Large Paraumbilical Vein Shunts Increase the Risk of Overt Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Placement
Abstract
:1. Introduction
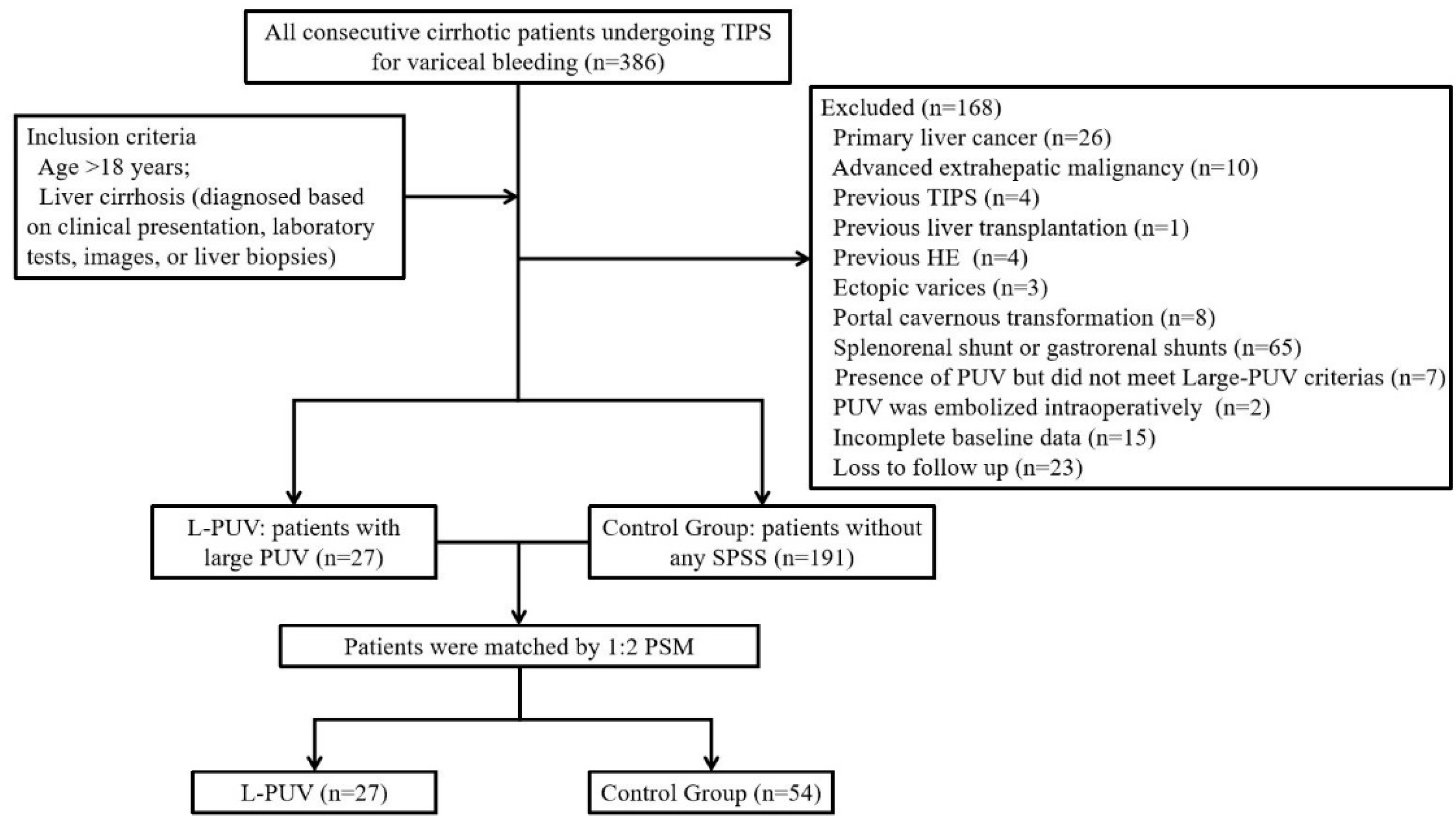
2. Materials and Methods
2.1. Patients
2.2. Radiological Data Acquisition
2.3. Outcomes and Definitions
2.4. TIPS Procedure and Follow-Up
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Propensity Score Matching
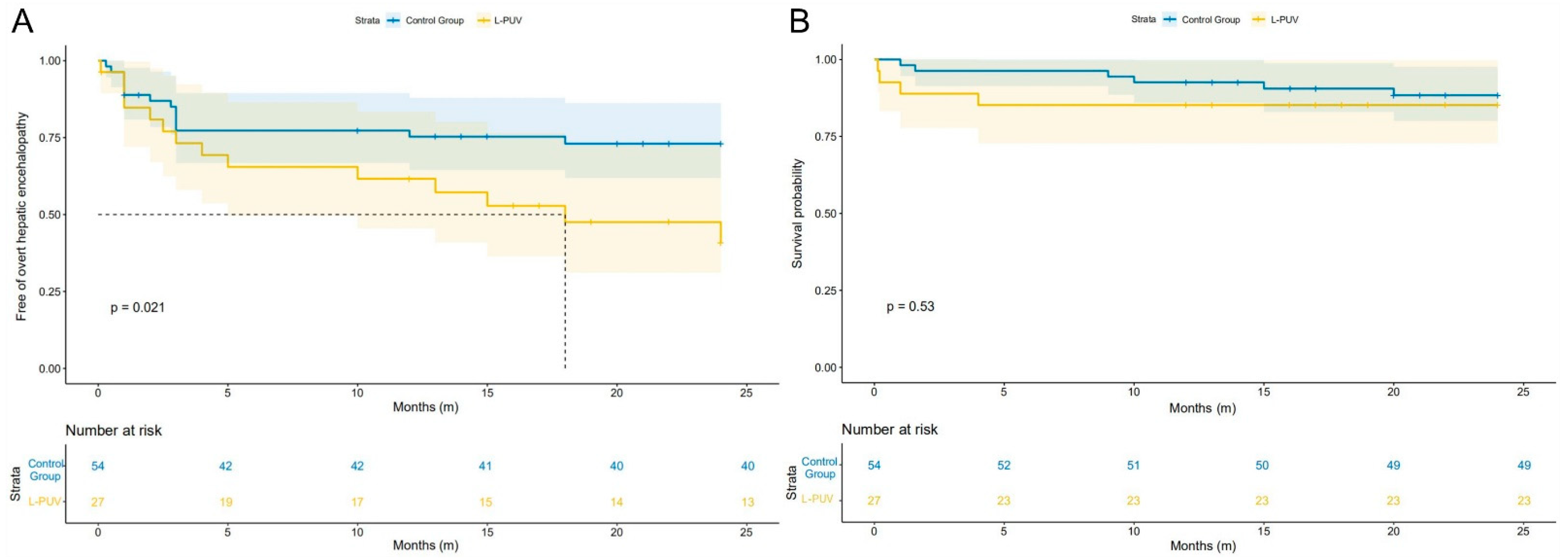
3.3. Overt Hepatic Encephalopathy
3.4. Mortality, Rebleeding, and Shunt Dysfunction
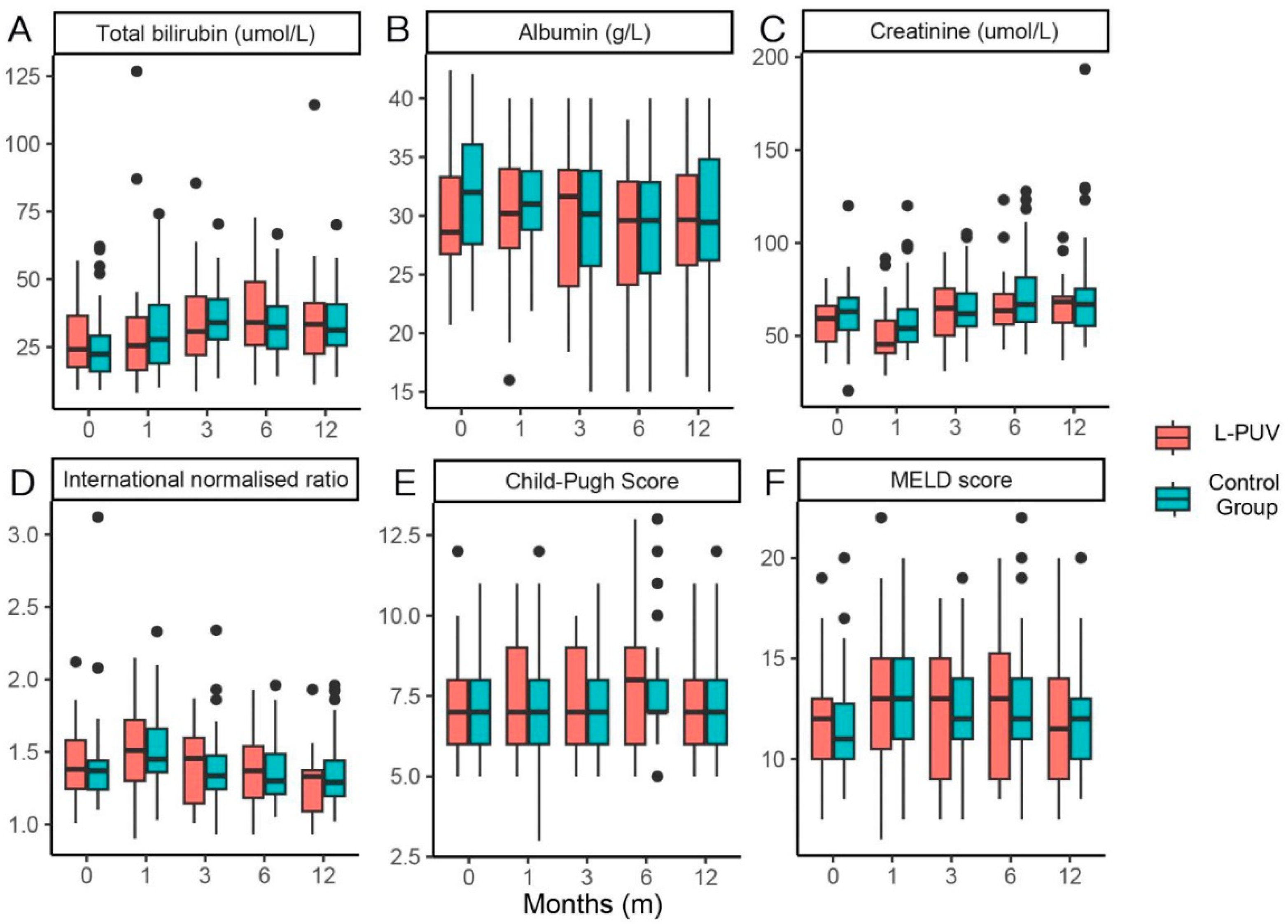
3.5. Changes in Liver Function and L-PUV Diameter
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Praktiknjo, M.; Simón-Talero, M.; Römer, J.; Roccarina, D.; Martínez, J.; Lampichler, K.; Baiges, A.; Low, G.; Llop, E.; Maurer, M.H.; et al. Total area of spontaneous portosystemic shunts independently predicts hepatic encephalopathy and mortality in liver cirrhosis. J. Hepatol. 2020, 72, 1140–1150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turco, L.; Garcia-Tsao, G. Portal Hypertension: Pathogenesis and Diagnosis. Clin. Liver Dis. 2019, 23, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Vidal-González, J.; Quiroga, S.; Simón-Talero, M.; Genescà, J. Spontaneous portosystemic shunts in liver cirrhosis: New approaches to an old problem. Ther. Adv. Gastroenterol. 2020, 13, 1320571904. [Google Scholar] [CrossRef] [PubMed]
- Simón-Talero, M.; Roccarina, D.; Martínez, J.; Lampichler, K.; Baiges, A.; Low, G.; Llop, E.; Praktiknjo, M.; Maurer, M.H.; Zipprich, A.; et al. Association Between Portosystemic Shunts and Increased Complications and Mortality in Patients With Cirrhosis. Gastroenterology 2018, 154, 1694–1705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, Y.; Yang, Z.; Liu, L.; Li, K.; He, C.; Wang, Z.; Bai, W.; Guo, W.; Yu, T.; Yuan, X.; et al. Early TIPS with covered stents versus standard treatment for acute variceal bleeding in patients with advanced cirrhosis: A randomised controlled trial. Lancet Gastroenterol. 2019, 4, 587–598. [Google Scholar] [CrossRef]
- Garcia-Tsao, G.; Abraldes, J.G.; Berzigotti, A.; Bosch, J. Portal hypertensive bleeding in cirrhosis: Risk stratification, diagnosis, and management: 2016 practice guidance by the American Association for the study of liver diseases. Hepatology 2017, 65, 310–335. [Google Scholar] [CrossRef] [Green Version]
- Yanny, B.; Winters, A.; Boutros, S.; Saab, S. Hepatic Encephalopathy Challenges, Burden, and Diagnostic and Therapeutic Approach. Clin. Liver Dis. 2019, 23, 607–623. [Google Scholar] [CrossRef]
- Gairing, S.J.; Müller, L.; Kloeckner, R.; Galle, P.R.; Labenz, C. Review article: Post-TIPSS hepatic encephalopathy—Current knowledge and future perspectives. Aliment. Pharmacol. Ther. 2022, 55, 1265–1276. [Google Scholar] [CrossRef]
- Lv, Y.; Chen, H.; Luo, B.; Bai, W.; Li, K.; Wang, Z.; Xia, D.; Guo, W.; Wang, Q.; Li, X.; et al. Concurrent large spontaneous portosystemic shunt embolization for the prevention of overt hepatic encephalopathy after TIPS: A randomized controlled trial. Hepatology 2022, 76, 676–688. [Google Scholar] [CrossRef]
- He, C.; Lv, Y.; Wang, Z.; Guo, W.; Tie, J.; Li, K.; Niu, J.; Zuo, L.; Yu, T.; Yuan, X.; et al. Association between non-variceal spontaneous portosystemic shunt and outcomes after TIPS in cirrhosis. Dig. Liver Dis. 2018, 50, 1315–1323. [Google Scholar] [CrossRef]
- Leng, X.; Zhang, F.; Zhang, M.; Guo, H.; Yin, X.; Xiao, J.; Wang, Y.; Zou, X.; Zhuge, Y. Comparison of transjugular intrahepatic portosystemic shunt for treatment of variceal bleeding in patients with cirrhosis with or without spontaneous portosystemic shunt. Eur. J. Gastroenterol. Hepatol. 2019, 31, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Sacerdoti, D.; Bolognesi, M.; Bombonato, G.; Gatta, A. Paraumbilical vein patency in cirrhosis: Effects on hepatic hemodynamics evaluated by Doppler sonography. Hepatology 1995, 22, 1689–1694. [Google Scholar] [PubMed]
- Kondo, T.; Maruyama, H.; Sekimoto, T.; Shimada, T.; Takahashi, M.; Okugawa, H.; Yokosuka, O.; Yamaguchi, T. Influence of paraumbilical vein patency on the portal hemodynamics of patients with cirrhosis. J. Clin. Gastroenterol. 2014, 48, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Li, W.C.; Zhong, B.Y.; Zhang, S.; Shen, J.; Wang, W.; Zhu, X.L. Emergent TIPS as a first-line therapy in advanced cirrhotic patients with acute gastroesophageal variceal bleeding. J. Vasc. Interv. Radiol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Raman, S.S.; Yu, N.C.; To’o, K.J.; Jutabha, R.; Lu, D.S. Esophageal varices in cirrhotic patients: Evaluation with liver CT. Am. J. Roentgenol. 2007, 188, 139–144. [Google Scholar] [CrossRef]
- Vilstrup, H.; Amodio, P.; Bajaj, J.; Cordoba, J.; Ferenci, P.; Mullen, K.D.; Weissenborn, K.; Wong, P. Hepatic encephalopathy in chronic liver disease: 2014 Practice Guideline by the American Association for the Study of Liver Diseases and the European Association for the Study of the Liver. Hepatology 2014, 60, 715–735. [Google Scholar] [CrossRef]
- Riggio, O.; Nardelli, S.; Moscucci, F.; Pasquale, C.; Ridola, L.; Merli, M. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin. Liver Dis. 2012, 16, 133–146. [Google Scholar] [CrossRef]
- de Franchis, R.; Baveno VI Faculty. Expanding consensus in portal hypertension: Report of the Baveno VI Consensus Workshop: Stratifying risk and individualizing care for portal hypertension. J. Hepatol. 2015, 63, 743–752. [Google Scholar] [CrossRef] [Green Version]
- Trebicka, J.; Bastgen, D.; Byrtus, J.; Praktiknjo, M.; Terstiegen, S.; Meyer, C.; Thomas, D.; Fimmers, R.; Treitl, M.; Euringer, W.; et al. Smaller-Diameter Covered Transjugular Intrahepatic Portosystemic Shunt Stents Are Associated With Increased Survival. Clin. Gastroenterol. Hepatol. 2019, 17, 2793–2799.e1. [Google Scholar] [CrossRef] [Green Version]
- Dariushnia, S.R.; Haskal, Z.J.; Midia, M.; Martin, L.G.; Walker, T.G.; Kalva, S.P.; Clark, T.W.; Ganguli, S.; Krishnamurthy, V.; Saiter, C.K.; et al. Quality Improvement Guidelines for Transjugular Intrahepatic Portosystemic Shunts. J. Vasc. Interv. Radiol. 2016, 27, 1–7. [Google Scholar] [CrossRef]
- Yi, F.; Guo, X.; Wang, L.; Xu, X.; An, Y.; Tang, Y.; Zhang, W.; Tacke, F.; Arora, A.; Qi, X. Impact of spontaneous splenorenal shunt on liver volume and long-term survival of liver cirrhosis. J. Gastroenterol. Hepatol. 2021, 36, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Nardelli, S.; Gioia, S.; Ridola, L.; Riggio, O. Radiological Intervention for Shunt Related Encephalopathy. J. Clin. Exp. Hepatol. 2018, 8, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, S.K.; Mitchell, D.G.; Lakhman, Y.; Bergin, D.; Dolin, R.J.; Doria, C.; Parker, L. Paraumbilical collateral veins on MRI as possible protection against portal venous thrombosis in candidates for liver transplantation. Abdom. Imaging. 2008, 33, 536–541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morin, C.; Lafortune, M.; Pomier, G.; Robin, M.; Breton, G. Patent paraumbilical vein: Anatomic and hemodynamic variants and their clinical importance. Radiology 1992, 185, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Borentain, P.; Soussan, J.; Resseguier, N.; Botta-Fridlund, D.; Dufour, J.C.; Gérolami, R.; Vidal, V. The presence of spontaneous portosystemic shunts increases the risk of complications after transjugular intrahepatic portosystemic shunt (TIPS) placement. Diagn. Interv. Imaging 2016, 97, 643–650. [Google Scholar] [CrossRef]
- Greinert, R.; Zipprich, A.; Simón-Talero, M.; Stangl, F.; Ludwig, C.; Wienke, A.; Praktiknjo, M.; Höhne, K.; Trebicka, J.; Genescà, J.; et al. Covert hepatic encephalopathy and spontaneous portosystemic shunts increase the risk of developing overt hepatic encephalopathy. Liver Int. 2020, 40, 3093–3102. [Google Scholar] [CrossRef]
- Philips, C.A.; Rajesh, S.; Augustine, P.; Padsalgi, G.; Ahamed, R. Portosystemic shunts and refractory hepatic encephalopathy: Patient selection and current options. Hepatic Med.-Evid. Res. 2019, 11, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Bai, M.; Qi, X.; Yang, Z.; Yin, Z.; Nie, Y.; Yuan, S.; Wu, K.; Han, G.; Fan, D. Predictors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt in cirrhotic patients: A systematic review. J. Gastroenterol. Hepatol. 2011, 26, 943–951. [Google Scholar] [CrossRef]
- Yin, X.; Zhang, F.; Xiao, J.; Wang, Y.; He, Q.; Zhu, H.; Leng, X.; Zou, X.; Zhang, M.; Zhuge, Y. Diabetes mellitus increases the risk of hepatic encephalopathy after a transjugular intrahepatic portosystemic shunt in cirrhotic patients. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1264–1269. [Google Scholar] [CrossRef]
- Luo, X.; Wang, X.; Zhu, Y.; Xi, X.; Zhao, Y.; Yang, J.; Li, X.; Yang, L. Clinical Efficacy of Transjugular Intrahepatic Portosystemic Shunt Created with Expanded Polytetrafluoroethylene-Covered Stent-Grafts: 8-mm Versus 10-mm. Cardiovasc. Interv. Radiol. 2019, 42, 737–743. [Google Scholar] [CrossRef]
- Fonio, P.; Discalzi, A.; Calandri, M.; Breatta, A.D.; Bergamasco, L.; Martini, S.; Ottobrelli, A.; Righi, D.; Gandini, G. Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol. Med. 2017, 122, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Gao, F.; Wu, X.; Cai, W.; Chen, X.; Huang, Z. Efficacy of albumin-bilirubin score to predict hepatic encephalopathy in patients underwent transjugular intrahepatic portosystemic shunt. Eur. J. Gastroenterol. Hepatol. 2021, 33, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, J.; Zhou, C.; Yang, C.; Huang, S.; Shi, Q.; Xiong, B. Potential Benefits of Underdilation of 8-mm Covered Stent in Transjugular Intrahepatic Portosystemic Shunt Creation. Clin. Transl. Gastroenterol. 2021, 12, e00376. [Google Scholar] [CrossRef] [PubMed]
- Calame, P.; Ronot, M.; Bouveresse, S.; Cervoni, J.P.; Vilgrain, V.; Delabrousse, É. Predictive value of CT for first esophageal variceal bleeding in patients with cirrhosis: Value of para-umbilical vein patency. Eur. J. Radiol. 2017, 87, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Wang, J.H.; Lu, S.N.; Tung, W.C.; Hung, C.H.; Lee, C.M.; Changchien, C.S. Comparison of prevalence for paraumbilical vein patency in patients with viral and alcoholic liver cirrhosis. Am. J. Gastroenterol. 2002, 97, 2415–2418. [Google Scholar] [CrossRef]
- Luo, S.H.; Chu, J.G.; Huang, H.; Zhao, G.R.; Yao, K.C. Targeted puncture of left branch of intrahepatic portal vein in transjugular intrahepatic portosystemic shunt to reduce hepatic encephalopathy. World J. Gastroenterol. 2019, 25, 1088–1099. [Google Scholar] [CrossRef]

| Parameters | L-PUV (n = 27) | Control Group (n = 54) | p-Value |
|---|---|---|---|
| Sex (male), n (%) | 15 (55.6%) | 28 (51.9%) | 0.753 |
| Age (years) | 54.0 (42.0–66.0) | 55.0 (48.0–66.5) | 0.426 |
| Viral hepatitis | 15 (55.6%) | 30 (55.6%) | 1.000 |
| Ascites (present), n (%) | 13 (48.1%) | 29 (53.7%) | 0.637 |
| Previous diabetes, n (%) | 4 (14.8%) | 8 (14.8%) | 1.000 |
| Spleen diameter (mm) | 167.0 (133.9–185.9) | 167.5 (150.4–199.5) | 0.241 |
| Presence of gastric varices, n (%) | 5 (18.5%) | 7 (13.0%) | 0.422 |
| Portal vein thrombosis, n (%) | 7 (25.9%) | 16 (29.6%) | 0.726 |
| Total bilirubin (μmol/L) | 24.1 (16.0–39.5) | 22.4 (15.8–29.6) | 0.344 |
| Albumin (g/L) | 28.6 (26.7–33.6) | 32.0 (27.5–36.1) | 0.110 |
| Creatinine (μmol/L) | 59.4 (45.9–67.2) | 63.0 (53.0–70.1) | 0.233 |
| Prothrombin time (S) | 16.2 (14.7–18.5) | 15.6 (14.4–17.6) | 0.370 |
| International normalized ratio | 1.38 (1.23–1.60) | 1.37 (1.24–1.45) | 0.455 |
| Platelet count (×109/L) | 59.0 (40.0–73.0) | 50.0 (34.0–69.3) | 0.431 |
| White blood cell (×109/L) | 3.3 (2.0–7.23) | 2.6 (1.8–5.8) | 0.304 |
| Child–Pugh score (points) | 7.0 (6.0–8.0) | 7.0 (6.0–8.0) | 0.236 |
| Child–Pugh grade, n (%) | 0.226 | ||
| A | 8 (29.6%) | 23 (42.6%) | |
| B | 15 (55.6%) | 26 (48.1%) | |
| C | 4 (14.8%) | 5 (9.3%) | |
| MELD score (points) | 12.0 (10.0–13.0) | 11.0 (9.8–13.0) | 0.279 |
| Diameter of LPV | 13.2 (10.7–14.8) | 11.1 (10.0–12.6) | 0.010 |
| Diameter of RPV | 11.6 (8.1–13.0) | 11.0 (9.3–12.3) | 0.970 |
| Diameter of MPV | 16.9 (13.8–20.6) | 16.4 (14.2–18.4) | 0.702 |
| Esophageal variceal grade | 0.465 | ||
| 0 | 2 (7.4%) | 2 (3.7%) | |
| I | 1 (3.7%) | 0 | |
| II | 2 (7.4%) | 5 (9.3%) | |
| III | 22 (81.5%) | 47 (87.0%) | |
| Portal vein puncture | 0.648 | ||
| LPV | 19 (70.4%) | 37 (68.5%) | |
| RPV | 7 (25.9%) | 9 (16.7%) | |
| MPV | 1 (3.7%) | 8 (14.8%) | |
| Pre-TIPS PPG (mmHg) | 21.0 (18.0–21.0) | 21.9 (17.8–25.0) | 0.099 |
| Post-TIPS PPG (mmHg) | 7.0 (6.0–9.8) | 8.0 (6.0–10.0) | 0.536 |
| Diameter of balloon catheters, n (%) | 0.695 | ||
| 6 mm | 6 (22.2%) | 10 (18.5%) | |
| 8 mm | 21 (77.8%) | 44 (81.5%) | |
| Duration of follow-up (months) | 22.0 (12.0–43.0) | 32.0 (20.0–47.3) | 0.111 |
| Outcome | L-PUV (n = 27) | Control Group (n = 54) | p-Value |
|---|---|---|---|
| OHE, n (%) | 14 (51.9%) | 14 (25.9%) | 0.022 |
| Episodes per patient | 0.9 ± 1.2 | 0.7 ± 1.3 | 0.426 |
| Frequency of OHE | 0.056 | ||
| One episode | 9 (33.3%) | 5 (9.3%) | |
| More than one episode | 5 (18.5%) | 9 (16.7%) | |
| Severe HE (grade III/IV) | 3 (11.1%) | 7 (13.0%) | 0.810 |
| Refractory HE | 1 (3.7%) | 5 (9.3%) | 0.342 |
| 1-month OHE, n (%) | 4 (14.8%) | 9 (16.7%) | 0.830 |
| 3-month OHE, n (%) | 7 (25.9%) | 13 (24.1%) | 0.856 |
| 6-month OHE, n (%) | 9 (33.3%) | 13 (24.1%) | 0.382 |
| 1-year OHE, n (%) | 9 (33.3%) | 14 (25.9%) | 0.489 |
| Death, n (%) | 4 (14.8%) | 6 (11.1%) | 0.637 |
| Cause of death | |||
| Gastrointestinal bleeding | 2 (7.4%) | 2 (3.7%) | |
| Liver failure | 1 (3.7%) | 2 (3.7%) | |
| Sepsis/pneumonia | 1 (3.7%) | 1 (1.9%) | |
| Unrelated to liver disease | 0 | 1 (1.9%) | |
| Rebleeding, n (%) | 3 (11.1%) | 7 (13.0%) | 0.810 |
| Sources of bleeding | |||
| Variceal rebleeding | 2 (7.4%) | 3 (5.6%) | |
| Portal hypertensive gastropathy | 0 | 2 (3.7%) | |
| Unknown | 1 (3.7%) | 2 (3.7%) | |
| Shunt dysfunction, n (%) | 0 | 4 (7.4%) | 0.067 |
| Variables | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Total bilirubin | 1.029 | 1.001–1.058 | 0.043 | |||
| L-PUV | 2.301 | 1.094–4.839 | 0.028 | 2.217 | 1.050–4.682 | 0.037 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, H.-H.; Zhang, Z.-C.; Zhao, Z.-L.; Zhong, B.-Y.; Fan, C.; Zhu, X.-L.; Wang, W.-D. Large Paraumbilical Vein Shunts Increase the Risk of Overt Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Placement. J. Clin. Med. 2023, 12, 158. https://doi.org/10.3390/jcm12010158
Tang H-H, Zhang Z-C, Zhao Z-L, Zhong B-Y, Fan C, Zhu X-L, Wang W-D. Large Paraumbilical Vein Shunts Increase the Risk of Overt Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Placement. Journal of Clinical Medicine. 2023; 12(1):158. https://doi.org/10.3390/jcm12010158
Chicago/Turabian StyleTang, Hao-Huan, Zi-Chen Zhang, Zi-Le Zhao, Bin-Yan Zhong, Chen Fan, Xiao-Li Zhu, and Wei-Dong Wang. 2023. "Large Paraumbilical Vein Shunts Increase the Risk of Overt Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Placement" Journal of Clinical Medicine 12, no. 1: 158. https://doi.org/10.3390/jcm12010158
APA StyleTang, H.-H., Zhang, Z.-C., Zhao, Z.-L., Zhong, B.-Y., Fan, C., Zhu, X.-L., & Wang, W.-D. (2023). Large Paraumbilical Vein Shunts Increase the Risk of Overt Hepatic Encephalopathy after Transjugular Intrahepatic Portosystemic Shunt Placement. Journal of Clinical Medicine, 12(1), 158. https://doi.org/10.3390/jcm12010158





