Clinical Characteristics and Early Diagnosis of Spontaneous Fungal Peritonitis/Fungiascites in Hospitalized Cirrhotic Patients with Ascites: A Case–Control Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Ascitic Fungus and Bacteria Cultures
2.3. Diagnosis of SFP/Fungiascites and SBP
2.4. Clinical Data and Biochemistry
2.5. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Cirrhotic Patients with SFP/Fungiascites and SBP
3.2. Subgroup Analyses of Fungiascites, SFP with Fungus-Positive Ascites Only, and SFP Mixed with Bacteria-Positive Ascites
3.3. Prognosis of SFP/Fungiascites
3.4. Predictors for Occurrence of SFP/Fungiascites
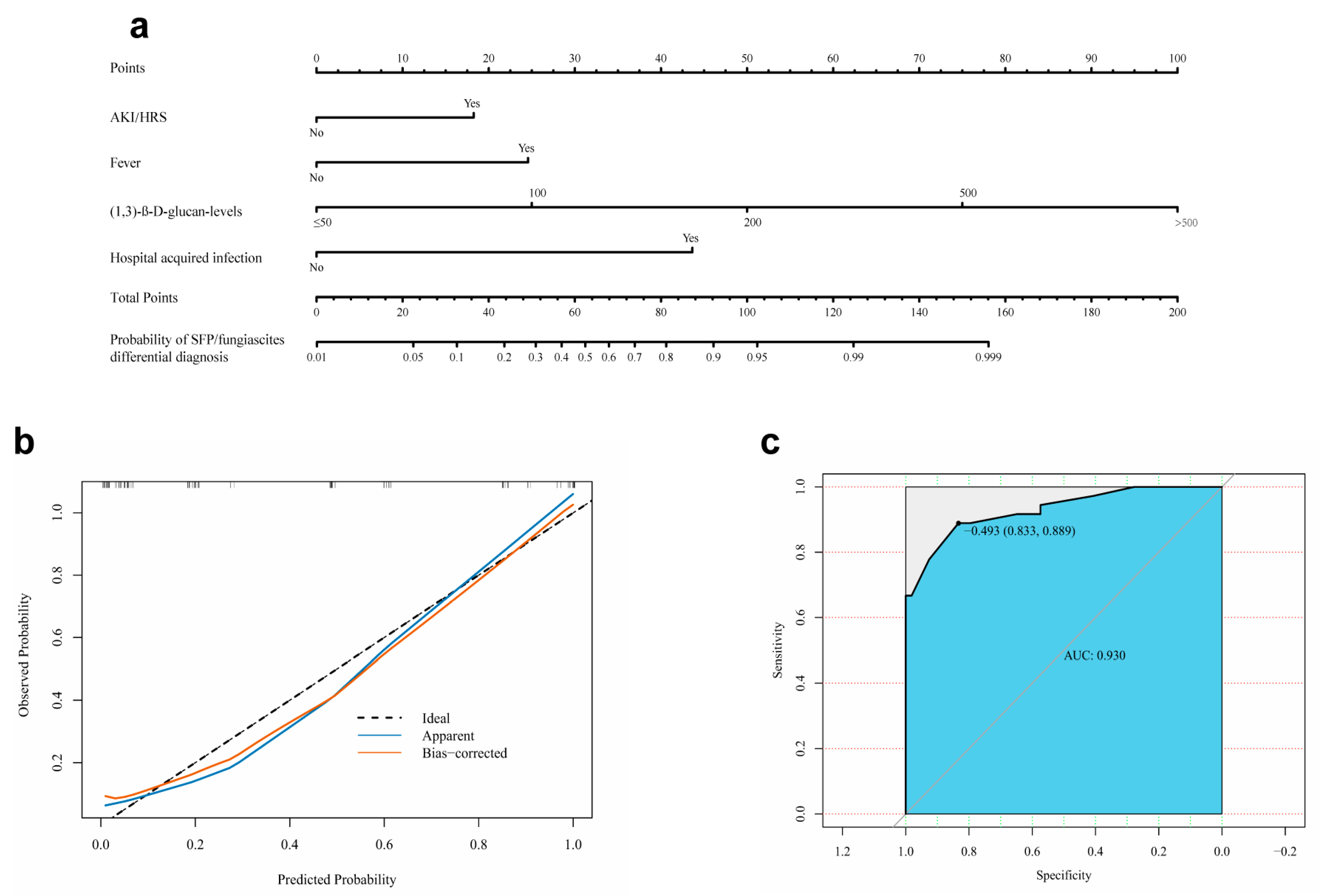
3.5. Development and Evaluation of a Nomogram for Early Differential Diagnosis of SFP/Fungiascites with SBP
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginès, P.; Krag, A.; Abraldes, J.; Solà, E.; Fabrellas, N.; Kamath, P.J.L. Liver cirrhosis. Lancet 2021, 398, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Mansour, D.; McPherson, S.J.C.M. Management of decompensated cirrhosis. Clin. Med. 2018, 18, s60–s65. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, C.; Clària, J.; Szabo, G.; Bosch, J.; Bernardi, M. Pathophysiology of decompensated cirrhosis: Portal hypertension, circulatory dysfunction, inflammation, metabolism and mitochondrial dysfunction. J. Hepatol. 2021, 75, S49–S66. [Google Scholar] [CrossRef]
- Arvaniti, V.; D’Amico, G.; Fede, G.; Manousou, P.; Tsochatzis, E.; Pleguezuelo, M.; Burroughs, A.K. Infections in Patients With Cirrhosis Increase Mortality Four-Fold and Should Be Used in Determining Prognosis. Gastroenterology 2010, 139, 1246–1256.e1245. [Google Scholar] [CrossRef] [PubMed]
- Alexopoulou, A.; Vasilieva, L.; Agiasotelli, D.; Dourakis, S.P. Fungal infections in patients with cirrhosis. J. Hepatol. 2015, 60, 1043–1045. [Google Scholar] [CrossRef]
- Würstle, S.; Hapfelmeier, A.; Karapetyan, S.; Studen, F.; Isaakidou, A.; Schneider, T.; Schmid, R.M.; von Delius, S.; Gundling, F.; Burgkart, R.; et al. Differentiation of Spontaneous Bacterial Peritonitis from Secondary Peritonitis in Patients with Liver Cirrhosis: Retrospective Multicentre Study. Diagnostics 2023, 13, 994. [Google Scholar] [CrossRef]
- Aithal, G.; Palaniyappan, N.; China, L.; Härmälä, S.; Macken, L.; Ryan, J.; Wilkes, E.; Moore, K.; Leithead, J.; Hayes, P.; et al. Guidelines on the management of ascites in cirrhosis. Gut 2021, 70, 9–29. [Google Scholar] [CrossRef]
- KASL. KASL clinical practice guidelines for liver cirrhosis: Ascites and related complications. Clin. Mol. Hepatol. 2018, 24, 230–277. [Google Scholar] [CrossRef]
- Biggins, S.; Angeli, P.; Garcia-Tsao, G.; Ginès, P.; Ling, S.; Nadim, M.; Wong, F.; Kim, W. Diagnosis, Evaluation, and Management of Ascites, Spontaneous Bacterial Peritonitis and Hepatorenal Syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef] [PubMed]
- Zoratti, C.; Moretti, R.; Rebuzzi, L.; Albergati, I.V.; Di Somma, A.; Decorti, G.; Di Bella, S.; Crocè, L.S.; Giuffrè, M. Antibiotics and liver cirrhosis: What the physicians need to know. Antibiotics 2021, 11, 31. [Google Scholar] [CrossRef]
- Zhang, G.; Jazwinski Faust, A.J.J. Spontaneous Bacterial Peritonitis. JAMA 2021, 325, 1118. [Google Scholar] [CrossRef]
- Fiore, M.; Maraolo, A.E.; Leone, S.; Gentile, I.; Cuomo, A.; Schiavone, V.; Bimonte, S.; Pace, M.C.; Cascella, M. Spontaneous peritonitis in critically ill cirrhotic patients: A diagnostic algorithm for clinicians and future perspectives. Ther. Clin. Risk Manag. 2017, 13, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Yu, S.; Lee, J.; Kim, J.; Yoon, J.; Kim, Y.; Yoon, J.; Kim, E.; Lee, H. Spontaneous fungal peritonitis: A severe complication in patients with advanced liver cirrhosis. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Righi, E. Management of bacterial and fungal infections in end stage liver disease and liver transplantation: Current options and future directions. World J. Gastroenterol. 2018, 24, 4311–4329. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Verma, N.; Singh, S.; Roy, A.; Valsan, A.; Garg, P.; Pradhan, P.; Chakrabarti, A.; Singh, M. Cirrhosis and fungal infections—A cocktail for catastrophe: A systematic review and meta-analysis with machine learning. Mycoses 2022, 65, 844–858. [Google Scholar] [CrossRef]
- Fiore, M.; Leone, S. Spontaneous fungal peritonitis: Epidemiology, current evidence and future prospective. World J. Gastroenterol. 2016, 22, 7742. [Google Scholar] [CrossRef]
- Bremmer, D.; Garavaglia, J.; Shields, R. Spontaneous fungal peritonitis: A devastating complication of cirrhosis. Mycoses 2015, 58, 387–393. [Google Scholar] [CrossRef]
- Marciano, S.; Díaz, J.M.; Dirchwolf, M.; Gadano, A. Spontaneous bacterial peritonitis in patients with cirrhosis: Incidence, outcomes, and treatment strategies. Hepat. Med. 2019, 11, 13–22. [Google Scholar] [CrossRef]
- Gravito-Soares, M.; Gravito-Soares, E.; Lopes, S.; Ribeiro, G.; Figueiredo, P. Spontaneous fungal peritonitis: A rare but severe complication of liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1010–1016. [Google Scholar] [CrossRef]
- Lahmer, T.; Brandl, A.; Rasch, S.; Schmid, R.; Huber, W. Fungal Peritonitis: Underestimated Disease in Critically Ill Patients with Liver Cirrhosis and Spontaneous Peritonitis. PLoS ONE 2016, 11, e0158389. [Google Scholar] [CrossRef]
- Fiore, M.; Chiodini, P.; Pota, V.; Sansone, P.; Passavanti, M.; Leone, S.; Aurilio, C.; Pace, M. Risk of spontaneous fungal peritonitis in hospitalized cirrhotic patients with ascites: A systematic review of observational studies and meta-analysis. Minerva Anestesiol. 2017, 83, 1309–1316. [Google Scholar] [CrossRef]
- Bassetti, M.; Peghin, M.; Carnelutti, A.; Righi, E.; Merelli, M.; Ansaldi, F.; Trucchi, C.; Alicino, C.; Sartor, A.; Toniutto, P.; et al. Clinical characteristics and predictors of mortality in cirrhotic patients with candidemia and intra-abdominal candidiasis: A multicenter study. Intensive Care Med. 2017, 43, 509–518. [Google Scholar] [CrossRef]
- Huang, C.; Pang, L.; Xu, L.; Ge, T.; Xu, Q.; Chen, Z. Risk factors, clinical features, and short-term prognosis of spontaneous fungal peritonitis in cirrhosis: A matched case-control study. World J. Clin. Cases 2019, 7, 2438–2449. [Google Scholar] [CrossRef]
- Hassan, E.A.; Abd El-Rehim, A.S.; Hassany, S.M.; Ahmed, A.O.; Elsherbiny, N.M.; Mohammed, M.H. Fungal infection in patients with end-stage liver disease: Low frequency or low index of suspicion. Int. J. Infect. Dis. 2014, 23, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, M.; Nishimoto, M.; Kokubu, M.; Matsui, M.; Eriguchi, M.; Samejima, K.; Akai, Y.; Tsuruya, K. Acute kidney injury as an independent predictor of infection and malignancy: The NARA-AKI cohort study. J. Nephrol. 2019, 32, 967–975. [Google Scholar] [CrossRef]
- Bajaj, J.S.; Reddy, R.K.; Tandon, P.; Wong, F.; Kamath, P.S.; Biggins, S.W.; Garcia-Tsao, G.; Fallon, M.; Maliakkal, B.; Lai, J. Prediction of fungal infection development and their impact on survival using the NACSELD cohort. Am. J. Gastroenterol. 2018, 113, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Kai, S.; Miller, L.; Ruiz-Velasco, V.; Kellum, J.A. Reversal of Acute Kidney Injury-Induced Neutrophil Dysfunction: A Critical Role for Resistin. Crit. Care Med. 2015, 44, e492. [Google Scholar]
- Gomes, C.L.; Silva, R.V.; Carrola, P.; Presa, J. Bacterial infections in patients with liver cirrhosis in an internal medicine department. GE Port. J. Gastroenterol. 2019, 26, 324–332. [Google Scholar]
- Fernández, J.; Piano, S.; Bartoletti, M.; Wey, E.Q. Management of bacterial and fungal infections in cirrhosis: The MDRO challenge. J. Hepatol. 2021, 75, S101–S117. [Google Scholar] [CrossRef]
- Gustot, T.; Durand, F.; Lebrec, D.; Vincent, J.L.; Moreau, R. Severe sepsis in cirrhosis. Hepatology 2009, 50, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Bunchorntavakul, C.; Chamroonkul, N.; Chavalitdhamrong, D. Bacterial infections in cirrhosis: A critical review and practical guidance. World J. Hepatol. 2016, 8, 307. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Maraolo, A.E.; Gentile, I.; Borgia, G.; Leone, S.; Sansone, P.; Passavanti, M.B.; Aurilio, C.; Pace, M.C. Nosocomial spontaneous bacterial peritonitis antibiotic treatment in the era of multi-drug resistance pathogens: A systematic review. World J. Gastroenterol. 2017, 23, 4654. [Google Scholar] [CrossRef] [PubMed]
- Fiore, M.; Gentile, I.; Maraolo, A.E.; Leone, S.; Simeon, V.; Chiodini, P.; Pace, M.C.; Gustot, T.; Taccone, F.S. Are third-generation cephalosporins still the empirical antibiotic treatment of community-acquired spontaneous bacterial peritonitis? A systematic review and meta-analysis. Eur. J. Gastroenterol. Hepatol. 2018, 30, 329–336. [Google Scholar] [CrossRef]
- Maindad, D.G.; Shenoy, S.; Shenoy, S.; Gopal, S.; Tantry, B.V. Treatment of Hospital-Acquired Infections in Patients with Cirrhosis-New Challenges. Infect. Drug Resist. 2022, 15, 1039. [Google Scholar] [CrossRef]


| Variable | SBP (n = 54) | SFP a/Fungiascites (n = 54) | p-Value |
|---|---|---|---|
| Ages (years) | 58.72 ± 11.72 | 58.30 ± 10.62 | 0.843 |
| Male n (%) | 40 (74.07%) | 39 (72.22%) | 0.828 |
| Antifungal therapy n (%) | - | 31 (57.41%) | - |
| Etiology | |||
| Viral n (%) | 22 (40.74%) | 27 (50.00%) | 0.334 |
| Alcoholic n (%) | 12 (22.22%) | 12 (22.22%) | 1 |
| Others n (%) | 20 (37.04%) | 15 (27.78%) | 0.304 |
| Fungus | |||
| Candida albicans n (%) | - | 23 (42.59%) | - |
| Candida glabrata n (%) | - | 10 (18.52%) | - |
| Candida tropicalis n (%) | - | 9 (16.67%) | - |
| Candida parapsilosis n (%) | - | 6 (11.11%) | - |
| Others n (%) | - | 6 (11.11%) | - |
| Time of ascites submitted for examination (day) | 7.5 (0, 16) | 6.5 (1, 18.25) | 0.599 |
| Time of ascites culture positive (day) | 4 (3,4) | 6 (5,7) | <0.001 |
| Hospital-acquired infection n (%) b | 16 (29.63%) | 45 (83.33%) | <0.001 |
| Fatigue n (%) | 53 (98.15%) | 54 (100%) | 1 |
| Fever n (%) | 18 (33.33%) | 33 (61.11%) | 0.004 |
| Abdominal swelling n (%) | 52 (96.3%) | 53 (98.15%) | 1 |
| Abdominal tenderness n (%) | 52 (96.3%) | 39 (72.2%) | 0.002 |
| Abdominal rebound tenderness n (%) | 30 (55.6%) | 22 (40.7%) | 0.123 |
| Malnutrition n (%) | 34 (62.96%) | 38 (70.37%) | 0.414 |
| HCC n (%) | 16 (29.63%) | 23 (42.59%) | 0.161 |
| HE n (%) | 16 (29.63%) | 18 (33.33%) | 0.727 |
| Variceal bleeding n (%) | 10 (18.52%) | 18 (33.33%) | 0.079 |
| AKI/HRS n (%) | 17 (31.48%) | 31 (57.41%) | 0.007 |
| Pleural effusion n (%) | 18 (33.33%) | 29 (53.7%) | 0.033 |
| Septic shock n (%) | 6 (11.11%) | 12 (22.22%) | 0.121 |
| Diabetes n (%) | 15 (27.78%) | 17 (31.48%) | 0.673 |
| Hypertension n (%) | 10 (18.52%) | 13 (24.07%) | 0.481 |
| SAAG, g/L | 21.13 ± 4.91 | 20.26 ± 7.17 | 0.349 |
| WBC, 109/L | 5.23 (3.25,7.42) | 7.75 (3.81,13.52) | 0.016 |
| NEU, % | 73.49 ± 15.47 | 77.96 ± 13.13 | 0.108 |
| PLT, 109/L | 77 (45.75,120.5) | 74.5 (44.25,101) | 0.958 |
| ALT, U/L | 21.5 (13,42.5) | 18.4 (11.83,37.83) | 0.408 |
| AST, U/L | 50.5 (25,88) | 44.6 (27.63,68.7) | 0.669 |
| TBIL, μmol/L | 54.05 (27.28,134.38) | 59.55(39.95,121.58) | 0.432 |
| ALB, g/L | 28.32 ± 4.86 | 29.24 ± 3.87 | 0.281 |
| PTA, % | 55.52 ± 22.62 | 52.76 ± 20.98 | 0.512 |
| Cr, μmol/L | 84.5 (62.25,124.5) | 107 (70.6,155) | 0.050 |
| PCT, ng/mL | 0.28 (0.15,2.48) | 1.71 (0.25,5.51) | 0.026 |
| CRP, mg/L | 17.55 (12.83,53.93) | 51.5 (19.45,96.03) | 0.126 |
| (1,3)-β-D-glucan levels, pg/mL | 10 (10,10) | 53.55 (11.53,249.75) | <0.001 |
| Child-Pugh | 10.59 ± 1.84 | 11.09 ± 1.65 | 0.140 |
| MELD | 14.65 ± 8.00 | 18.19 ± 9.56 | 0.041 |
| Variable | Fungiascites (n = 2) | SFP with Fungus-Positive Ascites Only (n = 23) | SFP Mixed with Bacteria-Positive Ascites (n = 29) | p-Value a |
|---|---|---|---|---|
| HE n (%) | 0 (0%) | 6 (26.08%) | 12 (41.38%) | 0.250 |
| Variceal bleeding n (%) | 0 (0%) | 8 (34.78%) | 10 (34.48%) | 0.982 |
| AKI/HRS n (%) | 0 (0%) | 15 (65.22%) | 16 (55.17%) | 0.463 |
| Pleural effusion n (%) | 1 (50%) | 13 (56.52%) | 15 (51.72%) | 0.730 |
| Septic shock n (%) | 1 (50%) | 4 (17.39%) | 7 (24.14%) | 0.803 |
| WBC, 109/L | 12.84 | 8.58 (3.46,16.03) | 7.62 (4.75,11.56) | 0.625 |
| NEU, % | 78.55 | 78.05 ± 13.28 | 77.86 ± 13.32 | 0.960 |
| PLT, 109/L | 102 | 72 (54,96) | 78 (31,119) | 0.706 |
| ALT, U/L | 7.6 | 28 (15,40.6) | 16.3 (7.5,31.15) | 0.068 |
| AST, U/L | 13.65 | 51.3 (30,74.7) | 42 (21,80) | 0.269 |
| TBIL, μmol/L | 62.15 | 65.4 (42.8,162.4) | 52.2 (39.9,86.6) | 0.507 |
| ALB, g/L | 32.7 | 30.33 ± 3.46 | 28.13 ± 3.69 | 0.033 |
| PTA, % | 77.5 | 52.26 ± 23.69 | 51.45 ± 15.14 | 0.881 |
| Cr, μmol/L | 315.25 | 126.16 (77,145) | 100.5 (66.25,155) | 0.478 |
| PCT, ng/mL | 0.9 | 2.12 (0.26,5.93) | 1.58 (0.2,4.93) | 0.465 |
| CRP, mg/L | 26.1 | 41.5 (17.6,90.1) | 71.65 (24.35,150.5) | 0.322 |
| (1,3)-β-D-glucan levels, pg/mL | 143.19 | 53.05 (10,431.93) | 50.4 (13.65,131.55) | 0.829 |
| Child–Pugh | 10 | 10.74 ± 1.60 | 11.45 ± 1.66 | 0.127 |
| MELD | 11.08 | 18.56 ± 9.19 | 18.54 ± 10.17 | 0.995 |
| Variable | HR | 95%CI | p Value |
|---|---|---|---|
| Univariate analysis | |||
| Septic shock | 3.482 | 1.584–7.653 | 0.002 |
| HCC | 2.316 | 1.103–4.865 | 0.027 |
| PCT | 1.010 | 1.001–1.020 | 0.037 |
| NEU | 1.045 | 1.010–1.080 | 0.010 |
| AST | 1.005 | 1.001–1.008 | 0.005 |
| Multivariate analysis | |||
| Septic shock | 4.171 | 1.363–12.765 | 0.012 |
| HCC | 3.087 | 1.196–7.973 | 0.020 |
| Variable | OR | 95% CI | p Value |
|---|---|---|---|
| Univariate analysis | |||
| AKI/HRS | 2.934 | 1.334–6.45 | 0.007 |
| Pleural effusion | 2.320 | 1.065–5.054 | 0.034 |
| Fever | 3.143 | 1.431–6.905 | 0.004 |
| (1,3)-β-D-glucan levels | 1.042 | 1.017–1.068 | 0.001 |
| MELD | 1.048 | 1.001–1.096 | 0.045 |
| Hospital-acquired infection | 11.875 | 4.715–2.911 | <0.001 |
| Multivariate analysis | |||
| AKI/HRS | 3.568 | 1.234–10.311 | 0.019 |
| Fever | 3.154 | 1.075–9.255 | 0.037 |
| (1,3)-β-D-glucan levels | 1.029 | 1.004–1.054 | 0.022 |
| Hospital-acquired infection | 10.386 | 3.515–30.682 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Fan, C.; Dang, Y.; Zhao, W.; Lv, L.; Lou, J.; Li, L.; Ding, H. Clinical Characteristics and Early Diagnosis of Spontaneous Fungal Peritonitis/Fungiascites in Hospitalized Cirrhotic Patients with Ascites: A Case–Control Study. J. Clin. Med. 2023, 12, 3100. https://doi.org/10.3390/jcm12093100
Jiang Y, Fan C, Dang Y, Zhao W, Lv L, Lou J, Li L, Ding H. Clinical Characteristics and Early Diagnosis of Spontaneous Fungal Peritonitis/Fungiascites in Hospitalized Cirrhotic Patients with Ascites: A Case–Control Study. Journal of Clinical Medicine. 2023; 12(9):3100. https://doi.org/10.3390/jcm12093100
Chicago/Turabian StyleJiang, Yingying, Chunlei Fan, Yan Dang, Wenmin Zhao, Lingna Lv, Jinli Lou, Lei Li, and Huiguo Ding. 2023. "Clinical Characteristics and Early Diagnosis of Spontaneous Fungal Peritonitis/Fungiascites in Hospitalized Cirrhotic Patients with Ascites: A Case–Control Study" Journal of Clinical Medicine 12, no. 9: 3100. https://doi.org/10.3390/jcm12093100
APA StyleJiang, Y., Fan, C., Dang, Y., Zhao, W., Lv, L., Lou, J., Li, L., & Ding, H. (2023). Clinical Characteristics and Early Diagnosis of Spontaneous Fungal Peritonitis/Fungiascites in Hospitalized Cirrhotic Patients with Ascites: A Case–Control Study. Journal of Clinical Medicine, 12(9), 3100. https://doi.org/10.3390/jcm12093100






