Can Blood Ammonia Level, Prehospital Time, and Return of Spontaneous Circulation Predict Neurological Outcomes of Out-of-Hospital Cardiac Arrest Patients? A Nationwide, Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection
2.3. The Emergency Medical Services System in Japan
2.4. Participants, Groups, and Endpoints
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Impact of Blood Ammonia Level and Time on Favorable Neurological Outcomes
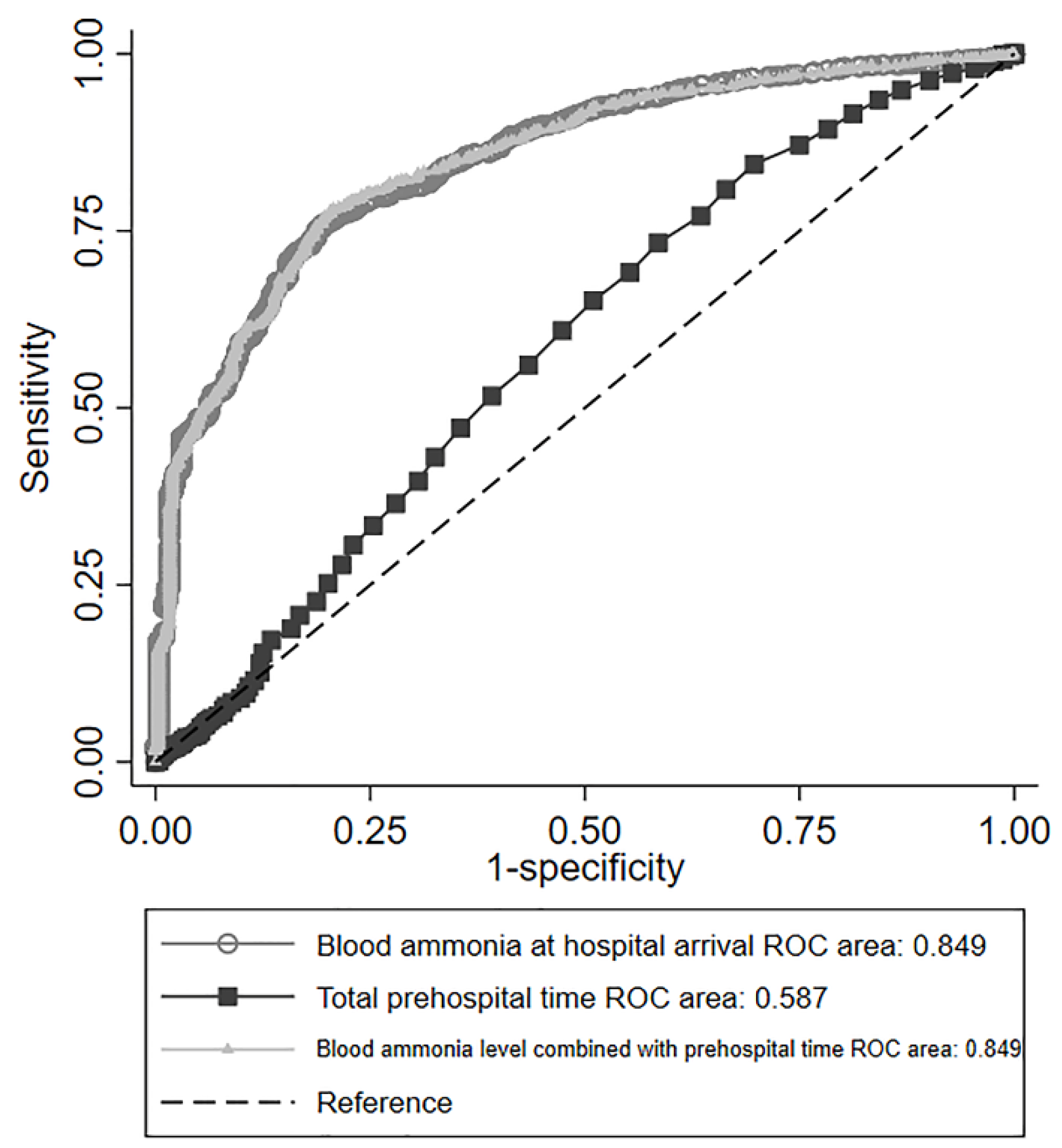
3.3. Prognostic Performance of Blood Ammonia Levels
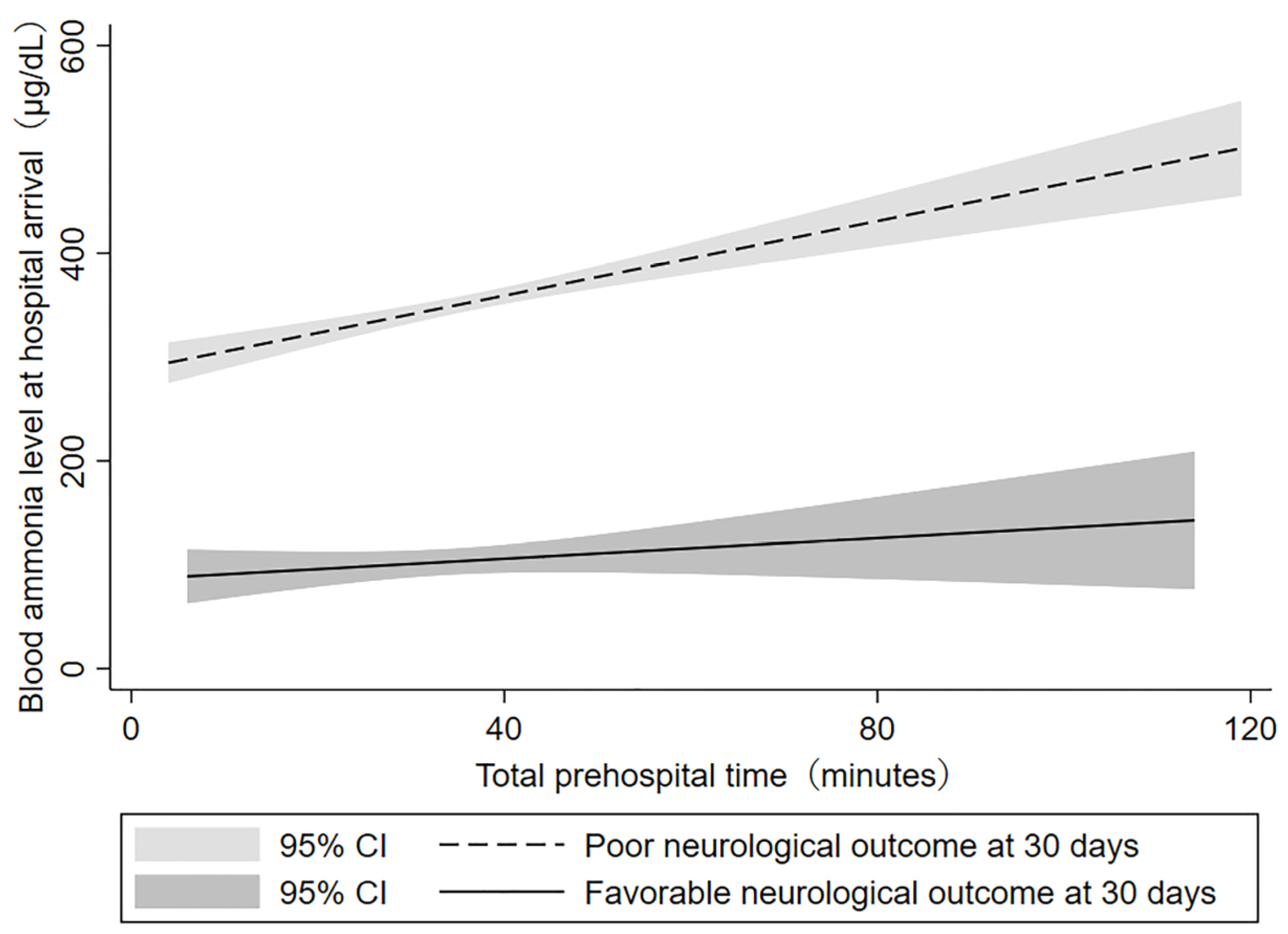
3.4. Correlation between Blood Ammonia Level and Total Prehospital Time
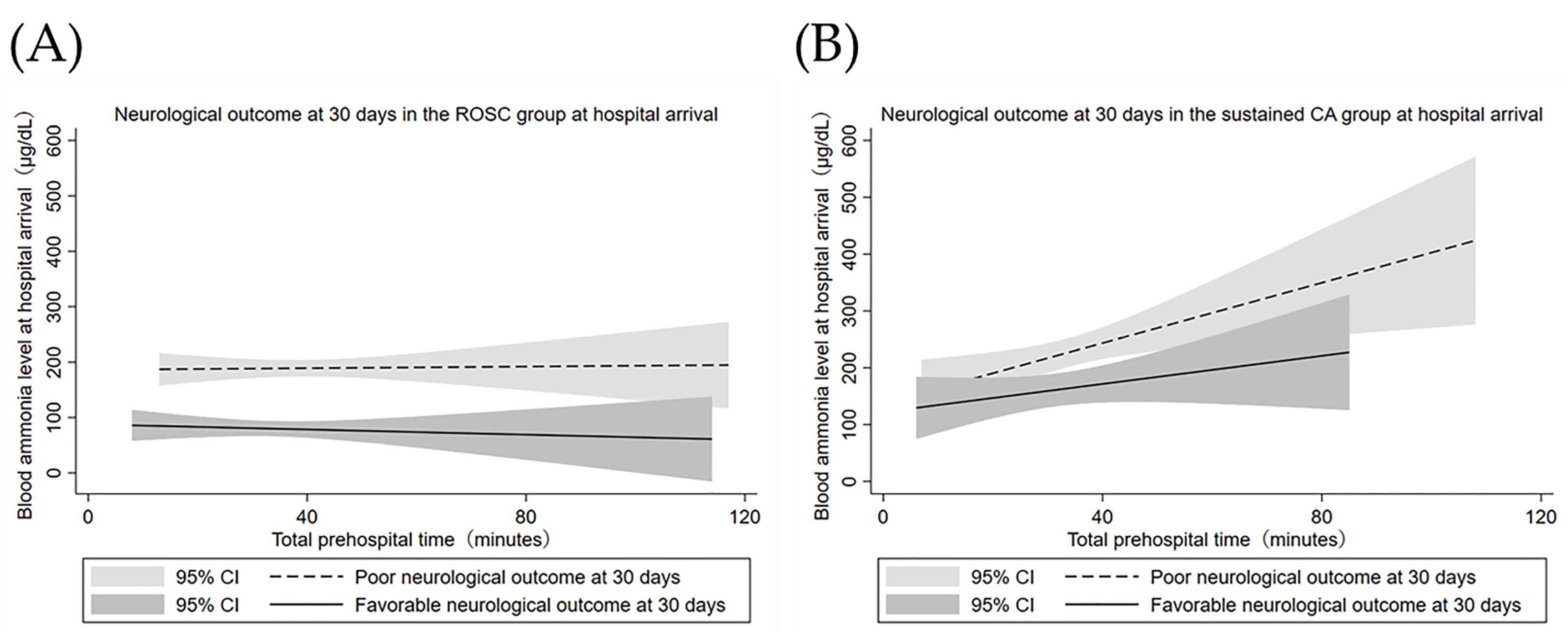
3.5. Cardiac Causes vs. Noncardiac Causes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Geocadin, R.G.; Callaway, C.W.; Fink, E.L.; Golan, E.; Greer, D.M.; Ko, N.U.; Lang, E.; Licht, D.J.; Marino, B.S.; McNair, N.D.; et al. Standards for Studies of Neurological Prognostication in Comatose Survivors of Cardiac Arrest: A Scientific Statement From the American Heart Association. Circulation 2019, 140, e517–e542. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Torres, C.; Aufderheide, T.P.; Austin, M.A.; Callaway, C.W.; Golan, E.; Herren, H.; Jasti, J.; Kudenchuk, P.J.; Scales, D.C.; et al. Association of early withdrawal of life-sustaining therapy for perceived neurological prognosis with mortality after cardiac arrest. Resuscitation 2016, 102, 127–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, J.P.; Sandroni, C.; Bottiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Sandroni, C.; D’Arrigo, S.; Cacciola, S.; Hoedemaekers, C.W.E.; Kamps, M.J.A.; Oddo, M.; Taccone, F.S.; Di Rocco, A.; Meijer, F.J.A.; Westhall, E.; et al. Prediction of poor neurological outcome in comatose survivors of cardiac arrest: A systematic review. Intensive Care Med. 2020, 46, 1803–1851. [Google Scholar] [CrossRef] [PubMed]
- Jakkula, P.; Skrifvars, M.B.; Pettila, V.; Hastbacka, J.; Reinikainen, M. NSE concentrations and haemolysis after cardiac arrest. Intensive Care Med. 2019, 45, 741–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung-Esaki, H.M.; Mui, G.; Mlynash, M.; Eyngorn, I.; Catabay, K.; Hirsch, K.G. The neuron specific enolase (NSE) ratio offers benefits over absolute value thresholds in post-cardiac arrest coma prognosis. J. Clin. Neurosci. 2018, 57, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Sondag, L.; Ruijter, B.J.; Tjepkema-Cloostermans, M.C.; Beishuizen, A.; Bosch, F.H.; van Til, J.A.; van Putten, M.; Hofmeijer, J. Early EEG for outcome prediction of postanoxic coma: Prospective cohort study with cost-minimization analysis. Crit. Care 2017, 21, 111. [Google Scholar] [CrossRef] [Green Version]
- Calderon, L.M.; Guyette, F.X.; Doshi, A.A.; Callaway, C.W.; Rittenberger, J.C. The Post Cardiac Arrest Service. Combining NSE and S100B with clinical examination findings to predict survival after resuscitation from cardiac arrest. Resuscitation 2014, 85, 1025–1029. [Google Scholar] [CrossRef] [Green Version]
- SOS-KANTO 2012 Study Group. Initial Blood Ammonia Level Is a Useful Prognostication Tool in Out-of-Hospital Cardiac Arrest- Multicenter Prospective Study (SOS-KANTO 2012 Study). Circ. J. 2017, 81, 1839–1845. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.H.; Chi, C.H.; Wu, S.Y.; Hsu, H.C.; Chang, Y.H.; Huang, Y.Y.; Chang, C.J.; Hong, M.Y.; Chan, T.Y.; Shih, H.I. Prognostic values of blood ammonia and partial pressure of ammonia on hospital arrival in out-of-hospital cardiac arrests. Am. J. Emerg. Med. 2013, 31, 8–15. [Google Scholar] [CrossRef]
- Yang, D.; Ryoo, E.; Kim, H.J. Combination of Early EEG, Brain CT, and Ammonia Level Is Useful to Predict Neurologic Outcome in Children Resuscitated From Cardiac Arrest. Front. Pediatrics 2019, 7, 223. [Google Scholar] [CrossRef] [PubMed]
- Khacho, M.; Tarabay, M.; Patten, D.; Khacho, P.; MacLaurin, J.G.; Guadagno, J.; Bergeron, R.; Cregan, S.P.; Harper, M.E.; Park, D.S.; et al. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat. Commun. 2014, 5, 3550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- JAAM OHCA Registry. Available online: http://www.jaamohca-web.com (accessed on 14 September 2011).
- Irisawa, T.; Matsuyama, T.; Iwami, T.; Yamada, T.; Hayakawa, K.; Yoshiya, K.; Noguchi, K.; Nishimura, T.; Uejima, T.; Yagi, Y.; et al. The effect of different target temperatures in targeted temperature management on neurologically favorable outcome after out-of-hospital cardiac arrest: A nationwide multicenter observational study in Japan (the JAAM-OHCA registry). Resuscitation 2018, 133, 82–87. [Google Scholar] [CrossRef]
- Naito, H.; Yumoto, T.; Yorifuji, T.; Tahara, Y.; Yonemoto, N.; Nonogi, H.; Nagao, K.; Ikeda, T.; Sato, N.; Tsutsui, H. Improved outcomes for out-of-hospital cardiac arrest patients treated by emergency life-saving technicians compared with basic emergency medical technicians: A JCS-ReSS study report. Resuscitation 2020, 153, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Shinozaki, K.; Oda, S.; Sadahiro, T.; Nakamura, M.; Hirayama, Y.; Watanabe, E.; Tateishi, Y.; Nakanishi, K.; Kitamura, N.; Sato, Y.; et al. Blood ammonia and lactate levels on hospital arrival as a predictive biomarker in patients with out-of-hospital cardiac arrest. Resuscitation 2011, 82, 404–409. [Google Scholar] [CrossRef]
- Nagamine, K. Does Blood Ammonia Level at Time of Initial Treatment Predict the Outcome of Patients in Cardiopulmonary Arrest on Arrival? Nihon Kyukyu Igakukai Zasshi 2005, 16, 283–288. [Google Scholar] [CrossRef] [Green Version]
- Nagao, K.; Nonogi, H.; Yonemoto, N.; Gaieski, D.F.; Ito, N.; Takayama, M.; Shirai, S.; Furuya, S.; Tani, S.; Kimura, T.; et al. Duration of Prehospital Resuscitation Efforts After Out-of-Hospital Cardiac Arrest. Circulation 2016, 133, 1386–1396. [Google Scholar] [CrossRef] [Green Version]
- Cooper, A.J.; Plum, F. Biochemistry and physiology of brain ammonia. Physiol. Rev. 1987, 67, 440–519. [Google Scholar] [CrossRef]
- Dasarathy, S.; Mookerjee, R.P.; Rackayova, V.; Rangroo Thrane, V.; Vairappan, B.; Ott, P.; Rose, C.F. Ammonia toxicity: From head to toe? Metab. Brain Dis. 2017, 32, 529–538. [Google Scholar] [CrossRef]
- Braissant, O.; McLin, V.A.; Cudalbu, C. Ammonia toxicity to the brain. J. Inherit. Metab. Dis. 2013, 36, 595–612. [Google Scholar] [CrossRef] [Green Version]
- Wihersaari, L.; Tiainen, M.; Skrifvars, M.B.; Bendel, S.; Kaukonen, K.M.; Vaahersalo, J.; Romppanen, J.; Pettila, V.; Reinikainen, M. Usefulness of neuron specific enolase in prognostication after cardiac arrest: Impact of age and time to ROSC. Resuscitation 2019, 139, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Hirlekar, G.; Jonsson, M.; Karlsson, T.; Hollenberg, J.; Albertsson, P.; Herlitz, J. Comorbidity and survival in out-of-hospital cardiac arrest. Resuscitation 2018, 133, 118–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roedl, K.; Wallmüller, C.; Drolz, A.; Horvatits, T.; Rutter, K.; Spiel, A.; Ortbauer, J.; Stratil, P.; Hubner, P.; Weiser, C.; et al. Outcome of in- and out-of-hospital cardiac arrest survivors with liver cirrhosis. Ann. Intensive Care 2017, 7, 103. [Google Scholar] [CrossRef] [PubMed]

| All Participants (n = 7426) | Favorable Outcomes (CPC 1–2) at 30 Days (n = 364) | Poor Outcomes (CPC 3–5) at 30 Days (n = 7062) | p-Value | |
|---|---|---|---|---|
| Age (year), median (IQR) | 76 (64–84) | 63 (48–73) | 76 (65–85) | <0.01 |
| Male, n (%) | 4538 (61%) | 281 (77%) | 4257 (60%) | <0.01 |
| Total prehospital time * (min), median (IQR) | 34 (28–41) | 31 (25–39) | 34 (28–42) | <0.01 |
| Witnessed collapse, n (%) | 3181 (43%) | 259 (71%) | 2922 (41%) | <0.01 |
| Bystander CPR, n (%) | 3202 (43%) | 183 (50%) | 3019 (43%) | <0.01 |
| AED used by bystander, n (%) | 149 (2.0%) | 78 (21%) | 71 (1.0%) | <0.01 |
| Adrenaline used by EMS | 2278 (31%) | 35 (9.6%) | 2243 (32%) | <0.01 |
| Cardiac cause, n (%) | 3719 (50%) | 302 (83%) | 3417 (48%) | <0.01 |
| Initial rhythm at hospital arrival | ||||
| VF/pulseless VT, n (%) | 392 (5.3%) | 69 (19%) | 323 (4.6%) | <0.01 |
| PEA/asystole, n (%) | 6124 (82%) | 46 (13%) | 6078 (86%) | <0.01 |
| ROSC during transport, n (%) | 910 (12%) | 249 (68%) | 661 (9.4%) | <0.01 |
| Blood ammonia level (μg/dL), median (IQR) | 253 (125–438) | 70 (42–122) | 265 (138–452) | <0.01 |
| Potassium (mEq/L), median (IQR) | 6.0 (4.6–7.9) | 3.8 (3.4–4.2) | 6.1 (4.8–8.1) | <0.01 |
| Lactate (mg/dL), median (IQR) | 118 (74–156) | 64 (17–117) | 119 (75–156) | <0.01 |
| TTM, n (%) | 634 (8.5%) | 203 (56%) | 431 (6.1%) | <0.01 |
| Patients admitted to the hospital, n (%) | 2457 (33%) | N/A | N/A | |
| Survival at 30 days, n (%) | 738 (10%) | N/A | N/A |
| Crude OR (95% CI) | Adjusted OR (95% CI) | |
|---|---|---|
| Blood ammonia level at hospital arrival | 0.988 (0.987–0.990) | 0.995 (0.994–0.998) |
| Total prehospital time | 0.996 (0.996–0.997) | 0.993 (0.981–1.006) |
| All Participants (n = 7426) | ROSC at Time of Hospital Arrival (n = 910) | Sustained CA at Hospital Arrival (n = 6516) | p-Value | |
|---|---|---|---|---|
| Age (year), median (IQR) | 76 (64–84) | 74 (62–83) | 76 (64–84) | 0.012 |
| Male, n (%) | 4538 (61%) | 576 (63%) | 3962 (61%) | 0.15 |
| Total prehospital time * (min), median (IQR) | 34 (28–41) | 34 (29–41) | 34 (28–41) | 0.08 |
| Witnessed collapse, n (%) | 3181 (43%) | 526 (58%) | 2655 (41%) | <0.01 |
| Bystander CPR, n (%) | 3202 (43%) | 411 (45%) | 2791 (43%) | <0.01 |
| AED used by bystander, n (%) | 149 (2.0%) | 76 (8.4%) | 73 (1.1%) | <0.01 |
| Adrenalin used by EMS | 2278 (31%) | 380 (42%) | 1898 (29%) | <0.01 |
| Cardiac cause, n (%) | 3719 (50%) | 426 (47%) | 3293 (51%) | 0.035 |
| Initial rhythm at hospital arrival | ||||
| VF/pulseless VT, n (%) | 392 (5.3%) | N/A | 392 (6 %) | N/A |
| PEA/Asystole, n (%) | 6124 (82%) | N/A | 6124 (94%) | N/A |
| ROSC during transport, n (%) | 910 (12%) | 910 (100%) | N/A | N/A |
| Blood ammonia level (μg/dL), median (IQR) | 253 (125–438) | 103 (54–206) | 278 (148–472) | <0.01 |
| Potassium (mEq/L), median (IQR) | 6.0 (4.6–7.9) | 3.9 (3.5–4.8) | 6.3 (4.9–8.2) | <0.01 |
| Lactate (mg/dL), median (IQR) | 118 (74–156) | 81 (38–110) | 119 (75–156) | <0.01 |
| TTM, n (%) | 634 (8.5%) | 246 (27%) | 388 (6.0%) | <0.01 |
| Patients admitted to the hospital, n (%) | 2457 (33%) | 788 (87%) | 1669 (26%) | <0.01 |
| Survival at 30 days, n (%) | 738 (10%) | 419 (46%) | 319 (4.9%) | <0.01 |
| Favorable neurological outcome at 30 days, n (%) | 364 (4.9%) | 249 (27%) | 115 (1.8%) | <0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nojima, T.; Naito, H.; Obara, T.; Ageta, K.; Yakushiji, H.; Yumoto, T.; Fujisaki, N.; Nakao, A. Can Blood Ammonia Level, Prehospital Time, and Return of Spontaneous Circulation Predict Neurological Outcomes of Out-of-Hospital Cardiac Arrest Patients? A Nationwide, Retrospective Cohort Study. J. Clin. Med. 2022, 11, 2566. https://doi.org/10.3390/jcm11092566
Nojima T, Naito H, Obara T, Ageta K, Yakushiji H, Yumoto T, Fujisaki N, Nakao A. Can Blood Ammonia Level, Prehospital Time, and Return of Spontaneous Circulation Predict Neurological Outcomes of Out-of-Hospital Cardiac Arrest Patients? A Nationwide, Retrospective Cohort Study. Journal of Clinical Medicine. 2022; 11(9):2566. https://doi.org/10.3390/jcm11092566
Chicago/Turabian StyleNojima, Tsuyoshi, Hiromichi Naito, Takafumi Obara, Kohei Ageta, Hiromasa Yakushiji, Tetsuya Yumoto, Noritomo Fujisaki, and Atsunori Nakao. 2022. "Can Blood Ammonia Level, Prehospital Time, and Return of Spontaneous Circulation Predict Neurological Outcomes of Out-of-Hospital Cardiac Arrest Patients? A Nationwide, Retrospective Cohort Study" Journal of Clinical Medicine 11, no. 9: 2566. https://doi.org/10.3390/jcm11092566
APA StyleNojima, T., Naito, H., Obara, T., Ageta, K., Yakushiji, H., Yumoto, T., Fujisaki, N., & Nakao, A. (2022). Can Blood Ammonia Level, Prehospital Time, and Return of Spontaneous Circulation Predict Neurological Outcomes of Out-of-Hospital Cardiac Arrest Patients? A Nationwide, Retrospective Cohort Study. Journal of Clinical Medicine, 11(9), 2566. https://doi.org/10.3390/jcm11092566






