The Utility of Pentraxin and Modified Prognostic Scales in Predicting Outcomes of Patients with End-Stage Heart Failure
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Measurements
2.2. Scales
- -
- The HFSS score was calculated based on the following equation incorporating seven variables: ([0.0216 × resting heart rhythm] + [−0.0255 × mean arterial blood pressure (MAP)] + [−0.0464 × left ventricular ejection fraction (LVEF)] + [−0.0470 × serum sodium] + [−0.0546 × peak VO2 ] + [0.6083 × presence (1) or absence (0) of interventricular conduction defect (QRS duration ≥ 0.12 due to any cause)] + [0.6931 × presence (1) or absence (0) of ischemic cardiomyopathy]), as described previously [3].
- -
- The MAGGIC score [5] was developed from 13 routinely available patient characteristics: (1) age, (2) sex, (3) body mass index (BMI), (4) systolic blood pressure, (5) creatinine concentration, (6) presence or absence of diabetes mellitus and (7) chronic obstructive pulmonary disease, (8) HF diagnosed in the last 18 months, (9) NYHA class, (10) LVEF, (11) current smoking status, (12) b-blockers, and (13) angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. From 18 September 2013, the integer score increased by 2 if HF was diagnosed >18 months ago, which is reflected in our analysis.
2.3. Outcome Data
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Freitas, P.; Aguiar, C.; Ferreira, A.; Tralhão, A.; Ventosa, A.; Mendes, M. Comparative analysis of four scores to stratify patients with heart failure and reduced ejection fraction. Am. J. Cardiol. 2017, 120, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Westerdahl, D.E.; Kobashigawa, J.A. Heart transplantation for advanced heart failure. Card. Intensive Care 2019, 504–524.e2. [Google Scholar] [CrossRef]
- Aaronson, K.D.; Schwartz, J.S.; Chen, T.-M.; Wong, K.-L.; Goin, J.E.; Mancini, D.M. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation 1997, 95, 2660–2667. [Google Scholar] [CrossRef] [PubMed]
- Levy, W.C.; Mozaffarian, D.; Linker, D.T.; Sutradhar, S.C.; Anker, S.D.; Cropp, A.B.; Anand, I.; Maggioni, A.; Burton, P.; Sullivan, M.D.; et al. The seattle heart failure model: Prediction of survival in heart failure. Circulation 2006, 113, 1424–1433. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.V.; Maggioni, A.; Køber, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Di Tanna, G.L.; Wirtz, H.; Burrows, K.L.; Globe, G. Evaluating risk prediction models for adults with heart failure: A systematic literature review. PLoS ONE 2020, 15, e0224135. [Google Scholar] [CrossRef]
- Szczurek, W.; Gąsior, M.; Skrzypek, M.; Szyguła-Jurkiewicz, B. Apelin improves prognostic value of HFSS (Heart Failure Survival Score) and MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) scales in ambulatory patients with end-stage heart failure. J. Clin. Med. 2020, 9, 2300. [Google Scholar] [CrossRef]
- Nadar, S.K.; Shaikh, M.M. Biomarkers in routine heart failure clinical care. Card. Fail. Rev. 2019, 5, 50–56. [Google Scholar] [CrossRef]
- Cabassi, A.; de Champlain, J.; Maggiore, U.; Parenti, E.; Coghi, P.; Vicini, V.; Tedeschi, S.; Cremaschi, E.; Binno, S.; Rocco, R.; et al. Prealbumin improves death risk prediction of BNP-added Seattle Heart Failure Model: Results from a pilot study in elderly chronic heart failure patients. Int. J. Cardiol. 2013, 168, 3334–3339. [Google Scholar] [CrossRef]
- Braunwald, E. Biomarkers in heart failure. N. Engl. J. Med. 2008, 358, 2148–2159. [Google Scholar] [CrossRef]
- Matsubara, J.; Sugiyama, S.; Nozaki, T.; Sugamura, K.; Konishi, M.; Ohba, K.; Matsuzawa, Y.; Akiyama, E.; Yamamoto, E.; Sakamoto, K.; et al. Pentraxin 3 is a new inflammatory marker correlated with left ventricular diastolic dysfunction and heart failure with normal ejection fraction. J. Am. Coll. Cardiol. 2011, 57, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Takeishi, Y.; Niizeki, T.; Koyama, Y.; Kitahara, T.; Sasaki, T.; Sagara, M.; Kubota, I. Pentraxin 3, a new marker for vascular inflammation, predicts adverse clinical outcomes in patients with heart failure. Am. Heart J. 2008, 155, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Norata, G.D.; Garlanda, C.; Catapano, A.L. The long pentraxin PTX3: A modulator of the immunoinflammatory response in atherosclerosis and cardiovascular diseases. Trends Cardiovasc. Med. 2010, 20, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Kaess, B.M.; Vasan, R.S. Heart failure: Pentraxin 3—A marker of diastolic dysfunction and HF? Nat. Rev. Cardiol. 2011, 8, 246–248. [Google Scholar] [CrossRef]
- Kotooka, N.; Inoue, T.; Aoki, S.; Anan, M.; Komoda, H.; Node, K. Prognostic value of pentraxin 3 in patients with chronic heart failure. Int. J. Cardiol. 2008, 130, 19–22. [Google Scholar] [CrossRef]
- Latini, R.; Gullestad, L.; Masson, S.; Nymo, S.H.; Ueland, T.; Cuccovillo, I.; Vårdal, M.; Bottazzi, B.; Mantovani, A.; Lucci, D.; et al. Investigators of the Controlled Rosuvastatin Multinational Trial in Heart Failure (CORONA) and GISSI-Heart Failure (GISSI-HF) trials. Pentraxin-3 in chronic heart failure: The CORONA and GISSI-HF trials. Eur. J. Heart Fail. 2012, 14, 992–999. [Google Scholar] [CrossRef]
- Pan, J.-P.; Liu, T.-Y.; Chiang, S.-C.; Lin, Y.-K.; Chou, C.-Y.; Chan, W.-L.; Lai, S.-T. The value of plasma levels of tumor necrosis factor-alpha and interleukin-6 in predicting the severity and prognosis in patients with congestive heart failure. J. Chin. Med Assoc. 2004, 67, 222–228. [Google Scholar]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune mechanisms in heart failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef]
- Sharma, R.; Coats, A.J.; Anker, S.D. The role of inflammatory mediators in chronic heart failure: Cytokines, nitric oxide, and endothelin-1. Int. J. Cardiol. 2000, 72, 175–186. [Google Scholar] [CrossRef]
- Ristagno, G.; Fumagalli, F.; Bottazzi, B.; Mantovani, A.; Olivari, D.; Novelli, D.; Latini, R. Pentraxin 3 in cardiovascular disease. front immunol. Front. Immunol. 2019, 10, 823. [Google Scholar] [CrossRef]
- Gullestad, L.; Ueland, T.; Vinge, L.E.; Finsen, A.; Yndestad, A.; Aukrust, P. Inflammatory cytokines in heart failure: Mediators and markers. Cardiology 2012, 122, 23–35. [Google Scholar] [CrossRef] [PubMed]
- Garlanda, C.; Bottazzi, B.; Magrini, E.; Inforzato, A.; Mantovani, A. PTX3, a humoral pattern recognition molecule, in innate immunity, tissue repair, and cancer. Physiol. Rev. 2018, 98, 623–639. [Google Scholar] [CrossRef] [PubMed]
- Nauta, A.J.; Bottazzi, B.; Mantovani, A.; Salvatori, G.; Kishore, U.; Schwaeble, W.J.; Gingras, A.R.; Tzima, S.; Vivanco, F.; Egido, J.; et al. Biochemical and functional characterization of the interaction between pentraxin 3 and C1q. Eur. J. Immunol. 2003, 33, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Complement activation: An emerging player in the pathogenesis of cardiovascular disease. Scientifica (Cairo) 2012, 2012, 402783. [Google Scholar] [CrossRef]
- Souza, D.G.; Amaral, F.A.; Fagundes, C.T.; Coelho, F.M.; Arantes, R.M.; Sousa, L.P.; Matzuk, M.M.; Garlanda, C.; Mantovani, A.; Dias, A.A.; et al. The long pentraxin PTX3 is crucial for tissue inflammation after intestinal ischemia and reperfusion in mice. Am. J. Pathol. 2009, 174, 1309–1318. [Google Scholar] [CrossRef]
- Suzuki, S.; Shishido, T.; Funayama, A.; Netsu, S.; Ishino, M.; Kitahara, T.; Sasaki, T.; Katoh, S.; Otaki, Y.; Watanabe, T.; et al. Long pentraxin PTX3 exacerbates pressure overload-induced left ventricular dysfunction. PLoS ONE 2013, 8, e53133. [Google Scholar] [CrossRef]
- Alba, A.C.; Agoritsas, T.; Jankowski, M.; Courvoisier, D.; Walter, S.D.; Guyatt, G.H.; Ross, H.J. Risk prediction models for mortality in ambulatory patients with heart failure: A systematic review. Circ. Heart Fail. 2013, 6, 881–889. [Google Scholar] [CrossRef]
- Mehra, M.R.; Canter, C.E.; Hannan, M.M.; Semigran, M.J.; Uber, P.A.; Baran, D.A.; Danziger-Isakov, L.; Kirklin, J.K.; Kirk, R.; Kushwaha, S.S.; et al. The 2016 International Society for Heart Lung Transplantation listing criteria for heart transplantation: A 10-year update. J. Heart Lung Transplant. 2016, 35, 1–23. [Google Scholar] [CrossRef]
- Khanam, S.S.; Choi, E.; Son, J.-W.; Lee, J.-W.; Youn, Y.J.; Yoon, J.; Lee, S.-H.; Kim, J.-Y.; Ahn, S.G.; Ahn, M.-S.; et al. Validation of the MAGGIC (Meta-Analysis Global Group in Chronic Heart Failure) heart failure risk score and the effect of adding natriuretic peptide for predicting mortality after discharge in hospitalized patients with heart failure. PLoS ONE 2018, 13, e0206380. [Google Scholar] [CrossRef]
- Goda, A.; Williams, P.; Mancini, D.; Lund, L.H. Selecting patients for heart transplantation: Comparison of the heart failure survival score (HFSS) and the seattle heart failure model (SHFM). J. Heart Lung Transplant. 2011, 30, 1236–1243. [Google Scholar] [CrossRef]
- AbouEzzeddine, O.F.; French, B.; Mirzoyev, S.A.; Jaffe, A.S.; Levy, W.C.; Fang, J.C.; Sweitzer, N.K.; Cappola, T.P.; Redfield, M.M. From statistical significance to clinical relevance: A simple algorithm to integrate brain natriuretic peptide and the Seattle Heart Failure Model for risk stratification in heart failure. J. Heart Lung Transplant. 2016, 35, 714–721. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chirinos, J.A.; Orlenko, A.; Zhao, L.; Basso, M.D.; Cvijic, M.E.; Li, Z.; Spires, T.E.; Yarde, M.; Wang, Z.; Seiffert, D.A.; et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J. Am. Coll. Cardiol. 2020, 75, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Lupón, J.; de Antonio, M.; Vila, J.; Peñafiel, J.; Galán, A.; Zamora, E.; Urrutia, A.; Bayes-Genis, A. Development of a novel heart failure risk tool: The barcelona bio-heart failure risk calculator (BCN bio-HF calculator). PLoS ONE 2014, 9, e85466. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Workicho, A.; Gudina, E.K. Hyponatremia in patients hospitalized with heart failure: A condition often overlooked in low-income settings. Int. J. Gen. Med. 2016, 9, 267–273. [Google Scholar] [CrossRef][Green Version]
- Kenchaiah, S.; Pocock, S.J.; Wang, D.; Finn, P.V.; Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; Yusuf, S.; Swedberg, K.; Michelson, E.L.; et al. Body mass index and prognosis in patients with chronic heart failure: Insights from the Candesartan in Heart failure: Assessment of Reduction in Mortality and morbidity (CHARM) program. Circulation 2007, 116, 627–636. [Google Scholar] [CrossRef]
- MacGowan, G.A.; Neely, D.; Peaston, R.; Wrightson, N.; Parry, G. Evaluation of NT-proBNP to predict outcomes in advanced heart failure. Int. J. Clin. Pract. 2010, 64, 892–899. [Google Scholar] [CrossRef]
- Lavie, C.J.; Alpert, M.A.; Arena, R.; Mehra, M.R.; Milani, R.V.; Ventura, H.O. Impact of obesity and the obesity paradox on prevalence and prognosis in heart failure. JACC Heart Fail. 2013, 1, 93–102. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Block, G.; Horwich, T.; Fonarow, G.C. Reverse epidemiology of conventional cardiovascular risk factors in patients with chronic heart failure. J. Am. Coll. Cardiol. 2004, 43, 1439–1444. [Google Scholar] [CrossRef]
- Torres, D.; Cuttitta, F.; Paterna, S.; Garofano, A.; Conti, G.; Pinto, A.; Parrinello, G. Bed-side inferior vena cava diameter and mean arterial pressure predict long-term mortality in hospitalized patients with heart failure: 36 months of follow-up. Eur. J. Intern. Med. 2016, 28, 80–84. [Google Scholar] [CrossRef]
- Remme, W.J. Overview of the relationship between ischemia and congestive heart failure. Clin. Cardiol. 2000, 23, 4–8. [Google Scholar] [CrossRef]
- Frazier, C.G.; Alexander, K.P.; Newby, L.K.; Anderson, S.; Iverson, E.; Packer, M.; Cohn, J.; Goldstein, S.; Douglas, P.S. Associations of gender and etiology with outcomes in heart failure with systolic dysfunction: A pooled analysis of 5 randomized control trials. J. Am. Coll. Cardiol. 2007, 49, 1450–1458. [Google Scholar] [CrossRef] [PubMed]

| Overall Population N = 343 # | Patients without Events N = 170 | Patients with Events N = 173 | p | |
|---|---|---|---|---|
| Baseline data | ||||
| Age, years | 56 (50–60) | 56 (49–61) | 56 (50–60) | 0.7533 |
| Male, n (%) | 297 (86.6) | 150 (88.2) | 147 (85) | 0.3751 |
| Ischemic etiology of HF, n (%) | 199 (58) | 73 (42.9) | 126 (72.8) | <0.0001 * |
| SBP, mmHg | 102.00 (92.00–116.00) | 113.00 (100.00–120.00) | 98.00 (90.00–105.00) | <0.0001 * |
| MAP, mmHg | 76.67 (71.67–85.33) | 81.33 (76.00–90.00) | 73.33 (68.67–78.33) | <0.0001 * |
| BMI, kg/m2 | 26.93 (23.85–30.08) | 27.47 (24.49–31.21) | 26.15 (23.25–29.05) | 0.0002 * |
| NYHA III, n (%) | 299 (87.2) | 163 (95.9) | 136 (78.6) | <0.0001 * |
| NYHA IV, n (%) | 44 (12.8) | 7 (4.1) | 37 (21.4) | |
| Comorbidities | ||||
| Hypertension, n (%) | 168 (49) | 82 (48.2) | 86 (49.7) | 0.7846 |
| Type 2 diabetes, n (%) | 177 (51.6) | 80 (47.1) | 97 (56.1) | 0.095 |
| Persistent FA, n (%) | 160 (46.6) | 85 (50) | 75 (43.4) | 0.2173 |
| COPD, n (%) | 42 (12.2) | 20 (11.8) | 22 (12.7) | 0.788 |
| Laboratory parameters | ||||
| WBC, ×109/L | 7.18 (6.02–8.46) | 6.96 (5.84–8.27) | 7.33 (6.21–8.72) | 0.1256 |
| Lymphocytes, % | 24.00 (17.70–30.06) | 22.75 (17.80–28.60) | 25.10 (17.70–32.50) | 0.1179 |
| Hemoglobin, mmol/L | 8.80 (8.20–9.60) | 8.80 (8.20–9.50) | 8.90 (8.20–9.70) | 0.4866 |
| Creatinine, µmol/L | 108.00 (93.00–126.00) | 103.00 (88.00–121.00) | 113.00 (102.00–134.00) | <0.0001 * |
| GFR, mL/min/1.73 m2 | 61.78 (51.63–75.73) | 68.11 (55.49–81.65) | 56.81 (50.15–68.78) | <0.0001 * |
| Platelets, ×109/L | 197.00 (172.00–228.00) | 193.00 (171.00–220.00) | 206.00 (175.00–237.00) | 0.0317 * |
| Total bilirubin, µmol/L | 18.40 (1220–24.10) | 17.35 (11.30–21.90) | 20.00 (13.40–25.90) | 0.0021 * |
| Albumin, g/L | 44.00 (41.00–46.00) | 44.00 (42.00–46.00) | 43.00 (41.00–46.00) | 0.0445 * |
| Uric acid, µmol/L | 441.00 (371.00–526.00) | 403.50 (339.00–483.00) | 470.00 (403.00–565.00) | <0.0001 * |
| Urea, µmol/L | 8.10 (5.90–12.60) | 7.35 (5.60–10.30) | 8.90 (6.20–13.80) | 0.0059 * |
| Sodium, mmol/L | 139.00 (136.00–140.00) | 140.00 (139.00–141.00) | 137.00 (135.00–139.00) | <0.0001 * |
| Fibrinogen | 379.00 (312.00–443.00) | 363.50 (296.00–424.00) | 396.00 (330.50–483.50) | 0.001 * |
| AST, U/L | 26.00 (20.00–31.00) | 26.00 (20.00–31.00) | 25.00 (20.00–33.00) | 0.6419 |
| ALT, U/L | 22.00 (16.00–32.00) | 23.00 (17.00–33.00) | 21.00 (16.00–30.00) | 0.1087 |
| ALP, U/L | 78.00 (62.00–102.00) | 75.50 (58.00–101.00) | 81.00 (65.00–102.00) | 0.0642 |
| GGTP, U/L | 73.00 (35.00–125.00) | 64.50 (34.00–111.00) | 84.00 (36.00–137.00) | 0.0256 * |
| Cholesterol, mmol/L | 4.54 (4.16–5.00) | 4.43 (4.02–4.86) | 4.62 (4.22–5.15) | 0.0019 * |
| LDL, mmol/L | 2.05 (1.58–2.83) | 2.04 (1.55–2.73) | 2.06 (1.61–2.93) | 0.43 |
| hs-CRP, mg/L | 3.40 (1.64–8.75) | 2.75 (1.50–5.42) | 4.52 (2.06–10.75) | 0.0003 * |
| HBA1c, % | 5.80 (5.40–6.30) | 5.80 (5.40–6.30) | 5.80 (5.30–6.30) | 0.2926 |
| NT-proBNP, pg/mL | 4334.00 (1965.00–7102.00) | 3023.00 (1743.00–6101.00) | 5539.00 (2916.00–8310.00) | <0.0001 * |
| Pentraxin-3, ng/mL | 3.65 (2.57–6.23) | 2.58 (2.10–3.25) | 6.22 (5.12–8.66) | <0.0001 * |
| Haemodynamic parameters | ||||
| mPAP, mmHg | 23.00 (17.00–30.00) | 23.50 (17.00–30.00) | 22.00 (18.00–30.00) | 0.7619 |
| CI, l/min/m2 | 1.84 (1.72–1.94) | 1.83 (1.70–1.94) | 1.84 (1.72–1.95) | 0.4959 |
| TPG, mmHg | 8.00 (6.00–10.00) | 8.00 (6.00–10.00) | 8.00 (6.00–10.50) | 0.9387 |
| PVR, Wood units | 1.97 (1.47–2.36) | 1.99 (1.56–2.35) | 1.95 (1.41–2.35) | 0.6186 |
| Echocardiographic parameters | ||||
| LA, mm | 52.00 (48.00–56.00) | 52.00 (46.00–56.00) | 53.00 (49.00–57.00) | 0.0708 |
| RVEDd, mm | 34.00 (30.00–40.00) | 33.00 (30.00–40.00) | 34.00 (31.00–41.00) | 0.065 |
| LVEDd, mm | 73.00 (68.00–80.00) | 73.00 (68.00–80.00) | 73.00 (69.00–81.00) | 0.2354 |
| LVEF, % | 18.00 (15.00–20.00) | 19.00 (16.00–21.00) | 17.00 (15.00–20.00) | <0.0001 * |
| Treatment | ||||
| B-blockers, n (%) | 320 (93.3) | 161 (94.7) | 159 (91.9) | 0.7176 |
| ACEI, n (%) | 244 (71.1) | 127 (74.7) | 117 (67.6) | 0.1482 |
| ARB, n (%) | 74 (21.6) | 31 (18.2) | 43 (24.9) | 0.1361 |
| Loop diuretics, n (%) | 343 (100) | 170 (100) | 173 (100) | |
| MRA, n (%) | 322 (93.9) | 159 (93.5) | 163 (94.2) | 0.7898 |
| Digoxin, n (%) | 102 (29.7) | 51 (30) | 33 (19.1) | 0.9161 |
| Ivabradine, n (%) | 63 (18.4) | 30 (17.8) | 33 (19.1) | 0.7522 |
| Statin, n (%) | 261 (76.1) | 135 (79.4) | 126 (72.8) | 0.1532 |
| Coumarin derivatives, n (%) | 186 (54.2) | 93 (54.7) | 93 (53.8) | 0.86 |
| Acetylsalicylic acid, n (%) | 124 (36.2) | 59 (34.7) | 65 (37.6) | 0.5806 |
| Allopurinol, (n%) | 163 (47.5) | 76 (44.7) | 87 (50.3) | 0.3006 |
| ICD n (%) | 201 (58.6) | 97 (57.1) | 104 (60.1) | 0.5655 |
| CRT-D n (%) | 142 (41.4) | 73 (42.9) | 69 (39.9) | |
| Other parameter | ||||
| VO2 max, mL/kg/min | 10.80 (10.00–11.50) | 10.90 (10.20–11.60) | 10.70 (9.70–11.30) | 0.0216 * |
| Current smoker, % | 40 (11.7) | 13 (7.6) | 27 (15.6) | 0.0217 * |
| QRS > 0.12 s | 136 (39.7) | 52 (30.6) | 84 (48.6) | 0.0007 * |
| Scales | ||||
| MAGGIC score | 26.00 (24.00–29.00) | 25.00 (23.00–27.00) | 28.00 (26.00–30.00) | <0.0001 * |
| SHFM score | 0.43 (−0.004–0.907) | 0.10 (−0.25–0.46) | 0.73 (0.39–1.26) | <0.0001 * |
| HFSS score | 7.65 (7.22–8.20) | 8.08 (0.52) | 7.31 (0.54) | <0.0001 * |
| AUC [±95 CI] | p | Cut-off | Sensitivity [±95 CI] | Specificity [±95 CI] | PPV [±95 CI] | NPV [±95 CI] | LR+ [±95 CI] | LR- [±95 CI] | Accuracy | |
|---|---|---|---|---|---|---|---|---|---|---|
| HFSS | 0.8481 [0.8079–0.8883] | <0.0001 | <7.86 | 0.88 [0.82–0.92] | 0.66 [0.59–0.74] | 0.73 [0.66–0.79] | 0.84 [0.77–0.90] | 2.62 [2.04–3.20] | 0.18 [0.11–0.26] | 0.77 [0.72–0.82] |
| SHFM | 0.7976 [0.7510–0.8442] | <0.0001 | ≥0.299 | 0.80 [0.73–0.85] | 0.66 [0.58–0.73] | 0.70 [0.63–0.77] | 0.76 [0.68–0.83] | 2.34 [1.82–2.86] | 0.31 [0.21–0.40] | 0.73 [0.68–0.78] |
| MAGGIC | 0.7491 [0.6979–0.8003] | <0.0001 | ≥27 | 0.69 [0.62–0.76] | 0.70 [0.63–0.77] | 0.70 [0.63–0.77] | 0.69 [0.62–0.76] | 2.31 [1.73–2.89] | 0.44 [0.33–0.55] | 0.70 [0.65–0.75] |
| PTX-3 | 0.9558 [0.9345–0.9772] | <0.0001 | ≥3.926 | 0.88 [0.83–0.93] | 0.95 [0.91–0.98] | 0.95 [0.91–0.98] | 0.89 [0.84–0.93] | 18.79 [5.95–31.63] | 0.12 [0.07–0.17] | 0.92 [0.88–0.94] |
| NT-proBNP | 0.6598 [0.6024–0.7171] | <0.0001 | ≥3136 | 0.73 [0.66–0.79] | 0.52 [0.44–0.59] | 0.61 [0.54–0.67] | 0.65 [0.56–0.73] | 1.51 [1.24–1.78] | 0.52 [0.37–0.67] | 0.62 [0.57–0.68] |
| HFSS+ PTX-3 | 0.9508 [0.9277–0.9838] | <0.0001 | <6.772 | 0.89 [0.83–0.93] | 0.91 [0.85–0.95] | 0.91 [0.85–0.94] | 0.89 [0.83–0.93] | 9.46 [4.99–0.1393] | 0.12 [0.07–0.17] | 0.90 [0.86–0.93] |
| SHFM+ PTX3 | 0.9727 [0.9588–0.9867] | <0.0001 | ≥1.062 | 0.89 [0.83–0.93] | 0.95 [0.90–0.98] | 0.94 [0.90–0.97] | 0.89 [0.84–0.94] | 16.81 [6.01–27.61] | 0.12 [0.07–0.17] | 0.92 [0.88–0.95] |
| MAGGIC+ PTX-3 | 0.9562 [0.9354–0.9770] | <0.0001 | ≥3.388 | 0.86 [0.79–0.90] | 0.96 [0.92–0.98] | 0.95 [0.91–0.98] | 0.87 [0.81–0.91] | 20.78 [5.55–36.00] | 0.15 [0.10–0.21] | 0.91 [0.87–0.94] |
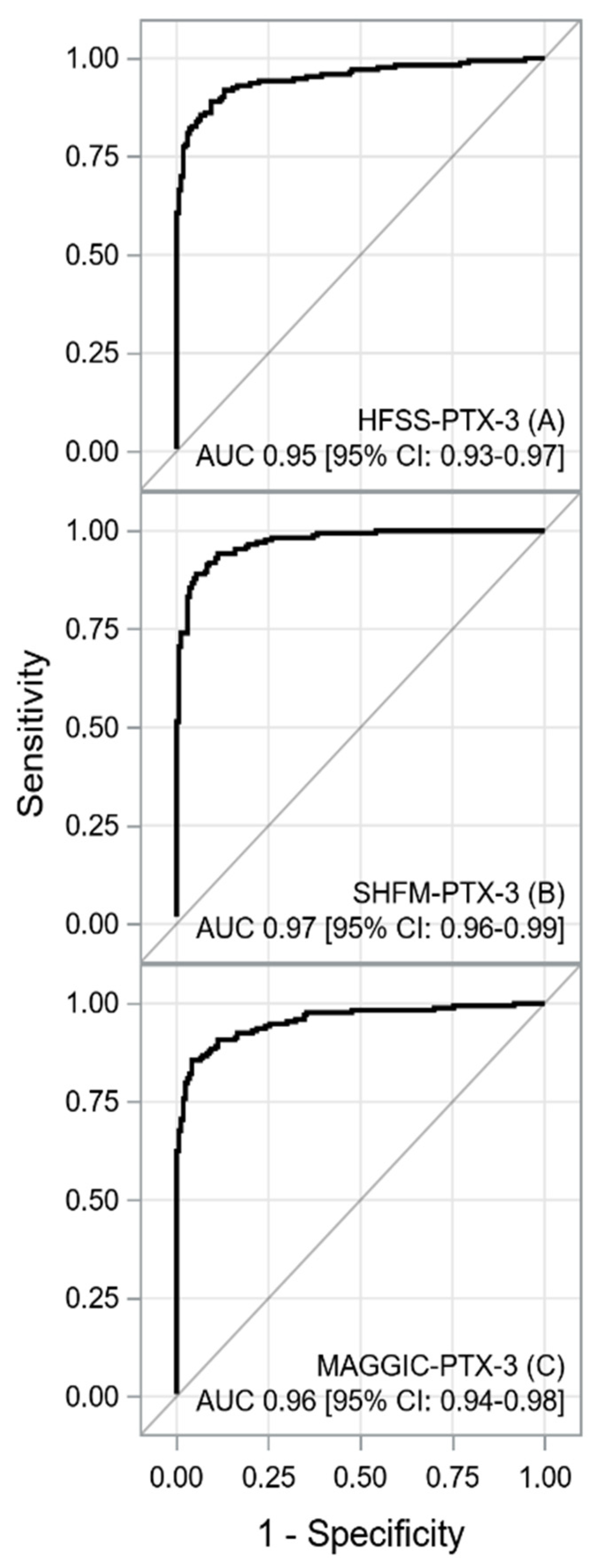
| HFSS-PTX-3, AUC [±95 CI] 1 | p | |
| HFSS, AUC [±95 CI] | 0.1027 [0.0754–0.1299] | <0.0001 |
| PTX-3, AUC [±95 CI] | −0.0051[−0.0285–0.0184] | 0.6720 |
| SHFM-PTX-3, AUC [±95 CI] | p | |
| SHFM, AUC [±95 CI] | 0.1751 [0.1320–0.2183] | <0.0001 |
| PTX-3, AUC [±95 CI] | 0.0169 [0.0014–0.0324] | 0.0330 |
| MAGGIC-PTX-3, AUC [±95 CI] | p | |
| MAGGIC, AUC [±95 CI] | 0.2071 [0.1637–0.2505] | <0.0001 |
| PTX-3, AUC [±95 CI] | 0.0004 [−0.0186–0.0194] | 0.9693 |
| Parameter | Univariable Data | Multivariable Data | ||
|---|---|---|---|---|
| Etiology of HF | 2.075 [1.477–2.914] | <0.0001 | 1.731 [1.227–2.441] | 0.0018 |
| MAP (−) | 1.044 [1.030–1.059] | <0.0001 | 1.026 [1.010–1.042] | 0.0011 |
| BMI (−) | 1.073 [1.034–1.114] | 0.0002 | 1.055 [1.014–1.098] | 0.0083 |
| NYHA IV (+) | 1.990 [1.354–2.924] | 0.0005 | ||
| Creatinine (+) | 1.012 [1.006–1.018] | 0.0001 | ||
| Bilirubin (+) | 1.016 [1.005–1.027] | 0.0030 | ||
| Uric acid (+) | 1.002 [1.001–1.003] | 0.0029 | ||
| hs-CRP (+) | 1.040 [1.019–1.061] | 0.0001 | ||
| Na (−) | 1.157 [1.107–1.209] | <0.0001 | 1.056 [1.007–1.109] | 0.0244 |
| GGTP (+) | 1.002 [1.000–1.004] | 0.0382 | ||
| Cholesterol (+) | 1.304 [1.087–1.059] | <0.0001 | ||
| PTX-3 (+) | 1.268 [1.212–1.326] | <0.0001 | 1.187 [1.126–1.251] | <0.0001 |
| NT-proBNP (a) | 1.007 [1.004–1.010] | <0.0001 | 1.004 [1.000–1.008] | 0.0259 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczurek-Wasilewicz, W.; Skrzypek, M.; Romuk, E.; Gąsior, M.; Szyguła-Jurkiewicz, B. The Utility of Pentraxin and Modified Prognostic Scales in Predicting Outcomes of Patients with End-Stage Heart Failure. J. Clin. Med. 2022, 11, 2567. https://doi.org/10.3390/jcm11092567
Szczurek-Wasilewicz W, Skrzypek M, Romuk E, Gąsior M, Szyguła-Jurkiewicz B. The Utility of Pentraxin and Modified Prognostic Scales in Predicting Outcomes of Patients with End-Stage Heart Failure. Journal of Clinical Medicine. 2022; 11(9):2567. https://doi.org/10.3390/jcm11092567
Chicago/Turabian StyleSzczurek-Wasilewicz, Wioletta, Michał Skrzypek, Ewa Romuk, Mariusz Gąsior, and Bożena Szyguła-Jurkiewicz. 2022. "The Utility of Pentraxin and Modified Prognostic Scales in Predicting Outcomes of Patients with End-Stage Heart Failure" Journal of Clinical Medicine 11, no. 9: 2567. https://doi.org/10.3390/jcm11092567
APA StyleSzczurek-Wasilewicz, W., Skrzypek, M., Romuk, E., Gąsior, M., & Szyguła-Jurkiewicz, B. (2022). The Utility of Pentraxin and Modified Prognostic Scales in Predicting Outcomes of Patients with End-Stage Heart Failure. Journal of Clinical Medicine, 11(9), 2567. https://doi.org/10.3390/jcm11092567






