Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment Preparation
2.3. Breath-Hold Procedure
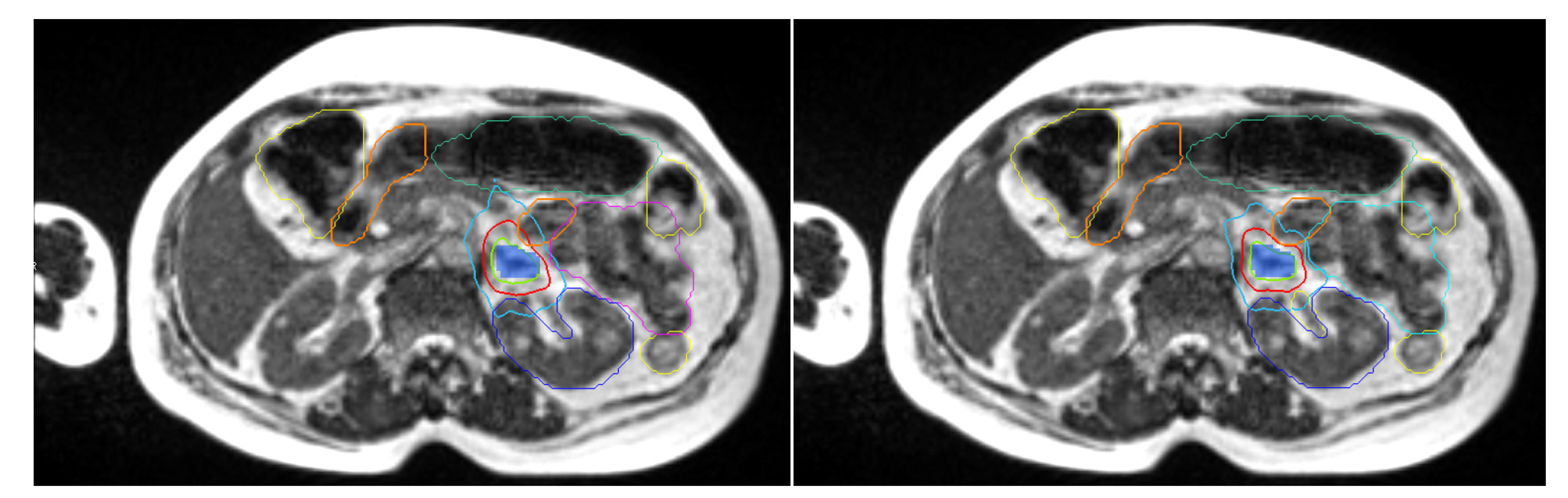
2.4. Treatment Planning
2.5. Daily Adaptive Treatment Workflow
2.6. Clinical Assessment, Dosimetric Evaluation, and Evolution of GTV
2.7. Statistical Analysis
3. Results
3.1. Patient and Treatment Characteristics at Inclusion
3.2. Initial Treatment Plans
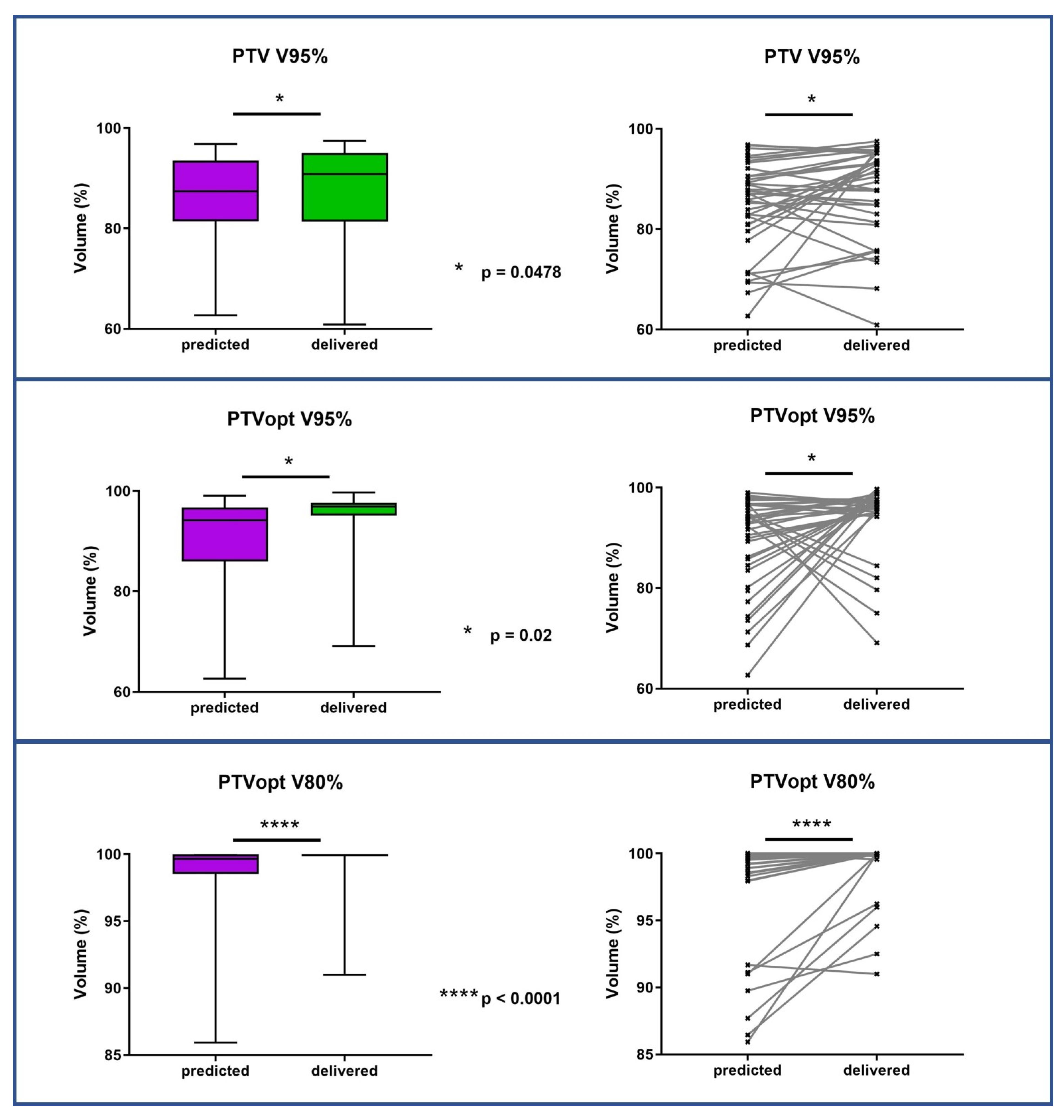
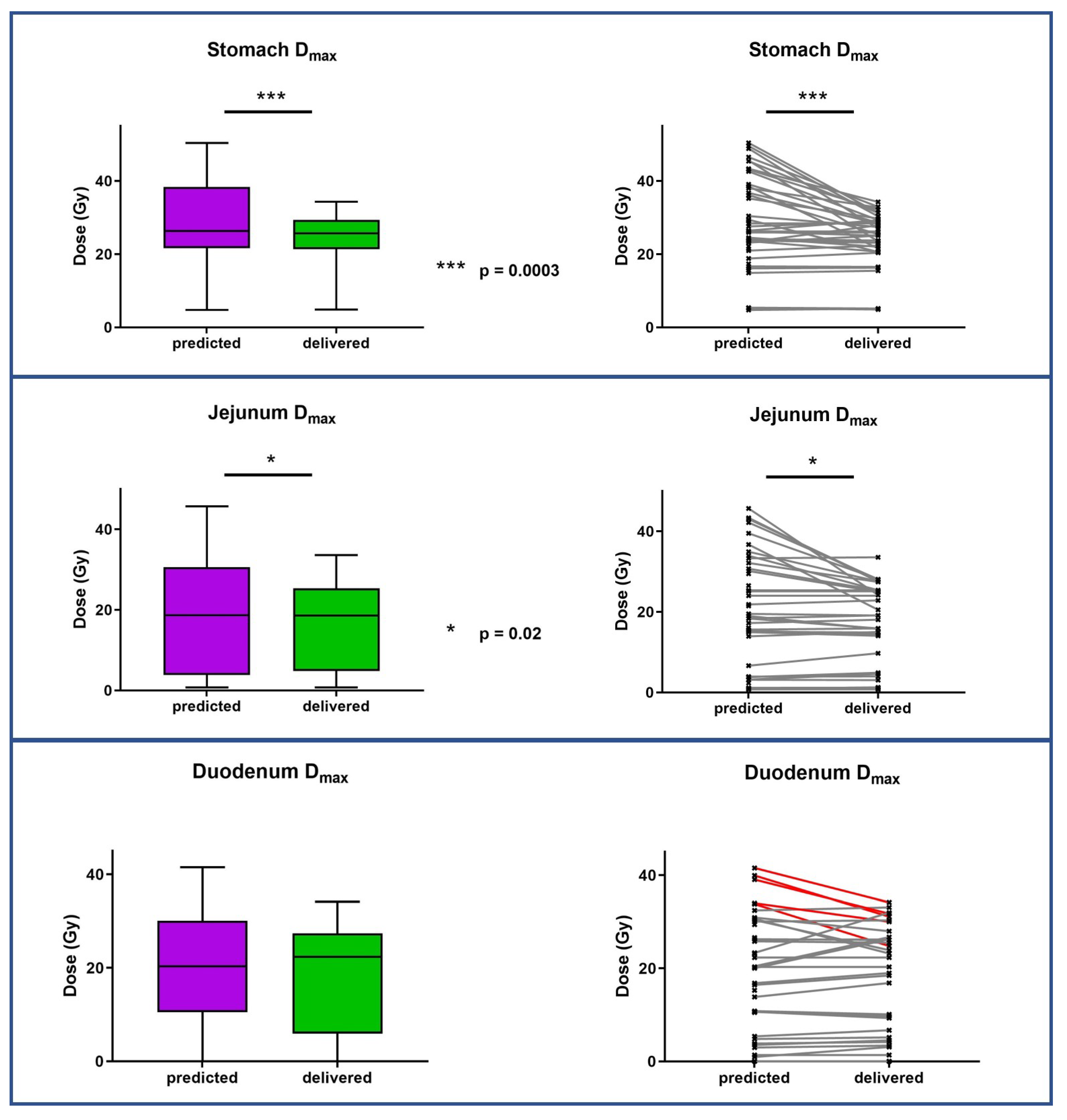
3.3. Dosimetric Benefits of Adaptive MRgRT
3.4. Evolution of GTV Size during Treatment
3.5. Evaluation of Tolerance
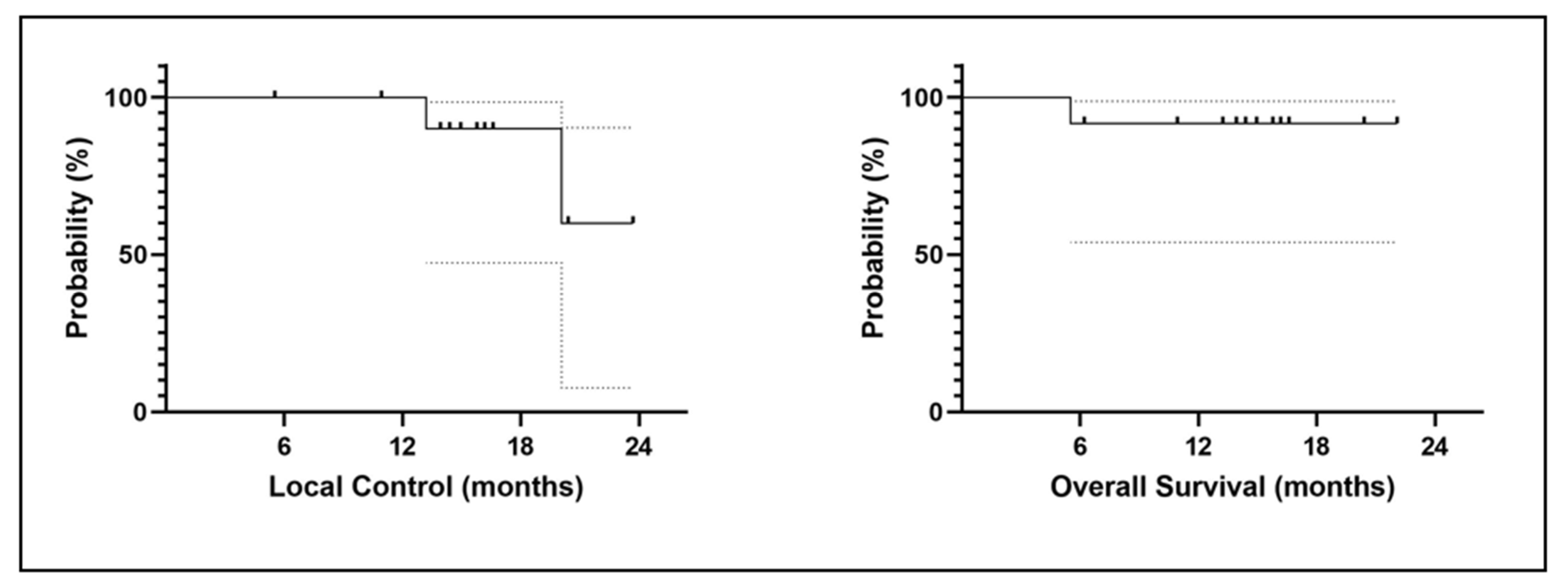
3.6. Clinical Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lam, K.-Y.; Lo, C.-Y. Metastatic tumours of the adrenal glands: A 30-year experience in a teaching hospital. Clin. Endocrinol. 2002, 56, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Franzese, C.; Franceschini, D.; Cozzi, L.; D’Agostino, G.; Comito, T.; De Rose, F.; Navarria, P.; Mancosu, P.; Tomatis, S.; Fogliata, A.; et al. Minimally Invasive Stereotactical Radio-ablation of Adrenal Metastases as an Alternative to Surgery. Cancer Res. Treat. 2017, 49, 20–28. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Oshiro, Y.; Takeda, Y.; Hirano, S.; Ito, H.; Aruga, T. Role of Radiotherapy for Local Control of Asymptomatic Adrenal Metastasis From Lung Cancer. Am. J. Clin. Oncol. 2011, 34, 249–253. [Google Scholar] [CrossRef]
- Coffin, A.; Boulay-Coletta, I.; Sebbag-Sfez, D.; Zins, M. Radio anatomie du rétro-péritoine. J. De Radiol. Diagn. Et Interv. 2015, 96, 44–59. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J. Clin. Oncol. 2020, 38, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Van Oirschot, M.; Bergman, A.; Verbakel, W.F.A.R.; Ward, L.; Gagne, I.; Huang, V.; Chng, N.; Houston, P.; Symes, K.; Thomas, C.G.; et al. Determining Planning Priorities for SABR for Oligometastatic Disease: A Secondary Analysis of the SABR-COMET Phase II Randomized Trial. Int. J. Radiat. Oncol. 2022, 114, 1016–1021. [Google Scholar] [CrossRef]
- Gunjur, A.; Duong, C.; Ball, D.; Siva, S. Surgical and ablative therapies for the management of adrenal ‘oligometastases’—A systematic review. Cancer Treat. Rev. 2014, 40, 838–846. [Google Scholar] [CrossRef]
- König, L.; Häfner, M.F.; Katayama, S.; Koerber, S.A.; Tonndorf-Martini, E.; Bernhardt, D.; von Nettelbladt, B.; Weykamp, F.; Hoegen, P.; Klüter, S.; et al. Stereotactic body radiotherapy (SBRT) for adrenal metastases of oligometastatic or oligoprogressive tumor patients. Radiat. Oncol. 2020, 15, 30. [Google Scholar] [CrossRef]
- Harrow, S.; Palma, D.A.; Olson, R.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int. J. Radiat. Oncol. 2022, 114, 611–616. [Google Scholar] [CrossRef]
- Dupic, G.; Biau, J.; Bellière-Calandry, A.; Lapeyre, M. Radiothérapie stéréotaxique hypofractionnée des métastases surrénaliennes. Cancer/Radiothérapie 2017, 21, 404–410. [Google Scholar] [CrossRef]
- Chance, W.W.; Nguyen, Q.-N.; Mehran, R.; Welsh, J.W.; Gomez, D.R.; Balter, P.; Komaki, R.; Liao, Z.; Chang, J.Y. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract. Radiat. Oncol. 2017, 7, e195–e203. [Google Scholar] [CrossRef]
- Mutic, S.; Dempsey, J.F. The ViewRay System: Magnetic Resonance–Guided and Controlled Radiotherapy. Semin. Radiat. Oncol. 2014, 24, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Oelfke, U. Magnetic Resonance Imaging-guided Radiation Therapy: Technological Innovation Provides a New Vision of Radiation Oncology Practice. Clin. Oncol. 2015, 27, 495–497. [Google Scholar] [CrossRef] [PubMed]
- Datta, A.; Aznar, M.C.; Dubec, M.; Parker, G.J.M.; O’Connor, J.P.B. Delivering Functional Imaging on the MRI-Linac: Current Challenges and Potential Solutions. Clin. Oncol. 2018, 30, 702–710. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, L.; Josset, S.; Moignier, A.; Delpon, G. Machines de radiothérapie hybrides: Évolution ou révolution ? Cancer/Radiothérapie 2019, 23, 761–764. [Google Scholar] [CrossRef]
- Michalet, M.; Bordeau, K.; Cantaloube, M.; Valdenaire, S.; Debuire, P.; Simeon, S.; Portales, F.; Draghici, R.; Ychou, M.; Assenat, E.; et al. Stereotactic MR-Guided Radiotherapy for Pancreatic Tumors: Dosimetric Benefit of Adaptation and First Clinical Results in a Prospective Registry Study. Front. Oncol. 2022, 12, 842402. [Google Scholar] [CrossRef]
- Raaymakers, B.W.; Jürgenliemk-Schulz, I.M.; Bol, G.H.; Glitzner, M.; Kotte, A.N.T.J.; van Asselen, B.; de Boer, J.C.J.; Bluemink, J.J.; Hackett, S.L.; Moerland, M.A.; et al. First patients treated with a 1.5 T MRI-Linac: Clinical proof of concept of a high-precision, high-field MRI guided radiotherapy treatment. Phys. Med. Biol. 2017, 62, L41–L50. [Google Scholar] [CrossRef]
- Lim-Reinders, S.; Keller, B.M.; Al-Ward, S.; Sahgal, A.; Kim, A. Online Adaptive Radiation Therapy. Int. J. Radiat. Oncol. 2017, 99, 994–1003. [Google Scholar] [CrossRef]
- Ippolito, E.; D’Angelillo, R.M.; Fiore, M.; Molfese, E.; Trodella, L.; Ramella, S. SBRT: A viable option for treating adrenal gland metastases. Rep. Pract. Oncol. Radiother. 2015, 20, 484–490. [Google Scholar] [CrossRef]
- Rudra, S.; Malik, R.; Ranck, M.C.; Farrey, K.; Golden, D.W.; Hasselle, M.D.; Weichselbaum, R.R.; Salama, J.K. Stereotactic Body Radiation Therapy for Curative Treatment of Adrenal Metastases. Technol. Cancer Res. Treat. 2013, 12, 217–224. [Google Scholar] [CrossRef]
- Chen, W.C.; Baal, J.D.; Baal, U.; Pai, J.; Gottschalk, A.; Boreta, L.; Braunstein, S.E.; Raleigh, D.R. Stereotactic Body Radiation Therapy of Adrenal Metastases: A Pooled Meta-Analysis and Systematic Review of 39 Studies with 1006 Patients. Int. J. Radiat. Oncol. 2020, 107, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, L.L.; Kotecha, R.; Tom, M.C.; Chuong, M.D.; Contreras, J.A.; Romaguera, T.; Alvarez, D.; McCulloch, J.; Herrera, R.; Hernandez, R.J.; et al. CT-guided versus MR-guided radiotherapy: Impact on gastrointestinal sparing in adrenal stereotactic body radiotherapy. Radiother. Oncol. 2022, 166, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Fischer-Valuck, B.W.; Kashani, R.; Parikh, P.; Yang, D.; Zhao, T.; Green, O.; Wooten, O.; Li, H.H.; Hu, Y.; et al. Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications. Int. J. Radiat. Oncol. 2016, 94, 394–403. [Google Scholar] [CrossRef] [PubMed]
- Fischer-Valuck, B.W.; Henke, L.; Green, O.; Kashani, R.; Acharya, S.; Bradley, J.D.; Robinson, C.G.; Thomas, M.; Zoberi, I.; Thorstad, W.; et al. Two-and-a-half-year clinical experience with the world’s first magnetic resonance image guided radiation therapy system. Adv. Radiat. Oncol. 2017, 2, 485–493. [Google Scholar] [CrossRef]
- van Sörnsen de Koste, J.R.; Palacios, M.A.; Chen, H.; Schneiders, F.L.; Bruynzeel, A.M.E.; Slotman, B.J.; Haasbeek, C.J.A.; Senan, S. Changes in gastric anatomy after delivery of breath-hold MR-guided SABR for adrenal metastases. Radiother. Oncol. 2020, 152, 26–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Schneiders, F.L.; Bruynzeel, A.M.E.; Lagerwaard, F.J.; van Sörnsen de Koste, J.R.; Cobussen, P.; Bohoudi, O.; Slotman, B.J.; Louie, A.V.; Senan, S. Impact of daily plan adaptation on organ-at-risk normal tissue complication probability for adrenal lesions undergoing stereotactic ablative radiation therapy. Radiother. Oncol. 2021, 163, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Henke, L.; Kashani, R.; Robinson, C.; Curcuru, A.; DeWees, T.; Bradley, J.; Green, O.; Michalski, J.; Mutic, S.; Parikh, P.; et al. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother. Oncol. 2018, 126, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Nierer, L.; Eze, C.; da Silva Mendes, V.; Braun, J.; Thum, P.; von Bestenbostel, R.; Kurz, C.; Landry, G.; Reiner, M.; Niyazi, M.; et al. Dosimetric benefit of MR-guided online adaptive radiotherapy in different tumor entities: Liver, lung, abdominal lymph nodes, pancreas and prostate. Radiat. Oncol. 2022, 17, 53. [Google Scholar] [CrossRef]
- van Vliet, C.; Dickhoff, C.; Bahce, I.; Engelsman, A.F.; Hashemi, S.M.S.; Haasbeek, C.J.A.; Bruynzeel, A.M.E.; Palacios, M.A.; Becker-Commissaris, A.; Slotman, B.J.; et al. Treatment patterns for adrenal metastases using surgery and SABR during a 10-year period. Radiother. Oncol. 2022, 170, 165–168. [Google Scholar] [CrossRef]
| Organ | Dose Constraints (5 Fractions) | Dose Contraints (3 Fractions) |
|---|---|---|
| Stomach | Dmax < 32 Gy V18 Gy < 10 cm3 | Dmax < 24 Gy V 16 Gy< 10 cc |
| Duodenum | Dmax <32 Gy V18 Gy < 5 cm3 | Dmax < 24 Gy V 15 Gy< 5 cc |
| Small intestine | Dmax < 32 Gy V 19.5 < 5 cm3 | Dmax < 27 Gy V 16 Gy < 5 cc |
| Large intestine | Dmax < 32 Gy V 25 Gy < 5 cm3 | Dmax < 30 Gy V 20 Gy < 20 cc |
| Kydneys | V 14.4 Gy < 178 cc | |
| Kidneyipsilateral | V18 Gy < 33% V < 14.5 Gy > 130 cm3 (if single kidney) | V 12.1 Gy < 59 cc |
| Liver | V 15 Gy > 700 cm3 | Dmoy < 15 Gy V 15 Gy < 700 cc |
| Spinal cord | Dmax < 25 Gy | Dmax < 22 cc |
| Gender | |
|---|---|
| Women Men | 6 (50%) 6 (50%) |
| Median age (range) | 74 years (51–86) |
| Primary tumor | |
| Lung Kidney Bladder Prostate | 8 (66,7%) 2 (16,7%) 1 (8.3%) 1 (8.3%) |
| Stage | |
| Oligometastatic Multimetastatic | 7 (58.3%) 5 (41.7%) |
| Side | |
| Right adrenal Left adrenal Bilateral | 6 (50%) 5 (41,7%) 1 (8.3%) |
| WHO score | |
| 0 1 | 0 12 (100%) |
| Previous treatment received | |
| Primary cancer surgery Chemotherapy Radiotherapy of the primary tumor Immune Checkpoint inhibitor or targeted therapy Hormone therapy | 2 (22%) 5 (56%) 2 (22%) 5 (56%) 1 (11%) |
| Concomitant treatment | |
| Chemotherapy Hormone therapy Immune Checkpoint inhibitor-Pembrolizumab -Nivolumab | 0% 0% 4 (33.3%) 3 (25%) 1 (8.3%) |
| Total dose | 45 Gy (35–50) |
| Median dose per fraction | 10 Gy (7–12) |
| MedianTreatment duration | 7 days (5–10) |
| Median Fraction duration | 78 min (45–170) |
| Median follow up | 15.5 months (5–23) |
| Evolution (1 year) | |
| Local complete response Local partial response Local Stable disease Local recurrence Metastatic progression Death | 1 (8.3%) 1 (8.3%) 2 (16.7%) 2(16.7%) 6 (50%) 1 (8.3%) |
| Target Volume Median (Min–Max) | OAR (3 Fractions) Median (Min–Max) | OAR (5 Fraction) Median (Min–Max) | |
|---|---|---|---|
| PTV-opti V100% V95% V80% | 67.7% (47.41–91.55) 95.26% (73.76–100) 99.27% (94.11–100) | ||
| PTV V100% V95% V80% | 64.84% (47.41–85.57) 95.74% (76.96–98.57) 99.44% (90.07–100) | ||
| GTV V100% V95% V80% | 81.16 (53.49–97.44) 99.90 (77.98–100) 100 (90.24–100) | ||
| Kidney | V12.1 Gy: 10.6 (1.13–52.59) | V18 Gy: 4.69 cc (0–49.56) | |
| Spinal cord | Dmax: 10.685 (9.63–11.65) | Dmax: 12.55 Gy (6.47–20.74) | |
| Stomach | Dmax: 20.79 (7.49–22.16) V16 Gy: 2.12 (0–2.2) | Dmax: 28.54 Gy (4.06–34.37) V18 Gy: 3.89 cc (0–7.72) | |
| Duodenum | Dmax: 12.28 (0.62–23.68) V15 Gy: 0 (0–3.96) | Dmax: 26.28 Gy (2.12–30.53) V18 Gy: 1.06 cc (0–4.4) | |
| Small intestine | Dmax: 7.49 (0.85–18.32) V16 Gy: 0 (0–0.82) | Dmax: 26.04 Gy (4.35–30.74) V19.5 Gy: 0.79 cc (0–3.86) | |
| Large intestine | Dmax: 0.55 V20 Gy: 0 | Dmax: 19.75 Gy (0.63–29.02) V25 Gy: 0 cc (0–0.45) |
| Target Volume | Predicted Plan Median (Min–Max) | Adapted/Delivered Plan Median (Min–Max) | p-Value |
|---|---|---|---|
| PTV-opti V100% V95% V80% | 75.69% (11.89–92.7) 94.91% (62.68–99.02) 99.83% (85.93–100) | 67.31% (43.99–92.12) 96.68%(69.11–99.71) 99.97% (91.01–100) | 0.44 0.02 <0.0001 |
| PTV V100% V95% V80% | 73.95% (11.51–88.22) 89.85% (62.68–96.82) 97.1% (84.92–100) | 63.75% (38.63–88.36) 91.17% (60.88–97.53) 96.1% (81.47–100) | 0.58 0.0478 0.68 |
| GTV V100% V95% V80% | 87.58% (15.07–99.35) 96.86% (79.67–100) 99.24% (90.55–100) | 76.83% (51–96.75) 96.44% (74.87–100) 98.46% (90.44–100) | 0.72 0.20 0.34 |
| OAR | Predicted Fractions Median (Min–Max) | Adapted/Delivered Fractions Median (Min–Max) | p-Value |
|---|---|---|---|
| Kidney 5 fractions V18 Gy Kidney 3 fractions V12.1 Gy | 2.98 cc (0–48.85) 31.76 cc (13.08–55,07) | 3.06 cc (0–49.1) 32.92 cc (14.2–62.16) | 0.20 |
| Spinal cord 5 fractions Dmax Spinal cord 3 fractions Dmax | 12.28 Gy (5.08–20.97) 11.1 Gy (8.87–13.05) | 12.15 Gy (0.21–21.77) 10.9 Gy (8.86–12.74) | 0.93 |
| Stomach 5 fractions Dmax Stomach 3 fractions Dmax | 28.41 Gy (14.86–50.38) 16,76 Gy (4.75–38.53) | 26.98 Gy (15.47–34.28) 17.89 Gy (4.86–23.7) | 0.0003 |
| Duodenum 5 fractions Dmax Duodenum 3 fractions Dmax | 19 Gy (1.8–36.3) 15.79 Gy (0–33.8) | 19.8 Gy (0.1–32) 13.29 Gy (0–24.81) | 0.64 |
| Small intestine 5 fractions Dmax Small intestine 3 fractions Dmax | 21.47 Gy (2.35–45.69) 9.58 Gy (0.76–19.54) | 15.88 Gy (3.07–57.17) 9.21 Gy (0.76–19.09) | 0.02 |
| Large intestine 5 fractions Dmax Large intestine 3 fractions Dmax | 18.34 Gy (4.98–26.55) 1.48 Gy (0–6.51) | 18.23 Gy (0–29.54) 1.38 Gy (0–6.89) | 0.73 |
| CTCAE v5.0 | Toxicity (0–90 Days) | Toxicity (6 Months) | Toxicity (9 Months) | Toxicity (1 Year) |
|---|---|---|---|---|
| Abdominal pain g0 g1 g2 g3 Ongoing | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
| Nausea/vomiting g0 g1 g2 g3 Ongoing | 10 (83.4%) 1 (8.3%) 1 (8.3%) 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
| Gastritis/Enteritis g0 g1 g2 g3 Ongoing | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
| Peptic ulcer g0 g1 g2 g3 Ongoing | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
| Digestive fistula g0 g1 g2 g3 Ongoing | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
| Diarrhea g0 g1 g2 g3 Ongoing | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
| Asthenia g0 g1 g2 g3 Ongoing | 8 (66.7%) 3 (25%) 1 (8.3%) 0 0 | 11 (91.7%) 1 (8.3%) 0 0 0 | 12 (100%) 0 0 0 0 | 12 (100%) 0 0 0 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalet, M.; Bettaïeb, O.; Khalfi, S.; Ghorbel, A.; Valdenaire, S.; Debuire, P.; Aillères, N.; Draghici, R.; De Méric De Bellefon, M.; Charissoux, M.; et al. Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results. J. Clin. Med. 2023, 12, 291. https://doi.org/10.3390/jcm12010291
Michalet M, Bettaïeb O, Khalfi S, Ghorbel A, Valdenaire S, Debuire P, Aillères N, Draghici R, De Méric De Bellefon M, Charissoux M, et al. Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results. Journal of Clinical Medicine. 2023; 12(1):291. https://doi.org/10.3390/jcm12010291
Chicago/Turabian StyleMichalet, Morgan, Ons Bettaïeb, Samia Khalfi, Asma Ghorbel, Simon Valdenaire, Pierre Debuire, Norbert Aillères, Roxana Draghici, Mailys De Méric De Bellefon, Marie Charissoux, and et al. 2023. "Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results" Journal of Clinical Medicine 12, no. 1: 291. https://doi.org/10.3390/jcm12010291
APA StyleMichalet, M., Bettaïeb, O., Khalfi, S., Ghorbel, A., Valdenaire, S., Debuire, P., Aillères, N., Draghici, R., De Méric De Bellefon, M., Charissoux, M., Boisselier, P., Demontoy, S., Marguerit, A., Cabaillé, M., Cantaloube, M., Keskes, A., Bouhafa, T., Farcy-Jacquet, M.-P., Fenoglietto, P., ... Riou, O. (2023). Stereotactic MR-Guided Radiotherapy for Adrenal Gland Metastases: First Clinical Results. Journal of Clinical Medicine, 12(1), 291. https://doi.org/10.3390/jcm12010291








