Tunneling of Mesh during Ventral Rectopexy: Technical Aspects and Long-Term Functional Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients’ Assessment
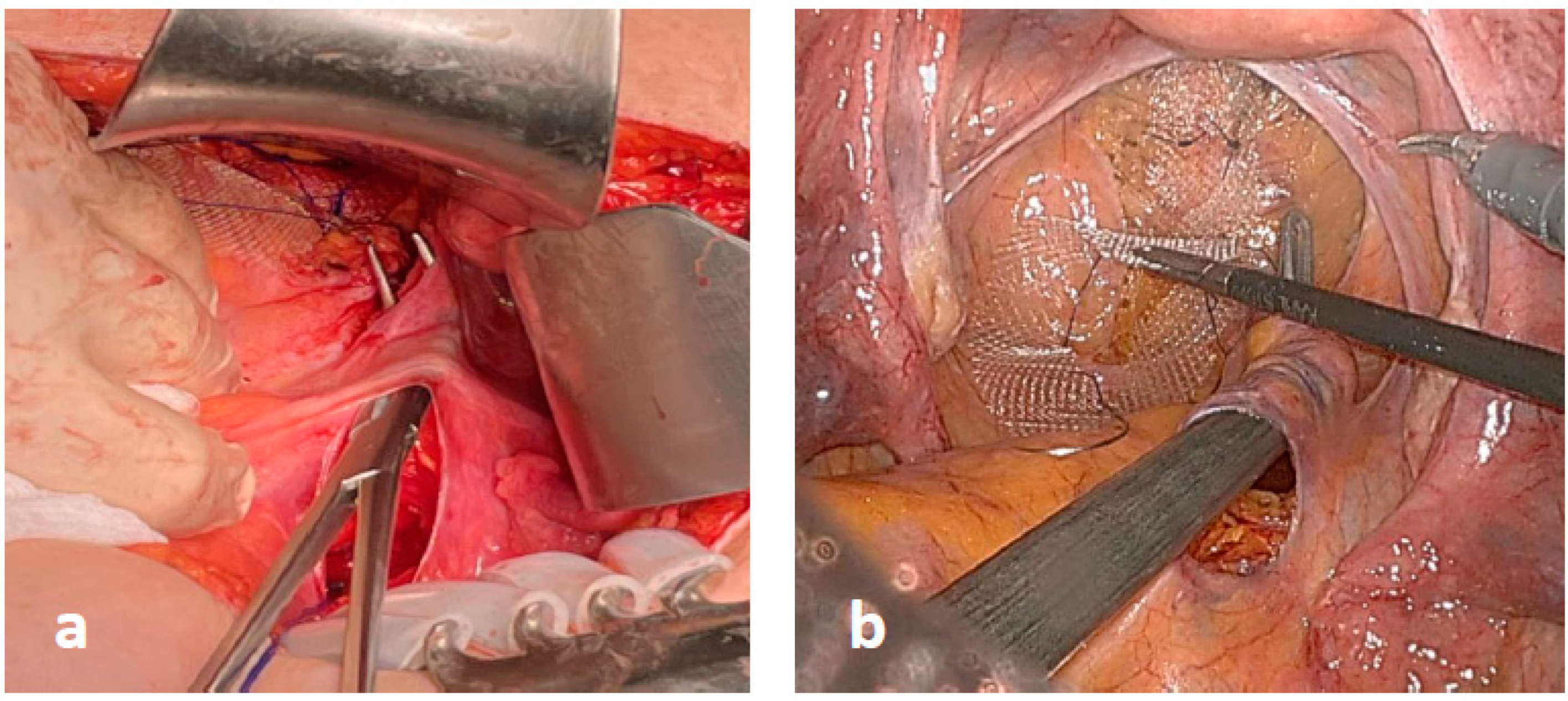
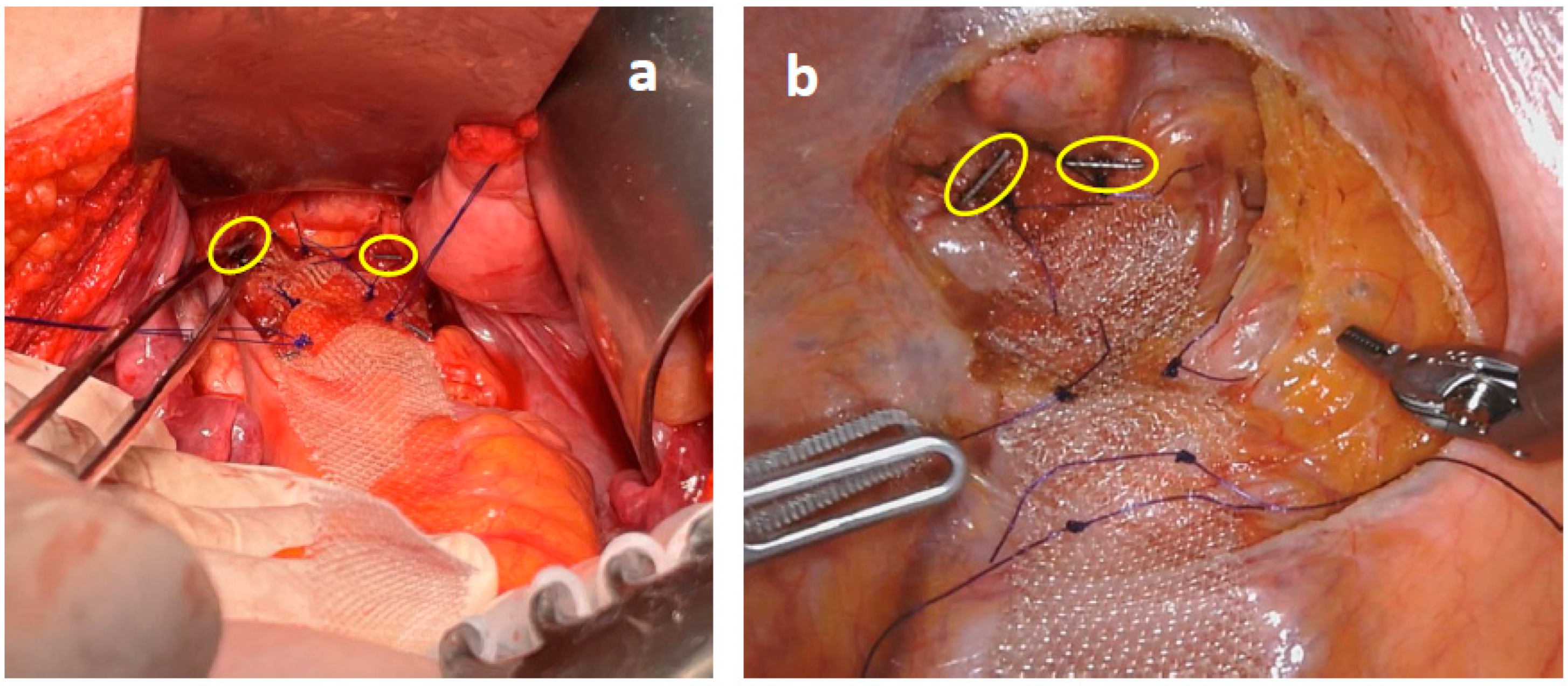
2.3. Surgical Technique
2.4. Modified VR: The Study Group
2.5. Standard VR: The Control Group
2.6. Postoperative Management
2.7. Data Collection
2.8. Statistical Analysis
3. Results
3.1. Demographic Data
3.2. Operation Data
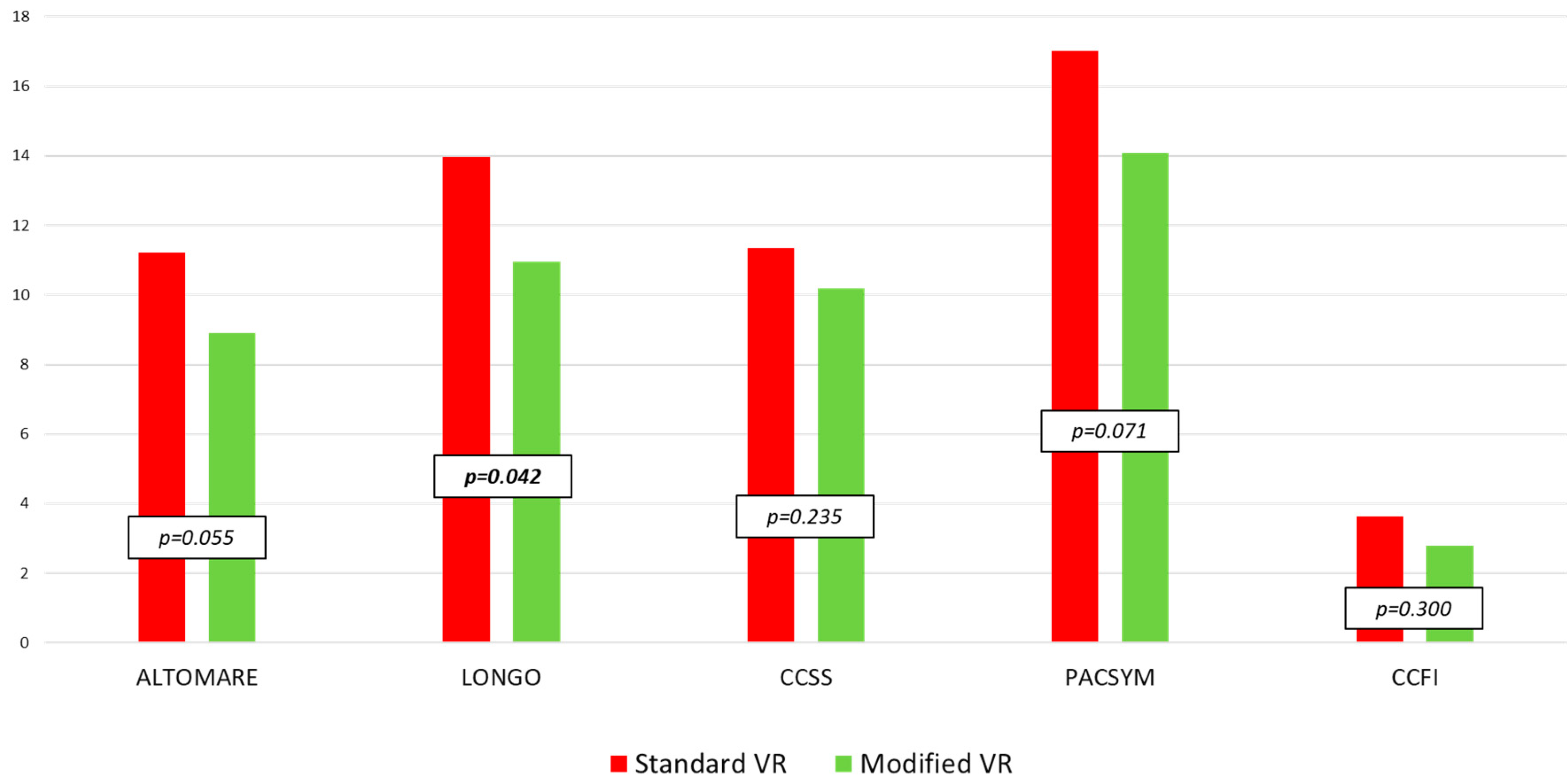
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Hoore, A.; Cadoni, R.; Penninckx, F. Long-term outcome of laparoscopic ventral rectopexy for total rectal prolapse. Br. J. Surg. 2004, 91, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, N.; Cunningham, C.; Dixon, A.; Greenslade, G.; Lindsey, I. Laparoscopic ventral rectopexy for external rectal prolapse is safe and effective in the elderly. Does this make perineal procedures obsolete? Color. Dis. Off. J. Assoc. Coloproctol. Great Br. Irel. 2011, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Gouvas, N.; Georgiou, P.A.; Agalianos, C.; Tan, E.; Tekkis, P.; Dervenis, C.; Xynos, E. Ventral colporectopexy for overt rectal prolapse and obstructed defaecation syndrome: A systematic review. Color. Dis. 2015, 17, O34–O46. [Google Scholar] [CrossRef] [PubMed]
- Mercer-Jones, M.A.; Brown, S.R.; Knowles, C.H.; Williams, A.B. Position statement by the Pelvic Floor Society on behalf of the Association of Coloproctology of Great Britain and Ireland on the use of mesh in ventral mesh rectopexy. Color. Dis. 2020, 22, 1429–1435. [Google Scholar] [CrossRef]
- Auguste, T.; Dubreuil, A.; Bost, R.; Bonaz, B.; Faucheron, J.L. Technical and functional results after laparoscopic rectopexy to the promontory for complete rectal prolapse. Prospective study in 54 consecutive patients. Gastroenterol. Clin. Biol. 2006, 30, 659–663. [Google Scholar] [CrossRef]
- Sileri, P.; Capuano, I.; Franceschilli, L.; Giorgi, F.; Gaspari, A.L. Modified laparoscopic ventral mesh rectopexy. Techol. Coloproctol. 2014, 18, 591–594. [Google Scholar] [CrossRef]
- Mäkelä-Kaikkonen, J.; Rautio, T.; Klintrup, K.; Takala, H.; Vierimaa, M.; Ohtonen, P.; Mäkelä, J. Robotic-assisted and laparoscopic ventral rectopexy in the treatment of rectal prolapse: A matched-pairs study of operative details and complications. Techol. Coloproctol. 2014, 18, 151–155. [Google Scholar] [CrossRef]
- Mantoo, S.; Podevin, J.; Regenet, N.; Rigaud, J.; Lehur, P.A.; Meurette, G. Is robotic-assisted ventral mesh rectopexy superior to laparoscopic ventral mesh rectopexy in the management of obstructed defaecation? Color. Dis. 2013, 15, e469–e475. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- Altomare, D.F.; Spazzafumo, L.; Rinaldi, M.; Dodi, G.; Ghiselli, R.; Piloni, V. Set-up and statistical validation of a new scoring system for obstructed defaecation syndrome. Color. Dis. 2008, 10, 84–88. [Google Scholar] [CrossRef]
- Jayne, D.G.; Schwandner, O.; Stuto, A. Stapled transanal rectal resection for obstructed defecation syndrome: One-year results of the European STARR Registry. Dis. Colon Rectum 2009, 52, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Agachan, F.; Chen, T.; Pfeifer, J.; Reissman, P.; Wexner, S.D. A constipation scoring system to simplify evaluation and management of constipated patients. Dis. Colon Rectum 1996, 39, 681–685. [Google Scholar] [CrossRef]
- Frank, L.; Kleinman, L.; Farup, C.; Taylor, L.; Miner, P., Jr. Psychometric validation of a constipation symptom assessment questionnaire. Scand. J. Gastroenterol. 1999, 34, 870–877. [Google Scholar] [CrossRef] [PubMed]
- Jorge, J.M.; Wexner, S.D. Etiology and management of fecal incontinence. Dis. Colon Rectum 1993, 36, 77–97. [Google Scholar] [CrossRef] [PubMed]
- Wijffels, N.A.; Collinson, R.; Cunningham, C.; Lindsey, I. What is the natural history of internal rectal prolapse? Color. Dis. 2010, 12, 822–830. [Google Scholar] [CrossRef] [PubMed]
- Ratto, C.; Marra, A.A.; Campennì, P.; De Simone, V.; Litta, F.; Parello, A. Modified robotic ventral mesh rectopexy—A video vignette. Color. Dis. 2022, 24, 142. [Google Scholar] [CrossRef] [PubMed]
- Dindo, D.; Demartines, N.; Clavien, P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004, 240, 205–213. [Google Scholar] [CrossRef]
- Picciariello, A.; O’Connell, P.R.; Hahnloser, D.; Gallo, G.; Munoz-Duyos, A.; Schwandner, O.; Sileri, P.; Milito, G.; Riss, S.; Boccasanta, P.A.; et al. Obstructed defaecation syndrome: European consensus guidelines on the surgical management. Br. J. Surg. 2021, 108, 1149–1153. [Google Scholar] [CrossRef]
- Bordeianou, L.; Paquette, I.; Johnson, E.; Holubar, S.D.; Gaertner, W.; Feingold, D.L.; Steele, S.R. Clinical Practice Guidelines for the Treatment of Rectal Prolapse. Dis. Colon Rectum 2017, 60, 1121–1131. [Google Scholar] [CrossRef]
- Bachoo, P.; Brazzelli, M.; Grant, A. Surgery for complete rectal prolapse in adults. Cochrane Database Syst. Rev. 2000, 2, CD001758. [Google Scholar] [CrossRef]
- Petros, P. The biomechanics of uterine prolapse impact rectal intussusception, ODS and surgical restoration. Techol. Coloproctol. 2022, 26, 161–162. [Google Scholar] [CrossRef] [PubMed]
- Brunenieks, I.; Pekarska, K.; Kasyanov, V.; Groma, V. Biomechanical and morphological peculiarities of the rectum in patients with obstructed defecation syndrome. Rom. J. Morphol. Embryol. Rev. Roum. Morphol. Embryol. 2017, 58, 1193–1200. [Google Scholar]
- Ren, X.H.; Yaseen, S.M.; Cao, Y.L.; Liu, W.C.; Shrestha, S.; Ding, Z.; Wu, Y.H.; Zheng, K.Y.; Qian, Q.; Jiang, C.Q. A transanal procedure using TST STARR Plus for the treatment of Obstructed Defecation Syndrome: ‘A mid-term study’. Int. J. Surg. 2016, 32, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Siri, S.; Zhao, Y.; Maier, F.; Pierce, D.M.; Feng, B. The Macro-and Micro-Mechanics of the Colon and Rectum I: Experimental Evidence. Bioengineering 2020, 7, 130. [Google Scholar] [CrossRef]
- Hidaka, J.; Elfeki, H.; Duelund-Jakobsen, J.; Laurberg, S.; Lundby, L. Functional Outcome after Laparoscopic Posterior Sutured Rectopexy Versus Ventral Mesh Rectopexy for Rectal Prolapse: Six-year Follow-up of a Double-blind, Randomized Single-center Study. EClinicalMedicine 2019, 16, 18–22. [Google Scholar] [CrossRef]
- van Iersel, J.J.; Paulides, T.J.; Verheijen, P.M.; Lumley, J.W.; Broeders, I.A.; Consten, E.C. Current status of laparoscopic and robotic ventral mesh rectopexy for external and internal rectal prolapse. World J. Gastroenterol. 2016, 22, 4977–4987. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.A.; Youssef, M.; Farid, M.; Wexner, S.D. Abdominal rectopexy for the treatment of internal rectal prolapse: A systematic review and meta-analysis. Color. Dis. 2017, 19, O13–O24. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Sileri, P.; Wexner, S.D. Outcome of laparoscopic ventral mesh rectopexy for full-thickness external rectal prolapse: A systematic review, meta-analysis, and meta-regression analysis of the predictors for recurrence. Surg. Endosc. 2019, 33, 2444–2455. [Google Scholar] [CrossRef]

| Modified VR—n° (%) | Standard VR—n° (%) | p | |
|---|---|---|---|
| Patients | 65 | 52 | |
| Female | 65 (100.0) | 51 (98.1) | 0.262 |
| Age (years) * | 60.9 ± 12.0 | 60.7 ± 13.9 | 0.251 |
| Previous abdominal surgery | 39 (60.0) | 32 (61.5) | 0.866 |
| Previous hysterectomy | 19 (29.2) | 18 (35.3) | 0.487 |
| Previous abdominal surgery for RP | 1 (1.5) | 1 (1.9) | 0.873 |
| Previous perineal surgery | 26 (40.0) | 21 (40.4) | 0.966 |
| Previous perineal surgery for RP | 12 (18.5) | 9 (17.3) | 0.645 |
| Vaginal delivery | 45 (69.2) | 40 (78.4) | 0.266 |
| Episiotomy | 35 (53.8) | 31 (60.8) | 0.454 |
| Obstetric anal sphincter injury | 9 (13.8) | 17 (33.3) | 0.188 |
| Forceps/vacuum | 1 (1.5) | 6 (11.8) | 0.402 |
| Caesarean delivery | 13 (20.0) | 10 (19.6) | 0.958 |
| Preoperative ODS | 61 (93.8) | 48 (92.3) | 0.743 |
| Preoperative FI | 37 (56.9) | 35 (67.3) | 0.251 |
| Preoperative ODS + FI | 33 (50.8) | 31 (59.6) | 0.339 |
| Preoperative UI | 39 (60.0) | 30 (57.7) | 0.801 |
| Oxford classification for RP | 0.508 | ||
| Grade I | 7 (10.8) | 4 (7.7) | |
| Grade II | 12 (18.5) | 5 (9.6) | |
| Grade III | 18 (27.7) | 13 (25.0) | |
| Grade IV | 9 (13.8) | 11 (21.2) | |
| Grade V | 19 (29.2) | 19 (36.5) | |
| Rectocele | 51 (78.5) | 30 (57.7) | 0.174 |
| Enterocele | 35 (53.8) | 35 (67.3) | 0.677 |
| Standard VR | SD | Modified VR | SD | p | |
|---|---|---|---|---|---|
| Altomare pre | 17.8 | 5.6 | 17.8 | 5.8 | 0.952 |
| Longo pre | 23.7 | 7.5 | 22.0 | 8.8 | 0.489 |
| CCSS pre | 18.2 | 4.9 | 18.7 | 5.4 | 0.383 |
| PAC-SYM pre | 23.9 | 5.5 | 23.2 | 5.6 | 0.643 |
| CCFI pre | 4.5 | 5.0 | 3.2 | 4.7 | 0.176 |
| Altomare post | 10.8 | 5.9 | 8.7 | 6.5 | 0.116 |
| Longo post | 13.5 | 7.8 | 10.5 | 8.4 | 0.050 |
| CCSS post | 11.4 | 5.4 | 10.0 | 6.3 | 0.289 |
| PAC-SYM post | 16.0 | 7.7 | 13.8 | 10.0 | 0.155 |
| CCFI post | 3.0 | 4.3 | 2.2 | 3.7 | 0.211 |
| Standard VR | SD | Modified VR | SD | p | |
|---|---|---|---|---|---|
| Altomare pre | 15.9 | 6.6 | 18.6 | 6.6 | 0.242 |
| Longo pre | 22.1 | 7.7 | 24.4 | 7.7 | 0.368 |
| CCSS pre | 16.3 | 6.2 | 19.0 | 4.6 | 0.106 |
| PAC-SYM pre | 23.8 | 7.4 | 23.7 | 5.8 | 0.927 |
| CCFI pre | 6.8 | 6.8 | 8.6 | 5.5 | 0.278 |
| Altomare post | 11.9 | 7.2 | 9.4 | 6.3 | 0.361 |
| Longo post | 14.9 | 10.2 | 12.2 | 7.6 | 0.447 |
| CCSS post | 11.2 | 6.4 | 10.5 | 5.8 | 0.703 |
| PAC-SYM post | 18.9 | 9.8 | 14.8 | 9.3 | 0.207 |
| CCFI post | 4.8 | 5.7 | 4.3 | 4.6 | 0.951 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campennì, P.; Marra, A.A.; De Simone, V.; Litta, F.; Parello, A.; Ratto, C. Tunneling of Mesh during Ventral Rectopexy: Technical Aspects and Long-Term Functional Results. J. Clin. Med. 2023, 12, 294. https://doi.org/10.3390/jcm12010294
Campennì P, Marra AA, De Simone V, Litta F, Parello A, Ratto C. Tunneling of Mesh during Ventral Rectopexy: Technical Aspects and Long-Term Functional Results. Journal of Clinical Medicine. 2023; 12(1):294. https://doi.org/10.3390/jcm12010294
Chicago/Turabian StyleCampennì, Paola, Angelo Alessandro Marra, Veronica De Simone, Francesco Litta, Angelo Parello, and Carlo Ratto. 2023. "Tunneling of Mesh during Ventral Rectopexy: Technical Aspects and Long-Term Functional Results" Journal of Clinical Medicine 12, no. 1: 294. https://doi.org/10.3390/jcm12010294
APA StyleCampennì, P., Marra, A. A., De Simone, V., Litta, F., Parello, A., & Ratto, C. (2023). Tunneling of Mesh during Ventral Rectopexy: Technical Aspects and Long-Term Functional Results. Journal of Clinical Medicine, 12(1), 294. https://doi.org/10.3390/jcm12010294






