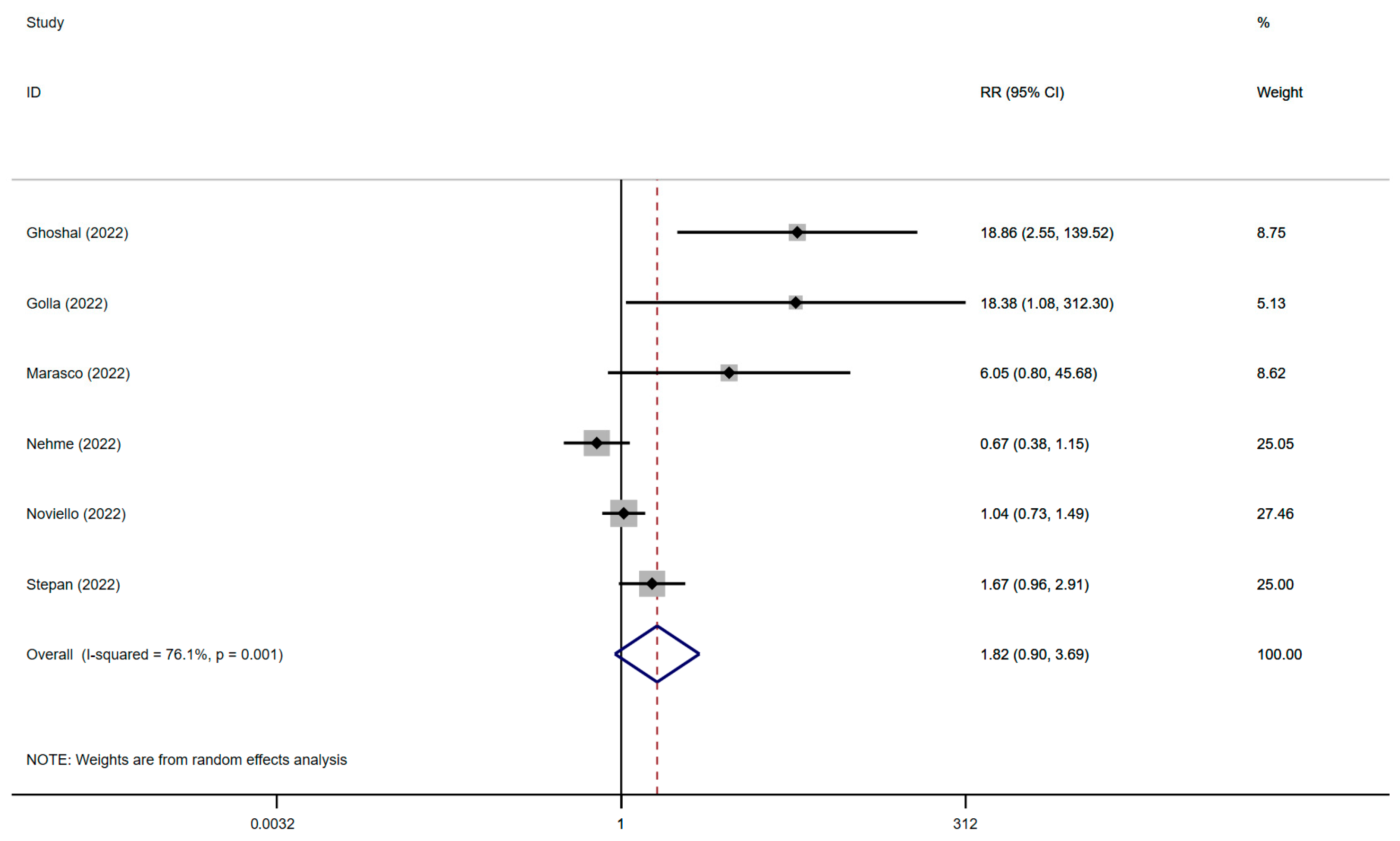
3.5. The Association between IBS and SARS-CoV-2 Infection
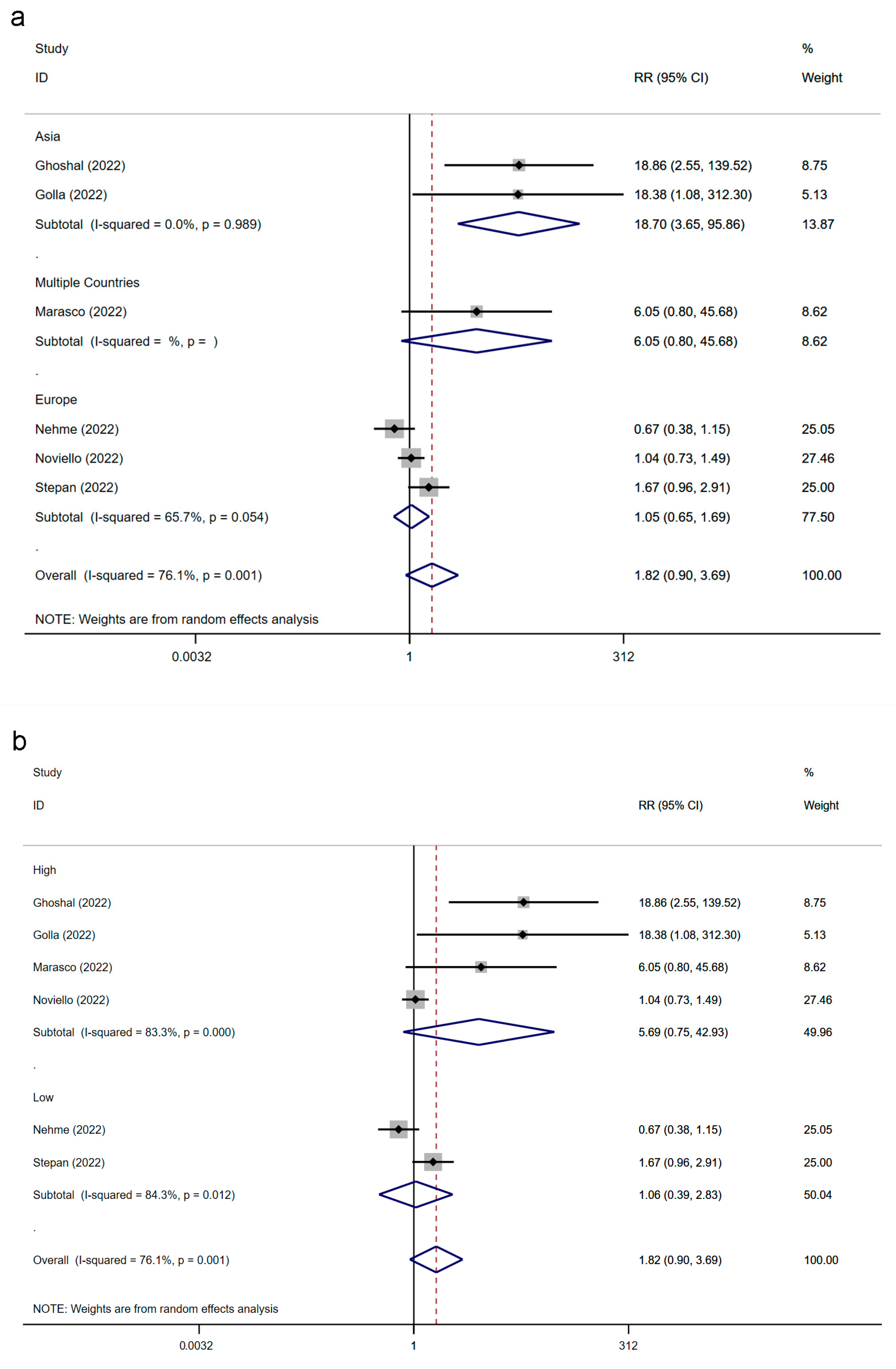
3.6. The Association between IBS and SARS-CoV-2 Infection Classified by Region and Study Quality
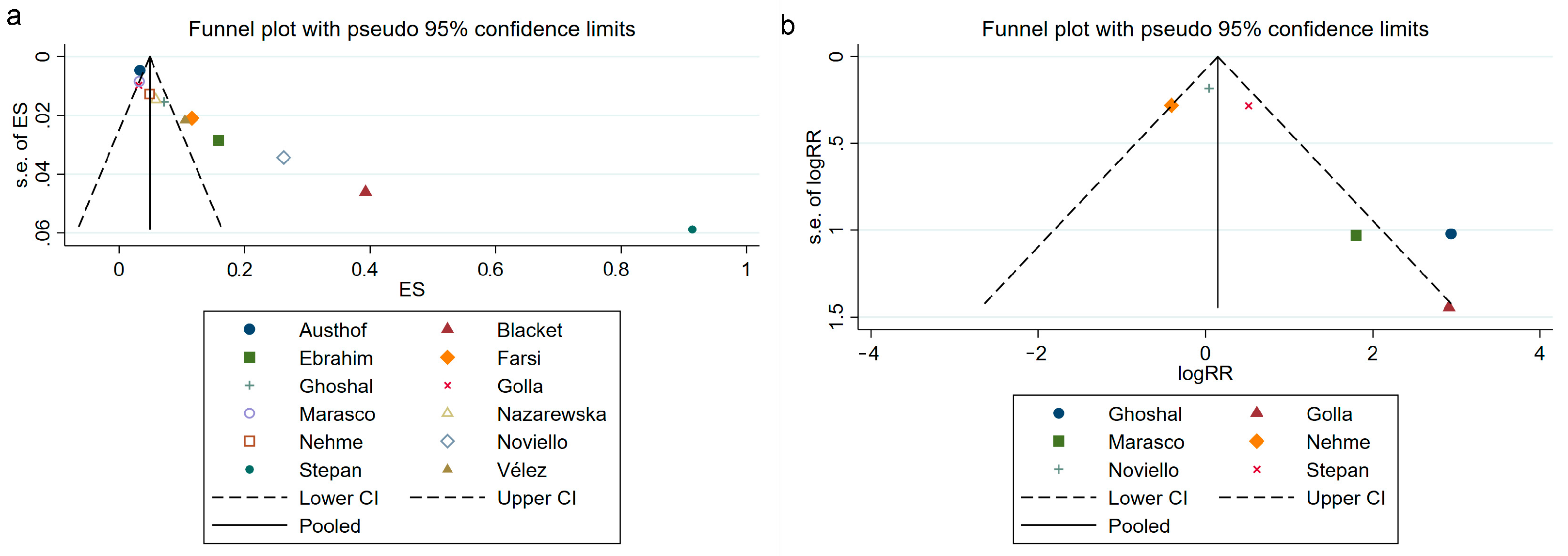
3.7. Publication Bias Assessment
3.8. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hu, B.; Guo, H.; Zhou, P.; Shi, Z.L. Characteristics of SARS-CoV-2 and COVID-19. Nat. Rev. Microbiol. 2021, 19, 141–154. [Google Scholar] [CrossRef] [PubMed]
- Settanni, C.R.; Ianiro, G.; Ponziani, F.R.; Bibbo, S.; Segal, J.P.; Cammarota, G.; Gasbarrini, A. COVID-19 as a trigger of irritable bowel syndrome: A review of potential mechanisms. World J. Gastroenterol. 2021, 27, 7433–7445. [Google Scholar] [CrossRef]
- Jackson, C.B.; Farzan, M.; Chen, B.; Choe, H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Mol. Cell Biol. 2022, 23, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Kruger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e278. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Gupta, A.; Madhavan, M.V.; Sehgal, K.; Nair, N.; Mahajan, S.; Sehrawat, T.S.; Bikdeli, B.; Ahluwalia, N.; Ausiello, J.C.; Wan, E.Y.; et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020, 26, 1017–1032. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Salvi, D.; Cacciari, G.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Prevalence of Gastrointestinal Symptoms in Severe Acute Respiratory Syndrome Coronavirus 2 Infection: Results of the Prospective Controlled Multinational GI-COVID-19 Study. Am. J. Gastroenterol. 2022, 117, 147–157. [Google Scholar] [CrossRef]
- Mao, R.; Qiu, Y.; He, J.S.; Tan, J.Y.; Li, X.H.; Liang, J.; Shen, J.; Zhu, L.R.; Chen, Y.; Iacucci, M.; et al. Manifestations and prognosis of gastrointestinal and liver involvement in patients with COVID-19: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 667–678. [Google Scholar] [CrossRef]
- Bai, T.; Xia, J.; Jiang, Y.; Cao, H.; Zhao, Y.; Zhang, L.; Wang, H.; Song, J.; Hou, X. Comparison of the Rome IV and Rome III criteria for IBS diagnosis: A cross-sectional survey. J. Gastroenterol. Hepatol. 2017, 32, 1018–1025. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, 150, 1393–1407.E5. [Google Scholar] [CrossRef]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Ford, A.C. Global burden of irritable bowel syndrome: Trends, predictions and risk factors. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 473–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Xiang, W.; Li, C.Y.; Li, S.C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 2016, 22, 10450–10460. [Google Scholar] [CrossRef]
- Ballou, S.; McMahon, C.; Lee, H.N.; Katon, J.; Shin, A.; Rangan, V.; Singh, P.; Nee, J.; Camilleri, M.; Lembo, A.; et al. Effects of Irritable Bowel Syndrome on Daily Activities Vary among Subtypes Based on Results from the IBS in America Survey. Clin. Gastroenterol. Hepatol. 2019, 17, 2471–2478.e2473. [Google Scholar] [CrossRef] [PubMed]
- Holtmann, G.J.; Ford, A.C.; Talley, N.J. Pathophysiology of irritable bowel syndrome. Lancet Gastroenterol. Hepatol. 2016, 1, 133–146. [Google Scholar] [CrossRef]
- Haagsma, J.A.; Siersema, P.D.; De Wit, N.J.; Havelaar, A.H. Disease burden of post-infectious irritable bowel syndrome in The Netherlands. Epidemiol. Infect. 2010, 138, 1650–1656. [Google Scholar] [CrossRef]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Klem, F.; Wadhwa, A.; Prokop, L.J.; Sundt, W.J.; Farrugia, G.; Camilleri, M.; Singh, S.; Grover, M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome after Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology 2017, 152, 1042–1054.e1041. [Google Scholar] [CrossRef]
- Austhof, E.; Bell, M.L.; Riddle, M.S.; Catalfamo, C.; McFadden, C.; Cooper, K.; Walter, E.S.; Jacobs, E.; Pogreba-Brown, K. Persisting gastrointestinal symptoms and post-infectious irritable bowel syndrome following SARS-CoV-2 infection: Results from the Arizona CoVHORT. Epidemiol. Infect. 2022, 150, e136. [Google Scholar] [CrossRef]
- Blackett, J.W.; Li, J.; Jodorkovsky, D.; Freedberg, D.E. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterol. Motil. 2022, 34, e14251. [Google Scholar] [CrossRef]
- Farsi, F.; Zonooz, S.R.; Ebrahimi, Z.; Jebraili, H.; Morvaridi, M.; Azimi, T.; Sikaroudi, M.K.; Heshmati, J.; Khorrami, S.; Mokhtare, M.; et al. The Incidence of Post-infectious Irritable Bowel Syndrome, Anxiety, and Depression in Iranian Patients with Coronavirus Disease 2019 Pandemic: A Cross-Sectional Study. Turk. J. Gastroenterol. 2022, 33, 1033. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, U.C.; Ghoshal, U.; Rahman, M.M.; Mathur, A.; Rai, S.; Akhter, M.; Mostafa, T.; Islam, M.S.; Haque, S.A.; Pandey, A.; et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: A case–control study. J. Gastroenterol. Hepatol. 2022, 37, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Golla, R.; Vuyyuru, S.; Kante, B.; Kumar, P.; Mathew, D.T.; Makharia, G.; Kedia, S.; Ahuja, V. Long-term gastrointestinal sequelae following COVID-19: A prospective follow-up cohort study. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Nazarewska, A.; Lewandowski, K.; Kaniewska, M.; Rosolowski, M.; Marlicz, W.; Rydzewska, G. Irritable bowel syndrome following COVID-19: Underestimated consequence of infection with SARS-CoV-2. Pol. Arch. Intern. Med. 2022, 132, 16323. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.W.; Grover, M. The COVID-19 Pandemic and Post-Infection Irritable Bowel Syndrome: What Lies Ahead for Gastroenterologists. Clin. Gastroenterol. Hepatol. 2022, 20, 2195–2197. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B. Meta-analysis of observational studies in epidemiology: A proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Fleiss, J.L. Analysis of data from multiclinic trials. Control. Clin. Trials 1986, 7, 267–275. [Google Scholar] [CrossRef]
- Dickersin, K.; Berlin, J.A. Meta-analysis: State-of-the-science. Epidemiol. Rev. 1992, 14, 154–176. [Google Scholar] [CrossRef]
- Egger, M.; Davey Smith, G.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [PubMed]
- Begg, C.B.; Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994, 50, 1088–1101. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim Nakhli, R.; Shanker, A.; Sarosiek, I.; Boschman, J.; Espino, K.; Sigaroodi, S.; Al Bayati, I.; Elhanafi, S.; Sadeghi, A.; Sarosiek, J.; et al. Gastrointestinal symptoms and the severity of COVID-19: Disorders of gut–brain interaction are an outcome. Neurogastroenterol. Motil. 2022, 34, e14368. [Google Scholar] [CrossRef] [PubMed]
- Nehme, M.; Braillard, O.; Chappuis, F.; Courvoisier, D.S.; Kaiser, L.; Soccal, P.M.; Reny, J.L.; Assal, F.; Bondolfi, G.; Tardin, A.; et al. One-year persistent symptoms and functional impairment in SARS-CoV-2 positive and negative individuals. J. Intern. Med. 2022, 292, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Noviello, D.; Costantino, A.; Muscatello, A.; Bandera, A.; Consonni, D.; Vecchi, M.; Basilisco, G. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: A controlled cohort study. Neurogastroenterol. Motil. 2022, 34, e14187. [Google Scholar] [CrossRef]
- Stepan, M.D.; Cioboata, R.; Vintilescu, S.B.; Vasile, C.M.; Osman, A.; Ciolofan, M.S.; Popescu, M.; Petrovici, I.L.; Zavate, A.C. Pediatric Functional Abdominal Pain Disorders following COVID-19. Life 2022, 12, 509. [Google Scholar] [CrossRef]
- Vélez, C.; Paz, M.; Silvernale, C.; Stratton, L.W.; Kuo, B.; Staller, K.; Barreto, E.; Vergara Cobos, J.; Buchanan, K.L.; Boyd, T.; et al. Factors Associated with Chronic De Novo Post-Coronavirus Disease Gastrointestinal Disorders in a Metropolitan US County. Clin. Gastroenterol. Hepatol. 2022, 20, e1488–e1492. [Google Scholar] [CrossRef]
- Marasco, G.; Cremon, C.; Barbaro, M.R.; Cacciari, G.; Falangone, F.; Kagramanova, A.; Bordin, D.; Drug, V.; Miftode, E.; Fusaroli, P.; et al. Post COVID-19 irritable bowel syndrome. Gut 2022, 72. [Google Scholar] [CrossRef]
- Choudhury, A.; Tariq, R.; Jena, A.; Vesely, E.K.; Singh, S.; Khanna, S.; Sharma, V. Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis. Ther. Adv. Gastroenterol. 2022, 15, 17562848221118403. [Google Scholar] [CrossRef]
- Oka, P.; Parr, H.; Barberio, B.; Black, C.J.; Savarino, E.V.; Ford, A.C. Global prevalence of irritable bowel syndrome according to Rome III or IV criteria: A systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2020, 5, 908–917. [Google Scholar] [CrossRef]
- Barberio, B.; Houghton, L.A.; Yiannakou, Y.; Savarino, E.V.; Black, C.J.; Ford, A.C. Symptom Stability in Rome IV vs. Rome III Irritable Bowel Syndrome. Am. J. Gastroenterol. 2021, 116, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Lovell, R.M.; Ford, A.C. Global prevalence of and risk factors for irritable bowel syndrome: A meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e714. [Google Scholar] [CrossRef] [PubMed]
- Schmulson, M.; Ghoshal, U.C.; Barbara, G. Managing the Inevitable Surge of Post-COVID-19 Functional Gastrointestinal Disorders. Am. J. Gastroenterol. 2021, 116, 4–7. [Google Scholar] [CrossRef] [PubMed]
- Price-Haywood, E.G.; Burton, J.; Fort, D.; Seoane, L. Hospitalization and Mortality among Black Patients and White Patients with Covid-19. N. Engl. J. Med. 2020, 382, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Foster, H.M.E.; Ho, F.K.; Mair, F.S.; Jani, B.D.; Sattar, N.; Katikireddi, S.V.; Pell, J.P.; Niedzwiedz, C.L.; Hastie, C.E.; Anderson, J.J.; et al. The association between a lifestyle score, socioeconomic status, and COVID-19 outcomes within the UK Biobank cohort. BMC Infect. Dis. 2022, 22, 273. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.G.; Amin, A.B.; Ali, A.R.; Hoots, B.; Cadwell, B.L.; Arora, S.; Avoundjian, T.; Awofeso, A.O.; Barnes, J.; Bayoumi, N.S.; et al. COVID-19 Incidence and Death Rates among Unvaccinated and Fully Vaccinated Adults with and without Booster Doses during Periods of Delta and Omicron Variant Emergence—25 U.S. Jurisdictions, April 4–December 25, 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 132–138. [Google Scholar] [CrossRef]
- Gao, Z.; Xu, Y.; Sun, C.; Wang, X.; Guo, Y.; Qiu, S.; Ma, K. A systematic review of asymptomatic infections with COVID-19. J. Microbiol. Immunol. Infect. 2021, 54, 12–16. [Google Scholar] [CrossRef]
- Guler, S.A.; Ebner, L.; Aubry-Beigelman, C.; Bridevaux, P.O.; Brutsche, M.; Clarenbach, C.; Garzoni, C.; Geiser, T.K.; Lenoir, A.; Mancinetti, M.; et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur. Respir. J. 2021, 57, 2003690. [Google Scholar] [CrossRef]
- Chen, C.; Haupert, S.R.; Zimmermann, L.; Shi, X.; Fritsche, L.G.; Mukherjee, B. Global Prevalence of Post-Coronavirus Disease 2019 (COVID-19) Condition or Long COVID: A Meta-Analysis and Systematic Review. J. Infect. Dis. 2022, 226, 1593–1607. [Google Scholar] [CrossRef]
- Xiao, F.; Tang, M.; Zheng, X.; Liu, Y.; Li, X.; Shan, H. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology 2020, 158, 1831–1833.e1833. [Google Scholar] [CrossRef]
- Ziegler, C.G.K.; Allon, S.J.; Nyquist, S.K.; Mbano, I.M.; Miao, V.N.; Tzouanas, C.N.; Cao, Y.; Yousif, A.S.; Bals, J.; Hauser, B.M.; et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Detected in Specific Cell Subsets across Tissues. Cell 2020, 181, 1016–1035.e1019. [Google Scholar] [CrossRef] [PubMed]
- Brierley, S.M.; Linden, D.R. Neuroplasticity and dysfunction after gastrointestinal inflammation. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 611–627. [Google Scholar] [CrossRef] [PubMed]
- Sinhamahapatra, P.; Saha, S.P.; Chowdhury, A.; Chakrabarti, S.K.; Ghosh, A.; Maiti, B. Visceral afferent hypersensitivity in irritable bowel syndrome--evaluation by cerebral evoked potential after rectal stimulation. Am. J. Gastroenterol. 2001, 96, 2150–2157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Mak, J.W.Y.; Su, Q.; Yeoh, Y.K.; Lui, G.C.; Ng, S.S.S.; Zhang, F.; Li, A.Y.L.; Lu, W.; Hui, D.S.; et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022, 71, 544–552. [Google Scholar] [CrossRef]
- Beović, B.; Doušak, M.; Ferreira-Coimbra, J.; Nadrah, K.; Rubulotta, F.; Belliato, M.; Berger-Estilita, J.; Ayoade, F.; Rello, J.; Erdem, H. Antibiotic use in patients with COVID-19: A ‘snapshot’ Infectious Diseases International Research Initiative (ID-IRI) survey. J. Antimicrob. Chemother. 2020, 75, 3386–3390. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association between Administration of Systemic Corticosteroids and Mortality among Critically Ill Patients with COVID-19: A Meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar] [CrossRef]
- Saravolatz, L.D.; Depcinski, S.; Sharma, M. Molnupiravir and Nirmatrelvir-Ritonavir: Oral Coronavirus Disease 2019 Antiviral Drugs. Clin. Infect. Dis. 2023, 76, 165–171. [Google Scholar] [CrossRef]
- Rogers, J.P.; Chesney, E.; Oliver, D.; Pollak, T.A.; McGuire, P.; Fusar-Poli, P.; Zandi, M.S.; Lewis, G.; David, A.S. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: A systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry 2020, 7, 611–627. [Google Scholar] [CrossRef]
- Janiri, D.; Kotzalidis, G.D.; Giuseppin, G.; Molinaro, M.; Modica, M.; Montanari, S.; Terenzi, B.; Carfi, A.; Landi, F.; Sani, G.; et al. Psychological Distress after Covid-19 Recovery: Reciprocal Effects with Temperament and Emotional Dysregulation. An Exploratory Study of Patients over 60 Years of Age Assessed in a Post-acute Care Service. Front. Psychiatry 2020, 11, 590135. [Google Scholar] [CrossRef]
- Ju, Y.; Chen, W.; Liu, J.; Yang, A.; Shu, K.; Zhou, Y.; Wang, M.; Huang, M.; Liao, M.; Liu, J.; et al. Effects of centralized isolation vs. home isolation on psychological distress in patients with COVID-19. J. Psychosom. Res. 2021, 143, 110365. [Google Scholar] [CrossRef]
- Guessoum, S.B.; Lachal, J.; Radjack, R.; Carretier, E.; Minassian, S.; Benoit, L.; Moro, M.R. Adolescent psychiatric disorders during the COVID-19 pandemic and lockdown. Psychiatry Res. 2020, 291, 113264. [Google Scholar] [CrossRef] [PubMed]
- Toubasi, A.A.; AbuAnzeh, R.B.; Tawileh, H.B.A.; Aldebei, R.H.; Alryalat, S.A.S. A meta-analysis: The mortality and severity of COVID-19 among patients with mental disorders. Psychiatry Res. 2021, 299, 113856. [Google Scholar] [CrossRef] [PubMed]
- Menni, C.; Valdes, A.M.; Polidori, L.; Antonelli, M.; Penamakuri, S.; Nogal, A.; Louca, P.; May, A.; Figueiredo, J.C.; Hu, C.; et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: A prospective observational study from the ZOE COVID Study. Lancet 2022, 399, 1618–1624. [Google Scholar] [CrossRef] [PubMed]



| Author | Year | Follow-Up Period | Region | Study Design | IBS Diagnostic Criteria | SARS-CoV-2 Detection Method | Age Information | Gender | The Number of IBS Patients Following SARS-CoV-2 Infection | The Number of IBS Patients in Control Group (Without SARS-CoV-2 Infection) | Study Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Austhof [19] | 2022 | 6 months | USA | Longitudinal | Rome IV | PCR test | Mean age (SD): No GI symptoms: 45.0 (15.9) years; GI symptoms: 42.7 (15.6) years | Male:487 Female:978 Non-Binary:7 Transgender female: 2 Transgender male: 1 | 49 of 1475 | NR | Low |
| Blackett [20] | 2022 | 6 months | USA | Longitudinal | Rome IV | PCR test/Antibody test/Symptom/Unclear methods | >18 years old | Male:28 Female:84 | 44 of 112 | NR | High |
| Ebrahim [33] | 2022 | NA | USA and Canada | Cross-sectional | Rome IV | PCR test or antibodies test | Age- group distribution was ≤65 years old: 79%, >65 years old: 4%, did not report their age: 17% | Male:14% Female:70% 16% did not specify their sex | 26 of 164 | NR | Low |
| Farsi [21] | 2022 | NA | Iran | Cross-sectional | Rome IV | PCR test/chest radiograph | Mean age (SD): 38.41 (11.51) years | Male:97 Female:136 | 27 of 233 | NR | Low |
| Ghoshal [22] | 2022 | 6 months | Bangladesh and India | Longitudinal | Rome III | RT-PCR test | Mean age (SD): SARS-CoV-2 infection group: 39.5 (15.4) years Control group: 36.8 (11.6) years | SARS-CoV-2 infection group: 204/76 (M/F) Control group: 193/71 (M/F) | 20 of 280 a | 1 of 264 a | High |
| Golla [23] | 2022 | 6 months | India | Longitudinal | Rome IV | RT-PCR/CBNAAT | Mean age (SD): SARS-CoV-2 infection group: 38.02 (11.4) years Control group: 38.47 (11.7) years | SARS-CoV-2 infection group: 163/157 (M/F) Control group: 172/108 (M/F) | 10 of 320 | 0 of 280 | High |
| Marasco [38] | 2022 | 6 to 12 months | Multiple countries * | Longitudinal | Rome IV | NAAT | Mean age (SD): SARS-CoV-2 infection group: 49.9 (16.1) years Control group: 50.9 (18.1) years | SARS-CoV-2 infection group: 164/105 (M/F) Control group: 364/250 (M/F) | 14 of 435 b | 1 of 188 b | High |
| Nazarewska [24] | 2022 | 6 months | Poland | Longitudinal | Rome IV | NR | SARS-CoV-2 infection patients with IBS: 67.5 years old (median age) SARS-CoV-2 infection patients without IBS: 71 years old (median age) | SARS-CoV-2 infection patients with IBS: 11/4 (M/F) SARS-CoV-2 infection patients without IBS: 130/112 (M/F) | 15 of 257 | NR | Low |
| Nehme [34] | 2022 | 12 months | Switzerland | Longitudinal | By a self-designed questionnaire | RT-PCR test | Mean age (SD): SARS-CoV-2 infection group: 44.2 (13.2) years Control group: 45.5 (14.8) years | SARS-CoV-2 infection group: 101/186 (M/F) Control group: 460/700 (M/F) | 14 of 287 | 85 of 1160 | Low |
| Noviello [35] | 2022 | 5 months | Italy | Longitudinal | Rome IV | PCR test | SARS-CoV-2 infection group: 44.1 (23–60) Control group: 39.6 (22–60) | SARS-CoV-2 infection group: 98/66 (M/F) Control group: 72/111 (M/F) | 43 of 164 | 46 of 183 | High |
| Stepan [36] | 2022 | 3 to 6 months | Romania | Longitudinal | Rome IV | RT-PCR test | 4 to 6 years old | SARS-CoV-2 infection group: 9/14 (M/F) Control group: 2/9 (M/F) | 21 of 23 | 6 of 11 | Low |
| Vélez [37] | 2022 | 6 months | USA | Longitudinal | Rome IV | NR | 47.2 | 75/125 (M/F) | 21 of 200 | NR | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Peng, Y.; Chen, M.; Peng, L.; Huang, Y.; Lin, W. The Prevalence of Irritable Bowel Syndrome after Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Their Association: A Systematic Review and Meta-Analysis of Observational Studies. J. Clin. Med. 2023, 12, 1865. https://doi.org/10.3390/jcm12051865
Wang Z, Peng Y, Chen M, Peng L, Huang Y, Lin W. The Prevalence of Irritable Bowel Syndrome after Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Their Association: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Clinical Medicine. 2023; 12(5):1865. https://doi.org/10.3390/jcm12051865
Chicago/Turabian StyleWang, Ziyan, Yinglong Peng, Minshan Chen, Liang Peng, Yongzhen Huang, and Wei Lin. 2023. "The Prevalence of Irritable Bowel Syndrome after Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Their Association: A Systematic Review and Meta-Analysis of Observational Studies" Journal of Clinical Medicine 12, no. 5: 1865. https://doi.org/10.3390/jcm12051865
APA StyleWang, Z., Peng, Y., Chen, M., Peng, L., Huang, Y., & Lin, W. (2023). The Prevalence of Irritable Bowel Syndrome after Severe Acute Respiratory Syndrome Coronavirus 2 Infection and Their Association: A Systematic Review and Meta-Analysis of Observational Studies. Journal of Clinical Medicine, 12(5), 1865. https://doi.org/10.3390/jcm12051865






