Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery
Abstract
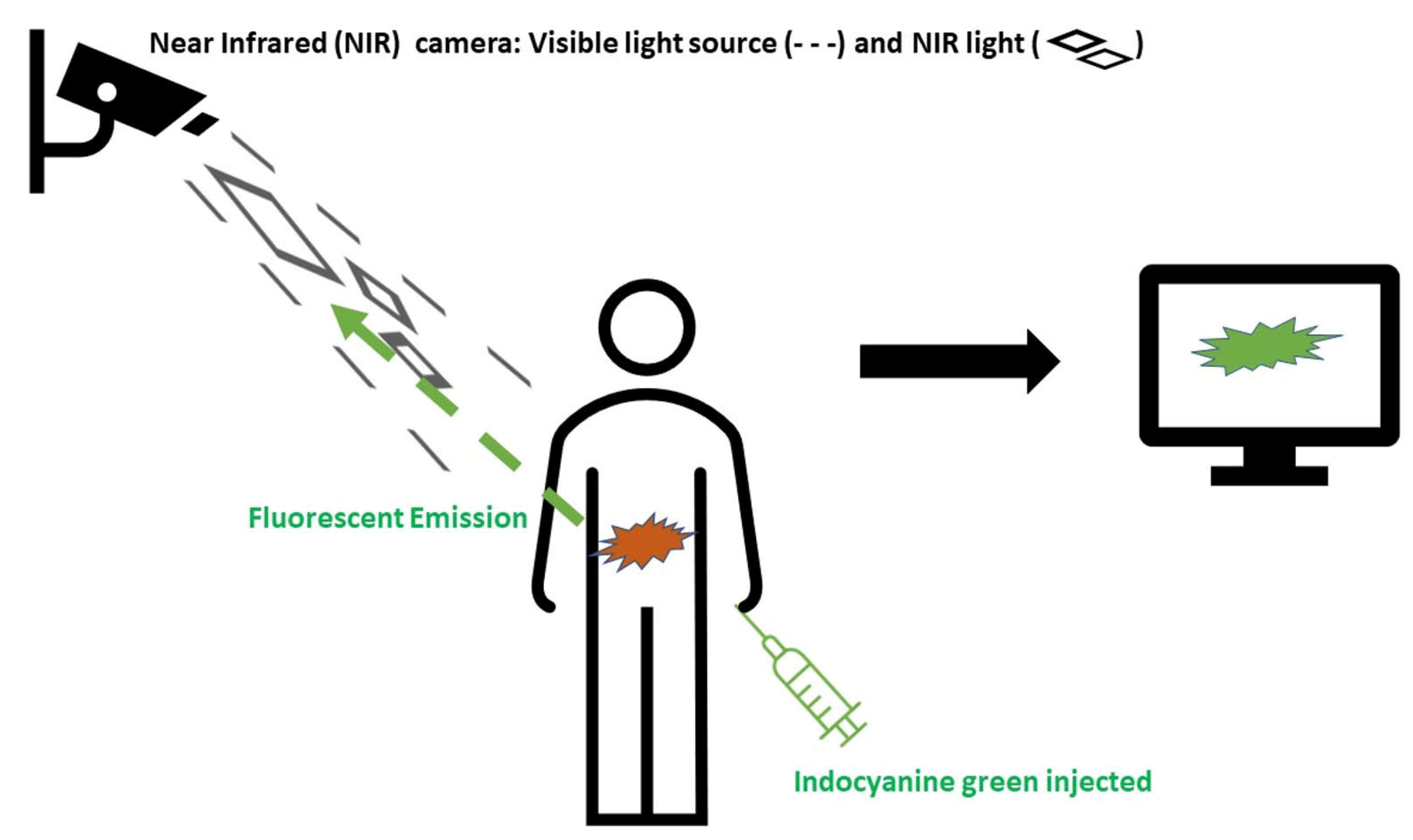
1. Introduction
2. Methods
3. Current Uses of ICG in Colorectal Surgery
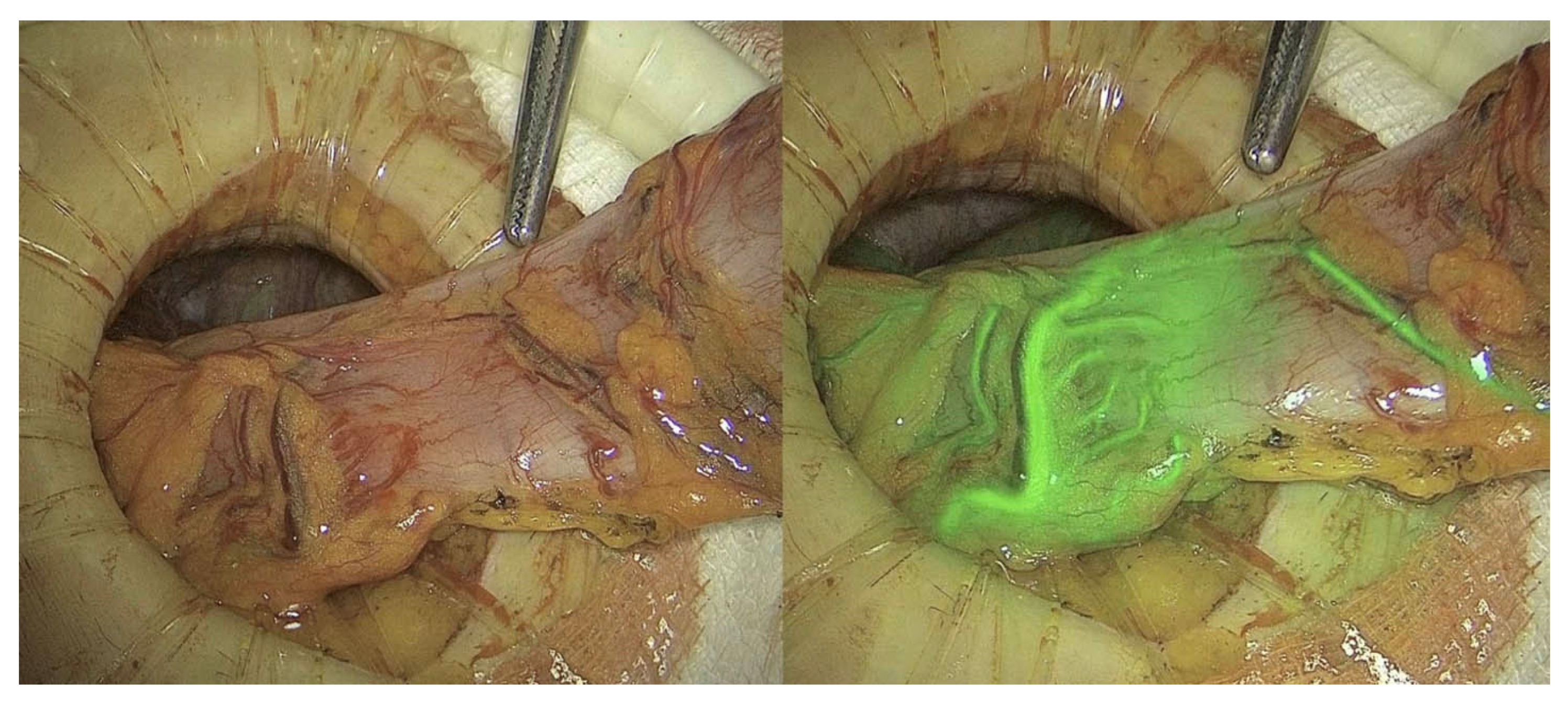
3.1. Perfusion Assessment
3.1.1. Anastomotic and Bowel Perfusion Assessment
3.1.2. Perfusion Assessment in Pedicled Omentoplasty
3.1.3. Perfusion Assessment in Gracilis Muscle Interposition
3.1.4. Perfusion Assessment in Anal Advancement Flaps
3.1.5. Perfusion Assessment in Ileal Pouch-Anal Anastomosis (IPAA)
3.2. Vital Structures Assessment
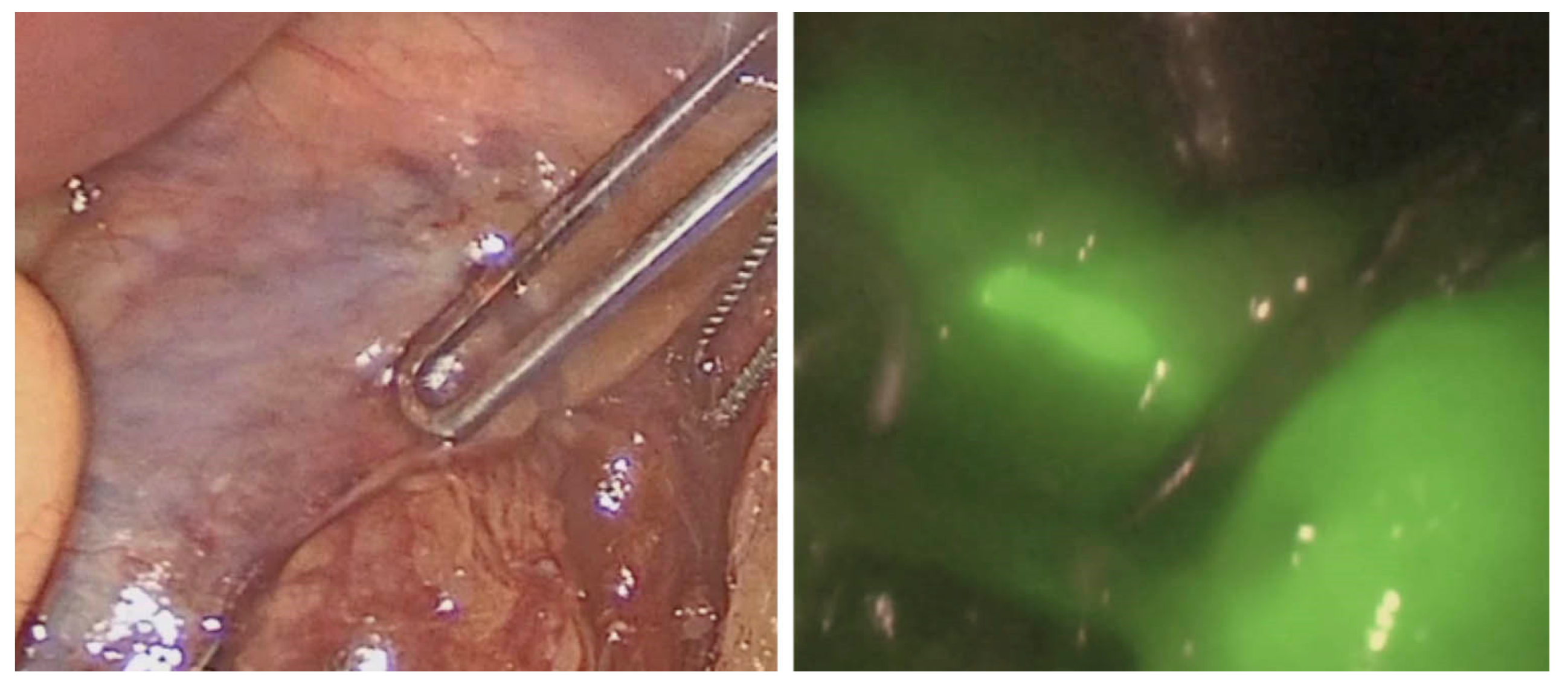
3.2.1. Intraoperative Ureteral Assessment
3.2.2. Intraoperative Urethral Assessment
3.2.3. Intraoperative Nerve Assessment
3.3. Tumor Assessment
3.4. Lymphatic Mapping
3.5. Sentinel Lymph Node Assessment
3.6. Lateral Pelvic Lymph Node Assessment
3.7. Distant Disease Assessment
3.7.1. Peritoneal Metastases Assessment
3.7.2. Liver Metastases Assessment
4. Discussions and Future Perspectives
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alander, J.T.; Kaartinen, I.; Laakso, A.; Pätilä, T.; Spillmann, T.; Tuchin, V.V.; Venermo, M.; Välisuo, P. A Review of Indocyanine Green Fluorescent Imaging in Surgery. Int. J. Biomed. Imaging 2012, 2012, 940585. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.cancer.gov/publications/dictionaries/cancer-drug/def/indocyanine-green-solution?redirect=true (accessed on 25 November 2022).
- Tajima, Y.; Murakami, M.; Yamazaki, K.; Masuda, Y.; Kato, M.; Sato, A.; Goto, S.; Otsuka, K.; Kato, T.; Kusano, M. Sentinel node mapping guided by indocyanine green fluorescence imaging during laparoscopic surgery in gastric cancer. Ann. Surg. Oncol. 2010, 17, 1787–1793. [Google Scholar] [CrossRef]
- Ellis, C.T.; Maykel, J.A. Defining Anastomotic Leak and the Clinical Relevance of Leaks. Clin. Colon Rectal Surg. 2021, 34, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Benčurik, V.; Škrovina, M.; Martínek, L.; Bartoš, J.; Macháčková, M.; Dosoudil, M.; Štěpánová, E.; Přibylová, L.; Briš, R.; Vomáčková, K. Intraoperative fluorescence angiography and risk factors of anastomotic leakage in mini-invasive low rectal resections. Surg. Endosc. 2021, 35, 5015–5023. [Google Scholar] [CrossRef]
- Bonadio, L.; Iacuzzo, C.; Cosola, D.; Cipolat Mis, T.; Giudici, F.; Casagranda, B.; Biloslavo, A.; de Manzini, N. Indocyanine green-enhanced fluorangiography (ICGf) in laparoscopic extraperitoneal rectal cancer resection. Updates Surg. 2020, 72, 477–482. [Google Scholar] [CrossRef] [PubMed]
- Dinallo, A.M.; Kolarsick, P.; Boyan, W.P.; Protyniak, B.; James, A.; Dressner, R.M.; Arvanitis, M.L. Does routine use of indocyanine green fluorescence angiography prevent anastomotic leaks? A retrospective cohort analysis. Am. J. Surg. 2019, 218, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Kin, C.; Vo, H.; Welton, L.; Welton, M. Equivocal effect of intraoperative fluorescence angiography on colorectal anastomotic leaks. Dis. Colon Rectum 2015, 58, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Jafari, M.D.; Wexner, S.D.; Martz, J.E.; McLemore, E.C.; Margolin, D.A.; Sherwinter, D.A.; Lee, S.W.; Senagore, A.J.; Phelan, M.J.; Stamos, M.J. Perfusion assessment in laparoscopic left-sided/anterior resection (PILLAR II): A multi-institutional study. J. Am. Coll. Surg. 2015, 220, 82–92.e1. [Google Scholar] [CrossRef]
- De Nardi, P.; Elmore, U.; Maggi, G.; Maggiore, R.; Boni, L.; Cassinotti, E.; Fumagalli, U.; Gardani, M.; De Pascale, S.; Parise, P.; et al. Intraoperative angiography with indocyanine green to assess anastomosis perfusion in patients undergoing laparoscopic colorectal resection: Results of a multicenter randomized controlled trial. Surg. Endosc. 2020, 34, 53–60. [Google Scholar] [CrossRef]
- Alekseev, M.; Rybakov, E.; Shelygin, Y.; Chernyshov, S.; Zarodnyuk, I. A study investigating the perfusion of colorectal anastomoses using fluorescence angiography: Results of the FLAG randomized trial. Color. Dis. 2020, 22, 1147–1153. [Google Scholar] [CrossRef]
- Jafari, M.D.; Pigazzi, A.; McLemore, E.C.; Mutch, M.G.; Haas, E.; Rasheid, S.H.; Wait, A.D.; Paquette, I.M.; Bardakcioglu, O.; Safar, B.; et al. Perfusion Assessment in Left-Sided/Low Anterior Resection (PILLAR III): A Randomized, Controlled, Parallel, Multicenter Study Assessing Perfusion Outcomes with PINPOINT Near-Infrared Fluorescence Imaging in Low Anterior Resection. Dis. Colon Rectum 2021, 64, 995–1002. [Google Scholar] [CrossRef] [PubMed]
- Safiejko, K.; Tarkowski, R.; Kozlowski, T.P.; Koselak, M.; Jachimiuk, M.; Tarasik, A.; Pruc, M.; Smereka, J.; Szarpak, L. Safety and Efficacy of Indocyanine Green in Colorectal Cancer Surgery: A Systematic Review and Meta-Analysis of 11,047 Patients. Cancers 2022, 14, 1036. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Khan, S.M.; Wexner, S.D. Impact of change in the surgical plan based on indocyanine green fluorescence angiography on the rates of colorectal anastomotic leak: A systematic review and meta-analysis. Surg. Endosc. 2022, 36, 2245–2257. [Google Scholar] [CrossRef] [PubMed]
- Meijer, R.P.J.; Faber, R.A.; Bijlstra, O.D.; Braak, J.P.B.M.; Meershoek-Klein Kranenbarg, E.; Putter, H.; Mieog, J.S.D.; Burggraaf, K.; Vahrmeijer, A.L.; Hilling, D.E.; et al. AVOID; a phase III, randomised controlled trial using indocyanine green for the prevention of anastomotic leakage in colorectal surgery. BMJ Open 2022, 12, e051144. [Google Scholar] [CrossRef] [PubMed]
- Serra-Aracil, X.; Lucas-Guerrero, V.; Garcia-Nalda, A.; Mora-López, L.; Pallisera-Lloveras, A.; Serracant, A.; Navarro-Soto, S. When should indocyanine green be assessed in colorectal surgery, and at what distance from the tissue? Quantitative measurement using the SERGREEN program. Surg. Endosc. 2022, 36, 8943–8949. [Google Scholar] [CrossRef]
- Gomez-Rosado, J.C.; Valdes-Hernandez, J.; Cintas-Catena, J.; Cano-Matias, A.; Perez-Sanchez, A.; Del Rio-Lafuente, F.J.; Torres-Arcos, C.; Lara-Fernandez, Y.; Capitan-Morales, L.C.; Oliva-Mompean, F. Feasibility of quantitative analysis of colonic perfusion using indocyanine green to prevent anastomotic leak in colorectal surgery. Surg. Endosc. 2022, 36, 1688–1695. [Google Scholar] [CrossRef]
- D’Urso, A.; Agnus, V.; Barberio, M.; Seeliger, B.; Marchegiani, F.; Charles, A.L.; Geny, B.; Marescaux, J.; Mutter, D.; Diana, M. Computer-assisted quantification and visualization of bowel perfusion using fluorescence-based enhanced reality in left-sided colonic resections. Surg. Endosc. 2021, 35, 4321–4331. [Google Scholar] [CrossRef]
- Hayami, S.; Matsuda, K.; Iwamoto, H.; Ueno, M.; Kawai, M.; Hirono, S.; Okada, K.; Miyazawa, M.; Tamura, K.; Mitani, Y.; et al. Visualization and quantification of anastomotic perfusion in colorectal surgery using near-infrared fluorescence. Tech. Coloproctol. 2019, 23, 973–980. [Google Scholar] [CrossRef]
- Liu, R.Q.; Elnahas, A.; Tang, E.; Alkhamesi, N.A.; Hawel, J.; Alnumay, A.; Schlachta, C.M. Cost analysis of indocyanine green fluorescence angiography for prevention of anastomotic leakage in colorectal surgery. Surg. Endosc. 2022, 36, 9281–9287. [Google Scholar] [CrossRef]
- Slooter, M.D.; Blok, R.D.; Wisselink, D.D.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J.; Hompes, R. Near-infrared fluorescence angiography for intra-operative assessment of pedicled omentoplasty for filling of a pelvic cavity: A pilot study. Tech. Coloproctol. 2019, 23, 723–728. [Google Scholar] [CrossRef]
- Slooter, M.D.; Blok, R.D.; de Krom, M.A.; Buskens, C.J.; Bemelman, W.A.; Tanis, P.J.; Hompes, R. Optimizing omentoplasty for management of chronic pelvic sepsis by intra-operative fluorescence angiography: A comparative cohort study. Color. Dis. 2020, 22, 2252–2259. [Google Scholar] [CrossRef] [PubMed]
- Garoufalia, Z.; Gefen, R.; Emile, S.H.; Silva-Alvarenga, E.; Horesh, N.; Freund, M.R.; Wexner, S.D. Gracilis Muscle Interposition for Complex Perineal Fistulae: A Systematic Review and Meta-Analysis of the Literature. Color. Dis. 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, L.A.; Hoveling, R.J.M.; Schmidt, L.R.; Berns, S.; Weixler, B. Objective Perfusion Assessment in Gracilis Muscle Interposition—A Novel Software-Based Approach to Indocyanine Green Derived Near-Infrared Fluorescence in Reconstructive Surgery. Life 2022, 12, 278. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.S.; Okonkwo, A.; Chase, A.; Clark, C.E. Early outcomes of fluorescence angiography in the setting of endorectal mucosa advancement flaps. Tech. Coloproctol. 2018, 22, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Joosten, J.J.; Reijntjes, M.A.; Slooter, M.D.; Duijvestein, M.; Buskens, C.J.; Bemelman, W.A.; Hompes, R. Fluorescence angiography after vascular ligation to make the ileo-anal pouch reach. Tech. Coloproctol. 2021, 25, 875–878. [Google Scholar] [CrossRef] [PubMed]
- Freund, M.R.; Kent, I.; Agarwal, S.; Wexner, S.D. Use of indocyanine green fluorescence angiography during ileal J-pouch surgery requiring lengthening maneuvers. Tech. Coloproctol. 2022, 26, 181–186. [Google Scholar] [CrossRef]
- Slooter, M.D.; van der Does de Willebois, E.M.L.; Joosten, J.J.; Reijntjes, M.A.; Buskens, C.J.; Tanis, P.J.; Bemelman, W.A.; Hompes, R. Fluorescence Perfusion Assessment of Vascular Ligation during Ileal Pouch-Anal Anastomosis. Tech. Coloproctol. 2022; ahead of print. [Google Scholar] [CrossRef]
- Lobbes, L.A.; Hoveling, R.J.M.; Berns, S.; Schmidt, L.R.; Strobel, R.M.; Schineis, C.; Lauscher, J.C.; Beyer, K.; Weixler, B. Feasibility of Novel Software-Based Perfusion Indicators for the Ileal J-Pouch-On the Path towards Objective and Quantifiable Intraoperative Perfusion Assessment with Indocyanine Green Near-Infrared Fluorescence. Life 2022, 12, 1144. [Google Scholar] [CrossRef]
- Kanabur, P.; Chai, C.; Taylor, J. Use of indocyanine green for intraoperative ureteral identification in nonurologic surgery. JAMA Surg. 2020, 155, 520–521. [Google Scholar] [CrossRef]
- White, L.A.; Joseph, J.P.; Yang, D.Y.; Kelley, S.R.; Mathis, K.L.; Behm, K.; Viers, B.R. Intraureteral indocyanine green augments ureteral identification and avoidance during complex robotic-assisted colorectal surgery. Color. Dis. 2021, 23, 718–723. [Google Scholar] [CrossRef]
- Hamada, M.; Matsumi, Y.; Sekimoto, M.; Kurokawa, H.; Kita, M.; Kinoshita, H. Image navigation surgery with the fluorescent ureteral catheter of recurrent tumors in the pelvic cavity. Dis. Colon Rectum 2022, 65, e72–e76. [Google Scholar] [CrossRef] [PubMed]
- Mandovra, P.; Kalikar, V.; Patankar, R.V. Real-time visualization of ureters using indocyanine green during laparoscopic surgeries: Can we make surgery safer? Surg. Innov. 2019, 26, 464–468. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Hara, K.; Kitagawa, T.; Okamoto, A.; Marukuchi, R.; Ito, R.; Nakabayashi, Y. Fluorescence vessel and ureter navigation during laparoscopic lateral lymph node dissection. Langenbeck’s Arch. Surg. 2022, 407, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Satish, V.N.V.R.; Acharya, A.; Ramachandran, S.; Narasimhan, M.; Ardhanari, R. Fluorescent ureterography with indocyanine green in laparoscopic colorectal surgery: A safe method to prevent intraoperative ureteric injury. J. Minim. Access Surg. 2022, 18, 320–323. [Google Scholar]
- Soriano, C.R.; Cheng, R.R.; Corman, J.M.; Moonka, R.; Simianu, V.V.; Kaplan, J.A. Feasibility of injected indocyanine green for ureteral identification during robotic left-sided colorectal resections. Am. J. Surg. 2022, 223, 14–20. [Google Scholar] [CrossRef]
- Garoufalia, Z.; Wexner, S.D. Ureter identification utilizing indocyanine green (ICG) imaging in colorectal surgery: A systematic review of the literature. Mini-Invasive Surg. 2022, 6, 51. [Google Scholar] [CrossRef]
- Dip, F.D.; Nahmod, M.; Anzorena, F.S.; Moreira, A.; Sarotto, L.; Ampudia, C.; Kalaskar, S.N.; Ferraina, P.; Rosenthal, R.J.; Wexner, S.D. Novel technique for identification of ureters using sodium fluorescein. Surg. Endosc. 2014, 28, 2730–2733. [Google Scholar] [CrossRef]
- Mahalingam, S.M.; Dip, F.; Castillo, M.; Roy, M.; Wexner, S.D.; Rosenthal, R.J.; Low, P.S. Intraoperative Ureter Visualization Using a Novel Near-Infrared Fluorescent Dye. Mol. Pharm. 2018, 15, 3442–3447. [Google Scholar] [CrossRef]
- Penna, M.; Hompes, R.; Arnold, S.; Wynn, G.; Austin, R.; Warusavitarne, J.; Moran, B.; Hanna, G.B.; Mortensen, N.J.; Tekkis, P.P. Transanal total mesorectal excision: International registry results of the first 720 cases. Ann. Surg. 2017, 266, 111–117. [Google Scholar] [CrossRef]
- Rouanet, P.; Mourregot, A.; Azar, C.C.; Carrere, S.; Gutowski, M.; Quenet, F.; Saint-Aubert, B.; Colombo, P.E. Transanal endoscopic proctectomy: An innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis. Colon Rectum 2013, 56, 408–415. [Google Scholar] [CrossRef]
- Barnes, T.G.; Penna, M.; Hompes, R.; Cunningham, C. Fluorescence to highlight the urethra: A human cadaveric study. Tech. Coloproctol. 2017, 21, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Barnes, T.G.; Volpi, D.; Cunningham, C.; Vojnovic, B.; Hompes, R. Improved urethral fluorescence during low rectal surgery: A new dye and a new method. Tech. Coloproctol. 2018, 22, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Barberio, M.; Al-Taher, M.; Forgione, A.; Hoskere Ashoka, A.; Felli, E.; Agnus, V.; Marescaux, J.; Klymchenko, A.; Diana, M. A novel method for near-infrared fluorescence imaging of the urethra during perineal and transanal surgery: Demonstration in a cadaveric model. Color. Dis. 2020, 22, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Tanaka, K.; Kataoka, J.; Ohta, M.; Ishii, M.; Ishibashi, T.; Okuda, J. Novel technique with the IRIS U kit to prevent urethral injury in patients undergoing transanal total mesorectal excision. Ann. Med. Surg. 2019, 46, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zheng, L.; Lu, L.; Cui, M. Near-infrared intraoperative imaging of pelvic autonomic nerves: A pilot study. Surg. Endosc. 2022, 36, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Handgraaf, H.J.; Boogerd, L.S.; Verbeek, F.P.; Tummers, Q.R.; Hardwick, J.C.; Baeten, C.I.; Frangioni, J.V.; van de Velde, C.J.; Vahrmeijer, A.L. Intraoperative fluorescence imaging to localize tumors and sentinel lymph nodes in rectal cancer. Minim. Invasive Ther. Allied Technol. 2016, 25, 48–53. [Google Scholar] [CrossRef]
- Watanabe, M.; Tsunoda, A.; Narita, K.; Kusano, M.; Miwa, M. Colonic tattooing using fluorescence imaging with light-emitting diode-activated indocyanine green: A feasibility study. Surg. Today 2009, 39, 214–218. [Google Scholar] [CrossRef]
- Poris, S.P.; Tanishima, H.; Albert, M.R. Use of intraoperative submucosal tattooing with indocyanine green immunofluorescence angiography for tumor localization during colectomy. Tech. Coloproctol. 2017, 21, 165–166. [Google Scholar] [CrossRef]
- Kim, Y.J.; Park, J.W.; Lim, H.K.; Kwon, Y.H.; Kim, M.J.; Choe, E.K.; Moon, S.H.; Ryoo, S.B.; Jeong, S.Y.; Park, K.J. Preoperative Colonoscopic Tattooing Using a Direct Injection Method with Indocyanine Green for Localization of Colorectal Tumors: An Efficacy and Safety Comparison Study. J. Minim. Invasive Surg. 2020, 23, 186–190. [Google Scholar] [CrossRef]
- Kim, J.H. Small Efforts Can Prevent Big Mistakes: Preoperative Colonoscopic Tattooing Using Indocyanine Green. J. Minim. Invasive Surg. 2020, 23, 159–160. [Google Scholar] [CrossRef]
- Lee, S.J.; Sohn, D.K.; Han, K.S.; Kim, B.C.; Hong, C.W.; Park, S.C.; Kim, M.J.; Park, B.K.; Oh, J.H. Preoperative tattooing using indocyanine green in laparoscopic colorectal surgery. Ann. Coloproctol. 2018, 34, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Atallah, S.; Parra-Davila, E.; Melani, A.G.F.; Romagnolo, L.G.; Larach, S.W.; Marescaux, J. Robotic-assisted stereotactic real-time navigation: Initial clinical experience and feasibility for rectal cancer surgery. Tech. Coloproctol. 2019, 23, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Satoyoshi, T.; Okita, K.; Ishii, M.; Hamabe, A.; Usui, A.; Akizuki, E.; Okuya, K.; Nishidate, T.; Yamano, H.; Nakase, H.; et al. Timing of indocyanine green injection prior to laparoscopic colorectal surgery for tumor localization: A prospective case series. Surg. Endosc. 2021, 35, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.M.; Son, G.M.; Lee, I.Y.; Shin, D.H.; Kim, T.K.; Park, S.B.; Kim, H.W. Optimal ICG dosage of preoperative colonoscopic tattooing for fluorescence-guided laparoscopic colorectal surgery. Surg. Endosc. 2022, 36, 1152–1163. [Google Scholar] [CrossRef]
- Narihiro, S.; Yoshida, M.; Ohdaira, H.; Sato, T.; Suto, D.; Hoshimoto, S.; Suzuki, N.; Marukuchi, R.; Kamada, T.; Takeuchi, H.; et al. Effectiveness and safety of tumor site marking with near-infrared fluorescent clips in colorectal laparoscopic surgery: A case series study. Int. J. Surg. 2020, 80, 74–78. [Google Scholar] [CrossRef]
- Lee, D.W.; Sohn, D.K.; Han, K.S.; Hong, C.W.; Park, H.C.; Oh, J.H. Promising Novel Technique for Tumor Localization in Laparoscopic Colorectal Surgery Using Indocyanine Green-Coated Endoscopic Clips. Dis. Colon Rectum 2021, 64, e9–e13. [Google Scholar] [CrossRef]
- Ryu, S.; Okamoto, A.; Nakashima, K.; Hara, K.; Ishida, K.; Ito, R.; Nakabayashi, Y.; Eto, K.; Ikegami, T. Usefulness of Preoperative Endoscopic Fluorescent Clip Marking in Laparoscopic Gastrointestinal Surgery. Anticancer Res. 2020, 40, 6517–6523. [Google Scholar] [CrossRef]
- Nishigori, N.; Koyama, F.; Nakagawa, T.; Nakamura, S.; Ueda, T.; Inoue, T.; Kawasaki, K.; Obara, S.; Nakamoto, T.; Fujii, H.; et al. Visualization of Lymph/Blood Flow in Laparoscopic Colorectal Cancer Surgery by ICG Fluorescence Imaging (Lap-IGFI). Ann. Surg. Oncol. 2016, 23 (Suppl. S2), 266–274. [Google Scholar] [CrossRef]
- Ho, M.F.; Futaba, K.; Mak, T.W.C.; Ng, S.S.M. Personalized laparoscopic resection of colon cancer with the use of indocyanine green lymph node mapping: Technical and clinical outcomes. Asian J. Endosc. Surg. 2022, 15, 563–568. [Google Scholar] [CrossRef]
- Caprioli, M.; Garosio, I.; Botteri, E.; Vettoretto, N.; Molteni, B.; Molfino, S.; Yiu, D.; Portolani, N.; Baiocchi, G.L. Fluorescence-guided nodal navigation during colectomy for colorectal cancer. Minim. Invasive Ther. Allied Technol. 2022, 31, 879–886. [Google Scholar] [CrossRef]
- Soares, A.S.; Lovat, L.B.; Chand, M. Intracorporeal lymph node mapping in colon cancer surgery. Eur. J. Surg. Oncol. 2019, 45, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Chand, M.; Keller, D.S.; Joshi, H.M.; Devoto, L.; Rodriguez-Justo, M.; Cohen, R. Feasibility of fluorescence lymph node imaging in colon cancer: FLICC. Tech. Coloproctol. 2018, 22, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Goo, J.J.; Ryu, D.G.; Kim, H.W.; Park, S.B.; Kang, D.H.; Choi, C.W.; Kim, S.J.; Nam, H.S.; Kim, H.S.; Son, G.M.; et al. Efficacy of preoperative colonoscopic tattooing with indocyanine green on lymph node harvest and factors associated with inadequate lymph node harvest in colorectal cancer. Scand. J. Gastroenterol. 2019, 54, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Kakizoe, M.; Watanabe, J.; Suwa, Y.; Nakagawa, K.; Suwa, H.; Ozawa, M.; Ishibe, A.; Masui, H.; Nagahori, K. The histopathological evaluation based on the indocyanine green fluorescence imaging of regional lymph node metastasis of splenic flexural colon cancer by near-infrared observation. Int. J. Colorectal Dis. 2021, 36, 717–723. [Google Scholar] [CrossRef]
- Ushijima, H.; Kawamura, J.; Ueda, K.; Yane, Y.; Yoshioka, Y.; Daito, K.; Tokoro, T.; Hida, J.I.; Okuno, K. Visualization of lymphatic flow in laparoscopic colon cancer surgery using indocyanine green fluorescence imaging. Sci. Rep. 2020, 10, 14274. [Google Scholar] [CrossRef]
- Young, R.; Rajkomar, A.K.S.; Smart, P.; Warrier, S.K. Robotic complete mesocolic excision using indocyanine fluorescence imaging in colorectal cancer: A case study and technical approach. Int. J. Surg. Case Rep. 2020, 69, 32–34. [Google Scholar] [CrossRef]
- Petz, W.; Bertani, E.; Borin, S.; Fiori, G.; Ribero, D.; Spinoglio, G. Fluorescence-guided D3 lymphadenectomy in robotic right colectomy with complete mesocolic excision. Int. J. Med. Robot. 2021, 17, e2217. [Google Scholar] [CrossRef]
- Spinoglio, G.; Petz, W.; Borin, S.; Piccioli, A.N.; Bertani, E. Robotic right colectomy with complete mesocolic excision and indocyanine green guidance. Minerva Chir. 2019, 74, 165–169. [Google Scholar] [CrossRef]
- Trujillo-Díaz, J.J.; Pérez-Corbal, L.; Alarcón Del Agua, I.; Licardie Bolaños, E.; Senent Boza, A.; Morales-Conde, S. Complete mesocolon excision guided by indocyanine green for right colonic cancer. Color. Dis. 2021, 23, 2779–2780. [Google Scholar] [CrossRef]
- Park, S.Y.; Park, J.S.; Kim, H.J.; Woo, I.T.; Park, I.K.; Choi, G.S. Indocyanine Green Fluorescence Imaging-Guided Laparoscopic Surgery Could Achieve Radical D3 Dissection in Patients with Advanced Right-Sided Colon Cancer. Dis. Colon Rectum 2020, 63, 441–449. [Google Scholar] [CrossRef]
- Ribero, D.; Mento, F.; Sega, V.; Lo Conte, D.; Mellano, A.; Spinoglio, G. ICG-Guided Lymphadenectomy during Surgery for Colon and Rectal Cancer-Interim Analysis of the GREENLIGHT Trial. Biomedicines 2022, 10, 541. [Google Scholar] [CrossRef]
- van der Pas, M.H.; Meijer, S.; Hoekstra, O.S.; Riphagen, I.I.; de Vet, H.C.; Knol, D.L.; van Grieken, N.C.; Meijerink, W.J. Sentinel-lymph-node procedure in colon and rectal cancer: A systematic review and meta-analysis. Lancet Oncol. 2011, 12, 540–550. [Google Scholar] [CrossRef]
- Gould, E.A.; Winship, T.; Philbin, P.H.; Kerr, H.H. Observations on a “sentinel node” in cancer of the parotid. Cancer 1960, 13, 77–78. [Google Scholar] [CrossRef] [PubMed]
- Andersen, H.S.; Bennedsen, A.L.B.; Burgdorf, S.K.; Eriksen, J.R.; Eiholm, S.; Toxværd, A.; Riis, L.B.; Rosenberg, J.; Gögenur, I. In vivo and ex vivo sentinel node mapping does not identify the same lymph nodes in colon cancer. Int. J. Colorectal Dis. 2017, 32, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Cahill, R.A.; Anderson, M.; Wang, L.M.; Lindsey, I.; Cunningham, C.; Mortensen, N.J. Near-infrared (NIR) laparoscopy for intraoperative lymphatic road-mapping and sentinel node identification during definitive surgical resection of early-stage colorectal neoplasia. Surg. Endosc. 2012, 26, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Currie, A.C.; Brigic, A.; Thomas-Gibson, S.; Suzuki, N.; Moorghen, M.; Jenkins, J.T.; Faiz, O.D.; Kennedy, R.H. A pilot study to assess near infrared laparoscopy with indocyanine green (ICG) for intraoperative sentinel lymph node mapping in early colon cancer. Eur. J. Surg. Oncol. 2017, 43, 2044–2051. [Google Scholar] [CrossRef] [PubMed]
- Hirche, C.; Mohr, Z.; Kneif, S.; Doniga, S.; Murawa, D.; Strik, M.; Hünerbein, M. Ultrastaging of colon cancer by sentinel node biopsy using fluorescence navigation with indocyanine green. Int. J. Colorectal Dis. 2012, 27, 319–324. [Google Scholar] [CrossRef]
- Kusano, M.; Tajima, Y.; Yamazaki, K.; Kato, M.; Watanabe, M.; Miwa, M. Sentinel node mapping guided by indocyanine green fluorescence imaging: A new method for sentinel node navigation surgery in gastrointestinal cancer. Dig. Surg. 2008, 25, 103–108. [Google Scholar] [CrossRef]
- Liberale, G.; Galdon, M.G.; Moreau, M.; Vankerckhove, S.; El Nakadi, I.; Larsimont, D.; Donckier, V.; Bourgeois, P. Ex vivo detection of tumoral lymph nodes of colorectal origin with fluorescence imaging after intraoperative intravenous injection of indocyanine green. J. Surg. Oncol. 2016, 114, 348–353. [Google Scholar] [CrossRef]
- Liberale, G.; Vankerckhove, S.; Galdon, M.G.; Larsimont, D.; Ahmed, B.; Bouazza, F.; Moreau, M.; El Nakadi, I.; Donckier, V.; Bourgeois, P.; et al. Sentinel Lymph Node Detection by Blue Dye versus Indocyanine Green Fluorescence Imaging in Colon Cancer. Anticancer Res. 2016, 36, 4853–4858. [Google Scholar] [CrossRef]
- Noura, S.; Ohue, M.; Seki, Y.; Tanaka, K.; Motoori, M.; Kishi, K.; Miyashiro, I.; Ohigashi, H.; Yano, M.; Ishikawa, O.; et al. Feasibility of a lateral region sentinel node biopsy of lower rectal cancer guided by indocyanine green using a near-infrared camera system. Ann. Surg. Oncol. 2010, 17, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Weixler, B.; Rickenbacher, A.; Raptis, D.A.; Viehl, C.T.; Guller, U.; Rueff, J.; Zettl, A.; Zuber, M. Sentinel Lymph Node Mapping with Isosulfan Blue or Indocyanine Green in Colon Cancer Shows Comparable Results and Identifies Patients with Decreased Survival: A Prospective Single-Center Trial. World J. Surg. 2017, 41, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Sileri, P.; Laurberg, S.; Wexner, S.D. Sensitivity and specificity of indocyanine green near-infrared fluorescence imaging in detection of metastatic lymph nodes in colorectal cancer: Systematic review and meta-analysis. J. Surg. Oncol. 2017, 116, 730–740. [Google Scholar] [CrossRef] [PubMed]
- Villegas-Tovar, E.; Jimenez-Lillo, J.; Jimenez-Valerio, V.; Diaz-Giron-Gidi, A.; Faes-Petersen, R.; Otero-Piñeiro, A.; De Lacy, F.B.; Martinez-Portilla, R.J.; Lacy, A.M. Performance of Indocyanine green for sentinel lymph node mapping and lymph node metastasis in colorectal cancer: A diagnostic test accuracy meta-analysis. Surg. Endosc. 2020, 34, 1035–1047. [Google Scholar] [CrossRef]
- Burghgraef, T.A.; Zweep, A.L.; Sikkenk, D.J.; van der Pas, M.H.G.M.; Verheijen, P.M.; Consten, E.C.J. In vivo sentinel lymph node identification using fluorescent tracer imaging in colon cancer: A systematic review and meta-analysis. Crit. Rev. Oncol. Hematol. 2021, 158, 103149. [Google Scholar] [CrossRef]
- Picchetto, A.; Diana, M.; Swanström, L.L.; Magliocca, F.M.; Pronio, A.; Choppin, E.; Rocca, S.; Marescaux, J.; D’Ambrosio, G. Upstaging nodal status in colorectal cancer using ex vivo fluorescence sentinel lymph node mapping: Preliminary results. Minim. Invasive Ther. Allied Technol. 2022, 31, 223–229. [Google Scholar] [CrossRef]
- Kroon, H.M.; Hoogervorst, L.A.; Hanna-Rivero, N.; Traeger, L.; Dudi-Venkata, N.N.; Bedrikovetski, S.; Kusters, M.; Chang, G.J.; Thomas, M.L.; Sammour, T. Systematic review and meta-analysis of long-term oncological outcomes of lateral lymph node dissection for metastatic nodes after neoadjuvant chemoradiotherapy in rectal cancer. Eur. J. Surg. Oncol. 2022, 48, 1475–1482. [Google Scholar] [CrossRef]
- Emile, S.H.; Elfeki, H.; Shalaby, M.; Sakr, A.; Kim, N.K. Outcome of lateral pelvic lymph node dissection with total mesorectal excision in treatment of rectal cancer: A systematic review and meta-analysis. Surgery 2021, 169, 1005–1015. [Google Scholar] [CrossRef]
- Zhou, S.C.; Tian, Y.T.; Wang, X.W.; Zhao, C.D.; Ma, S.; Jiang, J.; Li, E.N.; Zhou, H.T.; Liu, Q.; Liang, J.W.; et al. Application of indocyanine green-enhanced near-infrared fluorescence-guided imaging in laparoscopic lateral pelvic lymph node dissection for middle-low rectal cancer. World J. Gastroenterol. 2019, 25, 4502–4511. [Google Scholar] [CrossRef]
- Dai, J.Y.; Han, Z.J.; Wang, J.D.; Liu, B.S.; Liu, J.Y.; Wang, Y.C. Short-term outcomes of near-infrared imaging using indocyanine green in laparoscopic lateral pelvic lymph node dissection for middle-lower rectal cancer: A propensity score-matched cohort analysis. Front. Med. 2022, 9, 1039928. [Google Scholar] [CrossRef]
- Kawada, K.; Yoshitomi, M.; Inamoto, S.; Sakai, Y. Indocyanine Green Fluorescence-Guided Laparoscopic Lateral Lymph Node Dissection for Rectal Cancer. Dis. Colon Rectum 2019, 62, 1401. [Google Scholar] [CrossRef] [PubMed]
- Yasui, M.; Ohue, M.; Noura, S.; Miyoshi, N.; Takahashi, Y.; Matsuda, C.; Nishimura, J.; Haraguchi, N.; Ushigome, H.; Nakai, N.; et al. Exploratory analysis of lateral pelvic sentinel lymph node status for optimal management of laparoscopic lateral lymph node dissection in advanced lower rectal cancer without suspected lateral lymph node metastasis. BMC Cancer 2021, 21, 911. [Google Scholar] [CrossRef] [PubMed]
- Ohya, H.; Watanabe, J.; Suwa, H.; Suwa, Y.; Ozawa, M.; Ishibe, A.; Kunisaki, C.; Endo, I. Near-Infrared Imaging Using Indocyanine Green for Laparoscopic Lateral Pelvic Lymph Node Dissection for Clinical Stage II/III Middle-Lower Rectal Cancer: A Propensity Score-Matched Cohort Study. Dis. Colon Rectum 2022, 65, 885–893. [Google Scholar] [CrossRef]
- Kazanowski, M.; Al Furajii, H.; Cahill, R.A. Near-infrared laparoscopic fluorescence for pelvic side wall delta mapping in patients with rectal cancer—’PINPOINT’ nodal assessment. Colorectal Dis. 2015, 17 (Suppl. S3), 32–35. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Choi, G.S.; Park, J.S.; Park, S.Y.; Cho, S.H.; Seo, A.N.; Yoon, G.S. S122: Impact of fluorescence and 3D images to completeness of lateral pelvic node dissection. Surg. Endosc. 2020, 34, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Liberale, G.; Vankerckhove, S.; Caldon, M.G.; Ahmed, B.; Moreau, M.; Nakadi, I.E.; Larsimont, D.; Donckier, V.; Bourgeois, P.; Group R & D for the Clinical Application of Fluorescence Imaging of the Jules Bordetʼs Institute. Fluorescence Imaging after Indocyanine Green Injection for Detection of Peritoneal Metastases in Patients Undergoing Cytoreductive Surgery for Peritoneal Carcinomatosis from Colorectal Cancer: A Pilot Study. Ann. Surg. 2016, 264, 1110–1115. [Google Scholar] [CrossRef]
- Baiocchi, G.L.; Gheza, F.; Molfino, S.; Arru, L.; Vaira, M.; Giacopuzzi, S. Indocyanine green fluorescence-guided intraoperative detection of peritoneal carcinomatosis: Systematic review. BMC Surg. 2020, 20, 158. [Google Scholar] [CrossRef]
- Barabino, G.; Klein, J.P.; Porcheron, J.; Grichine, A.; Coll, J.L.; Cottier, M. Intraoperative near-infrared fluorescence imaging using indocyanine green in colorectal carcinomatosis surgery: Proof of concept. Eur. J. Surg. Oncol. 2016, 42, 1931–1937. [Google Scholar] [CrossRef]
- Lieto, E.; Auricchio, A.; Cardella, F.; Mabilia, A.; Basile, N.; Castellano, P.; Orditura, M.; Galizia, G. Fluorescence-guided surgery in the combined treatment of peritoneal Carcinomatosis from colorectal Cancer: Preliminary results and considerations. World J. Surg. 2018, 42, 1154–1160. [Google Scholar] [CrossRef]
- González-Abós, C.; Selva, A.B.; de Lacy, F.B.; Valverde, S.; Almenara, R.; Lacy, A.M. Quantitative Indocyanine Green Fluorescence Imaging Assessment for Nonmucinous Peritoneal Metastases: Preliminary Results of the ICCP Study. Dis. Colon Rectum 2022, 65, 314–321. [Google Scholar] [CrossRef]
- Piccolo, G.; Barabino, M.; Pesce, A.; Diana, M.; Lecchi, F.; Santambrogio, R.; Opocher, E.; Bianchi, P.P.; Piozzi, G.N. Role of Indocyanine Green Fluorescence Imaging in Minimally Invasive Resection of Colorectal Liver Metastases. Surg. Laparosc. Endosc. Percutan Tech. 2022, 32, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Piccolo, G.; Barabino, M.; Diana, M.; Lo Menzo, E.; Epifani, A.G.; Lecchi, F.; Santambrogio, R.; Opocher, E. Application of Indocyanine Green Fluorescence as an Adjuvant to Laparoscopic Ultrasound in Minimally Invasive Liver Resection. J. Laparoendosc. Adv. Surg. Tech. A 2021, 31, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Patel, I.; Bartlett, D.; Dasari, B.V.; Chatzizacharias, N.; Isaac, J.; Marudanayagam, R.; Mirza, D.F.; Roberts, J.K.; Sutcliffe, R.P. Detection of Colorectal Liver Metastases Using Near-Infrared Fluorescence Imaging during Hepatectomy: Prospective Single Centre UK Study. J. Gastrointest. Cancer, 2022; ahead of print. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Hong, X.; Chi, C.; Cai, C.; An, Y.; Li, P.; Liu, X.; Shan, H.; Tian, J.; Li, J. Efficacy of Near-Infrared Fluorescence-Guided Hepatectomy for the Detection of Colorectal Liver Metastases: A Randomized Controlled Trial. J. Am. Coll. Surg. 2022, 234, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Nishino, H.; Turner, M.A.; Amirfakhri, S.; Hollandsworth, H.M.; Lwin, T.M.; Hosseini, M.; Framery, B.; Cailler, F.; Pèlegrin, A.; Hoffman, R.M.; et al. Proof of concept of improved fluorescence-guided surgery of colon cancer liver metastasis using color-coded imaging of a tumor-labeling fluorescent antibody and indocyanine green restricted to the adjacent liver segment. Surgery 2022, 172, 1156–1163. [Google Scholar] [CrossRef]
- Fretland, A.A.; Dagenborg, V.J.; Bjornelv, G.M.W.; Kazaryan, A.M.; Kristiansen, R.; Fagerland, M.W.; Hausken, J.; Tønnessen, T.I.; Abildgaard, A.; Barkhatov, L.; et al. Laparoscopic versus Open Resection for Colorectal Liver Metastases: The OSLO-COMET Randomized Controlled Trial. Ann. Surg. 2018, 267, 199–207. [Google Scholar] [CrossRef]
- Wexner, S.; Abu-Gazala, M.; Boni, L.; Buxey, K.; Cahill, R.; Carus, T.; Chadi, S.; Chand, M.; Cunningham, C.; Emile, S.H.; et al. Use of fluorescence imaging and indocyanine green during colorectal surgery: Results of an intercontinental Delphi survey. Surgery 2022, 172, S38–S45. [Google Scholar] [CrossRef] [PubMed]
- Dip, F.; Menzo, E.L.; Bouvet, M.; Schols, R.M.; Sherwinter, D.; Wexner, S.D.; White, K.P.; Rosenthal, R.J. Intraoperative fluorescence imaging in different surgical fields: Consensus among 140 intercontinental experts. Surgery 2022, 172, S54–S59. [Google Scholar] [CrossRef]
- Park, S.H.; Park, H.M.; Baek, K.R.; Ahn, H.M.; Lee, I.Y.; Son, G.M. Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery. World J. Gastroenterol. 2020, 26, 6945–6962. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, B.; Agnus, V.; Mascagni, P.; Barberio, M.; Longo, F.; Lapergola, A.; Mutter, D.; Klymchenko, A.S.; Chand, M.; Marescaux, J.; et al. Simultaneous computer-assisted assessment of mucosal and serosal perfusion in a model of segmental colonic ischemia. Surg. Endosc. 2020, 34, 4818–4827. [Google Scholar] [CrossRef]

| ICG Applications in Colorectal Surgery |
|---|
| Perfusion Assessment |
|
|
| Anatomic Visualization |
|
| Tumor Localization [47,48,49,50,51,52,53,54,55,56,57,58] |
| Lymphatic Mapping [59,60,61,62,63,64,65,66,67,68,69,70,71,72] |
| Sentinel Lymph Node Identification [75,76,77,78,79,80,81,82,83,84,85] |
| Lateral pelvic node dissection [88,89,90,91,92,93,94,95] |
| Distant Metastases Assessment |
| Important Parameters during Evaluation of Blood Flow and Lymphatic Mapping Using ICG |
|---|
| Dosage of ICG |
| Concentration of ICG |
| Route of administration |
| Assessment time (time from ICG injection until assessment) |
| Quantification of the result |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garoufalia, Z.; Wexner, S.D. Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery. J. Clin. Med. 2023, 12, 494. https://doi.org/10.3390/jcm12020494
Garoufalia Z, Wexner SD. Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery. Journal of Clinical Medicine. 2023; 12(2):494. https://doi.org/10.3390/jcm12020494
Chicago/Turabian StyleGaroufalia, Zoe, and Steven D. Wexner. 2023. "Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery" Journal of Clinical Medicine 12, no. 2: 494. https://doi.org/10.3390/jcm12020494
APA StyleGaroufalia, Z., & Wexner, S. D. (2023). Indocyanine Green Fluorescence Guided Surgery in Colorectal Surgery. Journal of Clinical Medicine, 12(2), 494. https://doi.org/10.3390/jcm12020494





