Multiple Primary Melanomas: Retrospective Review in a Tertiary Care Hospital
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Singh, D.; Laversanne, M.; Vignat, J.; Vaccarella, S.; Meheus, F.; Cust, A.E.; de Vries, E.; Whiteman, D.C.; Bray, F. Global Burden of Cutaneous Melanoma in 2020 and Projections to 2040. JAMA Dermatol. 2022, e220160. [Google Scholar] [CrossRef] [PubMed]
- Vogt, A.; Schmid, S.; Heinimann, K.; Frick, H.; Herrmann, C.; Cerny, T.; Omlin, A. Multiple primary tumours: Challenges and approaches, a review. ESMO Open 2017, 2, e000172. [Google Scholar] [CrossRef] [Green Version]
- Ferrone, C.R.; Ben Porat, L.; Panageas, K.S.; Berwick, M.; Halpern, A.C.; Patel, A.; Coit, D.G. Clinicopathological features of and risk factors for multiple primary melanomas. JAMA 2005, 294, 1647–1654. [Google Scholar] [CrossRef] [Green Version]
- Moore, M.M.; Geller, A.C.; Warton, E.M.; Schwalbe, J.; Asgari, M.M. Multiple primary melanomas among 16,570 patients with melanoma diagnosed at Kaiser Permanente Northern California, 1996 to 2011. J. Am. Acad Dermatol. 2015, 73, 630–636. [Google Scholar] [CrossRef]
- Helgadottir, H.; Isaksson, K.; Fritz, I.; Ingvar, C.; Lapins, J.; Höiom, V.; Newton-Bishop, J.; Olsson, H. Multiple Primary Melanoma Incidence Trends Over Five Decades: A Nationwide Population-Based Study. J. Natl. Cancer Inst. 2021, 113, 318–328. [Google Scholar] [CrossRef]
- Hwa, C.; Price, L.; Belitskaya-Levy, I.; Ma, M.W.; Shapiro, R.L.; Berman, R.; Kamino, H.; Darvishian, F.; Osman, I.; Stein, J.A. Single versus multiple primary melanomas: Old questions and new answers. Cancer 2012, 118, 4184–4192. [Google Scholar] [CrossRef]
- Pastor-Tomás, N.; Martínez-Franco, A.; Bañuls, J.; Peñalver, J.; Traves, V.; García-Casado, Z.; Requena, C.; Kumar, R.; Nagore, E. Risk factors for the development of a second melanoma in patients with cutaneous melanoma. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2295–2302. [Google Scholar] [CrossRef]
- Ungureanu, L.; Zboraș, I.; Vasilovici, A.; Vesa, Ș.; Cosgarea, I.; Cosgarea, R.; Șenilă, S. Multiple primary melanomas: Our experience. Exp. Ther. Med. 2021, 21, 88. [Google Scholar] [CrossRef]
- Salgüero Fernández, I.; Palma Marti, L.; Nájera Botello, L.; Roustan Gullón, G. Clinical and Histologic Features of Multiple Primary Melanoma in a Series of 31 Patients. Actas Dermosifiliogr. 2021, 112, 52–58. [Google Scholar] [CrossRef]
- Adler, N.R.; Kelly, J.W.; Haydon, A.; McLean, C.A.; Mar, V.J. Clinicopathological characteristics and prognosis of patients with multiple primary melanomas. Br. J. Dermatol. 2018, 178, e44–e45. [Google Scholar] [CrossRef]
- Balamurugan, A.; Rees, J.R.; Kosary, C.; Rim, S.H.; Li, J.; Stewart, S.L. Subsequent primary cancers among men and women with in situ and invasive melanoma of the skin. J. Am. Acad. Dermatol. 2011, 65, S69–S77. [Google Scholar] [CrossRef]
- Youlden, D.R.; Youl, P.H.; Soyer, H.P.; Aitken, J.F.; Baade, P.D. Distribution of subsequent primary invasive melanomas following a first primary invasive or in situ melanoma Queensland, Australia, 1982–2010. JAMA Dermatol. 2014, 150, 526–534. [Google Scholar] [CrossRef] [Green Version]
- Tryggvadóttir, L.; Gislum, M.; Hakulinen, T.; Klint, Å.; Engholm, G.; Storm, H.H.; Bray, F. Trends in the survival of patients diagnosed with malignant melanoma of the skin in the Nordic countries 1964-2003 followed up to the end of 2006. Acta Oncol. 2010, 49, 665–672. [Google Scholar] [CrossRef]
- Kurtansky, N.R.; Dusza, S.W.; Halpern, A.C.; Hartman, R.I.; Geller, A.C.; Marghoob, A.A.; Rotemberg, V.M.; Marchetti, M.A. An Epidemiologic Analysis of Melanoma Overdiagnosis in the United States, 1975–2017. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef]
- Olsen, C.M.; Whiteman, D.C. Cutaneous Melanoma in White Americans: A Tale of Two Epidemics. J. Investig. Dermatol. 2022. [Google Scholar] [CrossRef]
- Welch, H.G.; Mazer, B.L.; Adamson, A.S. The Rapid Rise in Cutaneous Melanoma Diagnoses. N. Engl. J. Med. 2021, 384, 72–79. [Google Scholar] [CrossRef]
- Semsarian, C.R.; Ma, T.; Nickel, B.; Scolyer, R.A.; Ferguson, P.M.; Soyer, H.P.; Parker, L.; Barratt, A.; Thompson, J.F.; Bell, K.J. Do we need to rethink the diagnoses melanoma in situ and severely dysplastic naevus? Br. J. Dermatol. 2022. [Google Scholar] [CrossRef]
- McCaul, K.A.; Fritschi, L.; Baade, P.; Coory, M. The incidence of second primary invasive melanoma in Queensland, 1982–2003. Cancer Causes Control 2008, 19, 451–458. [Google Scholar] [CrossRef]
- Avilés-Izquierdo, J.A.; Molina-López, I.; Rodríguez-Lomba, E.; Marquez-Rodas, I.; Suarez-Fernandez, R.; Lazaro-Ochaita, P. Who detects melanoma? Impact of detection patterns on characteristics and prognosis of patients with melanoma. J. Am. Acad. Dermatol. 2016, 75, 967–974. [Google Scholar] [CrossRef]
- Brobeil, A.; Rapaport, D.; Wells, K.; Cruse, C.W.; Glass, F.; Fenske, N.; Albertini, J.; Miliotis, G.; Messina, J.; DeConti, R.; et al. Multiple primary melanomas: Implications for screening and follow-up programs for melanoma. Ann. Surg. Oncol. 1997, 4, 19–23. [Google Scholar] [CrossRef]
- Müller, C.; Wendt, J.; Rauscher, S.; Sunder-Plassmann, R.; Richtig, E.; Fae, I.; Fischer, G.; Okamoto, I. Risk Factors of Subsequent Primary Melanomas in Austria. JAMA Dermatol. 2019, 155, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Menzies, S.; Barry, R.; Ormond, P. Multiple primary melanoma: A single centre retrospective review. Melanoma Res. 2017, 27, 638–640. [Google Scholar] [CrossRef]
- Betti, R.; Gualandri, L.; Vergani, R.; Menni, S.; Crosti, C. Really synchronous cutaneous melanomas: Serendipity or need for prevention? Eur. J. Dermatol. 2009, 19, 258–259. [Google Scholar] [CrossRef]
- Jones, M.S.; Torisu-Itakura, H.; Flaherty, D.C.; Schoellhammer, H.F.; Lee, J.; Sim, M.-S.; Faries, M.B. Second Primary Melanoma: Risk Factors, Histopathologic Features, Survival, and Implications for Follow-Up. Am. Surg. 2016, 82, 1009–1013. [Google Scholar] [CrossRef]
- Shain, A.H.; Bastian, B.C. From melanocytes to melanomas. Nat. Rev. Cancer. 2016, 16, 345–358. [Google Scholar] [CrossRef]
- De Giorgi, V.; Rossari, S.; Papi, F.; Gori, A.; Alfaioli, B.; Grazzini, M.; Crocetti, E.; Verdelli, A.; Foo, C.W.; Lotti, T. Multiple primary melanoma: The impact of atypical naevi and follow up. Br. J. Dermatol. 2010, 163, 1319–1322. [Google Scholar] [CrossRef]
- DiFronzo, L.A.; Wanek, L.A.; Morton, D.L. Earlier diagnosis of second primary melanoma confirms the benefits of patient education and routine postoperative follow-up. Cancer 2001, 91, 1520–1524. [Google Scholar] [CrossRef]
- De Summa, S.; Lasorella, A.; Strippoli, S.; Giudice, G.; Guida, G.; Elia, R.; Nacchiero, E.; Azzariti, A.; Silvestris, N.; Guida, M.; et al. The Genetic Germline Background of Single and Multiple Primary Melanomas. Front. Mol. Biosci. 2021, 7, 555630. [Google Scholar] [CrossRef]
- Ferguson, R.; Archambault, A.; Simpson, D.; Morales, L.; Chat, V.; Kazlow, E.; Lax, R.; Yoon, G.; Moran, U.; Shapiro, R.; et al. Immunomodulatory germline variation associated with the development of multiple primary melanoma (MPM). Sci. Rep. 2019, 9, 10173. [Google Scholar] [CrossRef] [Green Version]
- Requena, C.; Botella-Estrada, R.; Traves, V.; Nagore, E.; Almenar, S.; Guillén, C. Regresión en el melanoma: Problemas en su definición e implicación pronóstica. Actas Dermosifiliogr. 2009, 100, 759–766. [Google Scholar] [CrossRef]
- Gualano, M.R.; Osella-Abate, S.; Scaioli, G.; Marra, E.; Bert, F.; Faure, E.; Baduel, E.; Balagna, E.; Quaglino, P.; Fierro, M.; et al. Prognostic role of histological regression in primary cutaneous melanoma: A systematic review and meta-analysis. Br. J. Dermatol. 2018, 178, 357–362. [Google Scholar] [CrossRef]
- Ribero, S.; Torres-Navarro, I.; Botella-Estrada, R. Tumour-infiltrating lymphocyte and histological regression in primary melanoma. Arch. Dermatol. Res. 2021, 313, 63–64. [Google Scholar] [CrossRef]
- Botella-Estrada, R.; Traves, V.; Requena, C.; Guillen-Barona, C.; Nagore, E. Correlation of histologic regression in primary melanoma with sentinel node status. JAMA Dermatol. 2014, 150, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Saleh, F.H.; Crotty, K.A.; Hersey, P.; Menzies, S.W. Primary melanoma tumour regression associated with an immune response to the tumour-associated antigen melan-A/MART-1. Int J. Cancer 2001, 94, 551–557. [Google Scholar] [CrossRef]
- Martín, J.M.; Pinazo, I.; Mateo, J.F.; Escandell, I.; Jordá, E.; Monteagudo, C. Assessment of regression in successive primary melanomas. Actas Dermosifiliogr. 2014, 105, 768–773. [Google Scholar] [CrossRef]
- Zoller, L.; Mayer, E.; Itzhak, O.B.; Bergman, R. A lack of significantly increased incidence of regression in second primary melanomas does not support an ‘immunization effect’. J. Cutan. Pathol. 2010, 37, 1140–1144. [Google Scholar] [CrossRef]



| MPM (n = 58) n (%) | |
|---|---|
| Women | 20 (34.48%) |
| Age | |
| Mean (SD) | 69.07 (14.48) |
| Median (25–75th percentile) | 71 (63–80) |
| Fitzpatrick Skin phototype * | |
| I | 3 (5.26%) |
| II | 22 (38.60%) |
| III | 28 (49.12%) |
| IV | 4 (7.02%) |
| Severe sunburns * | 34 (59.65%) |
| Chronic sun exposure * | 8 (14.04%) |
| UVA rays exposure | 4 (7.02%) |
| Freckling * | 3 (5.26%) |
| Lentigines * | 46 (80.70%) |
| Actinic keratosis * | 21 (36.84%) |
| Non-skin cancer * | 5 (8.77%) |
| Non-melanoma skin cancer * | 17 (29.82%) |
| Congenital nevi * | 4 (7.02%) |
| Common nevi * | |
| <50 | 48 (84.21%) |
| >50 | 9 (15.79%) |
| History of histologically confirmed dysplastic nevi * | 4 (7.02%) |
| Family history of melanoma * | 4 (7.02%) |
| Family history of non-melanoma cancer * | 30 (53.57%) |
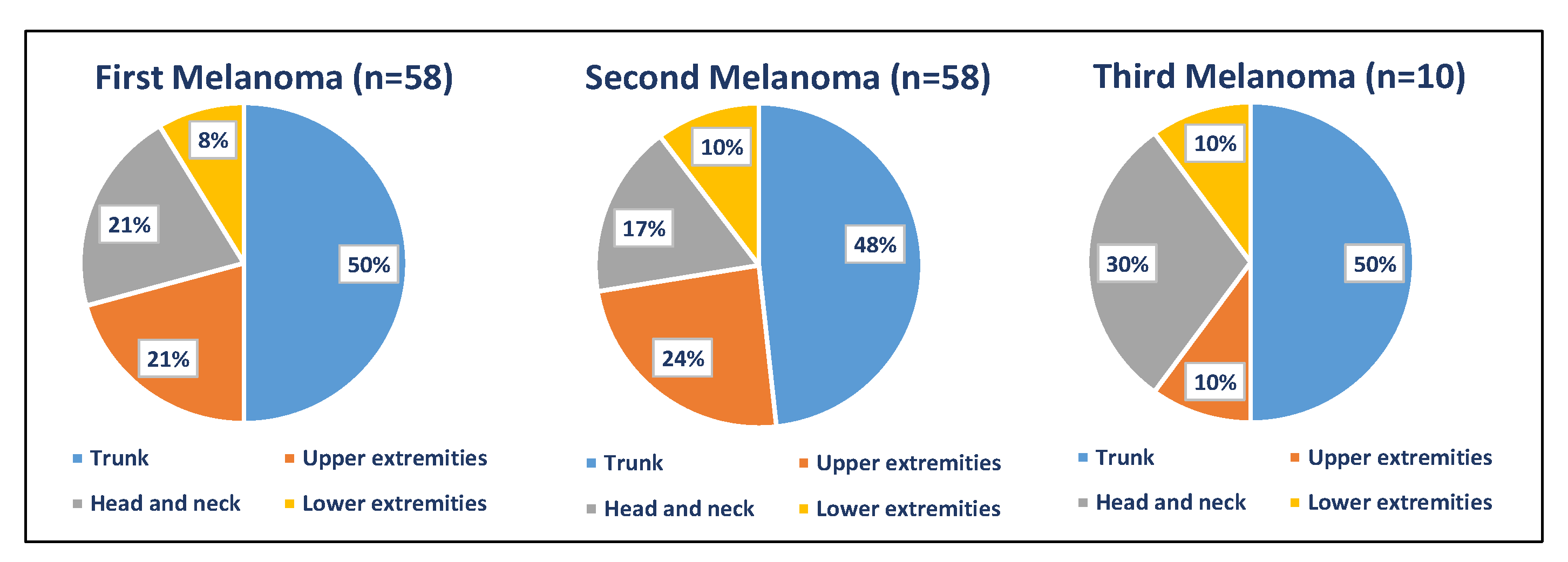
| First Melanoma n = 58, n (%) | Second Melanoma n = 58, n (%) | Third Melanoma n = 10, n (%) | Fourth Melanoma n = 2, n (%) | Fifth Melanoma n = 1, n (%) | |
|---|---|---|---|---|---|
| Location | |||||
| Trunk | 29 (50%) | 28 (48.28%) | 5 (50%) | 1 (100%) | |
| UE | 12 (20.69%) | 14 (24.14%) | 1 (10%) | 1 (50%) | |
| H&N | 12 (20.69%) | 10 (17.24%) | 3 (30%) | 1 (50%) | |
| LE | 5 (8.62%) | 6 (10.34%) | 1 (10%) | ||
| Histologic subtype | |||||
| SSM | 35 (60.34%) | 33 (56.90%) | 1 (10%) | 2 (100%) | |
| LMM | 14 (24.14%) | 23 (39.66%) | 9 (90%) | 1 (100%) | |
| NM | 8 (13.79%) | 1 (1.72%) | |||
| Other | 1 (1.72%) | 1 (1.72%) | |||
| In Situ Melanoma | 21/58 (36.21%) | 46/57 (80.70%) * | 9/10 (90%) | 2 (100%) | 1 (100%) |
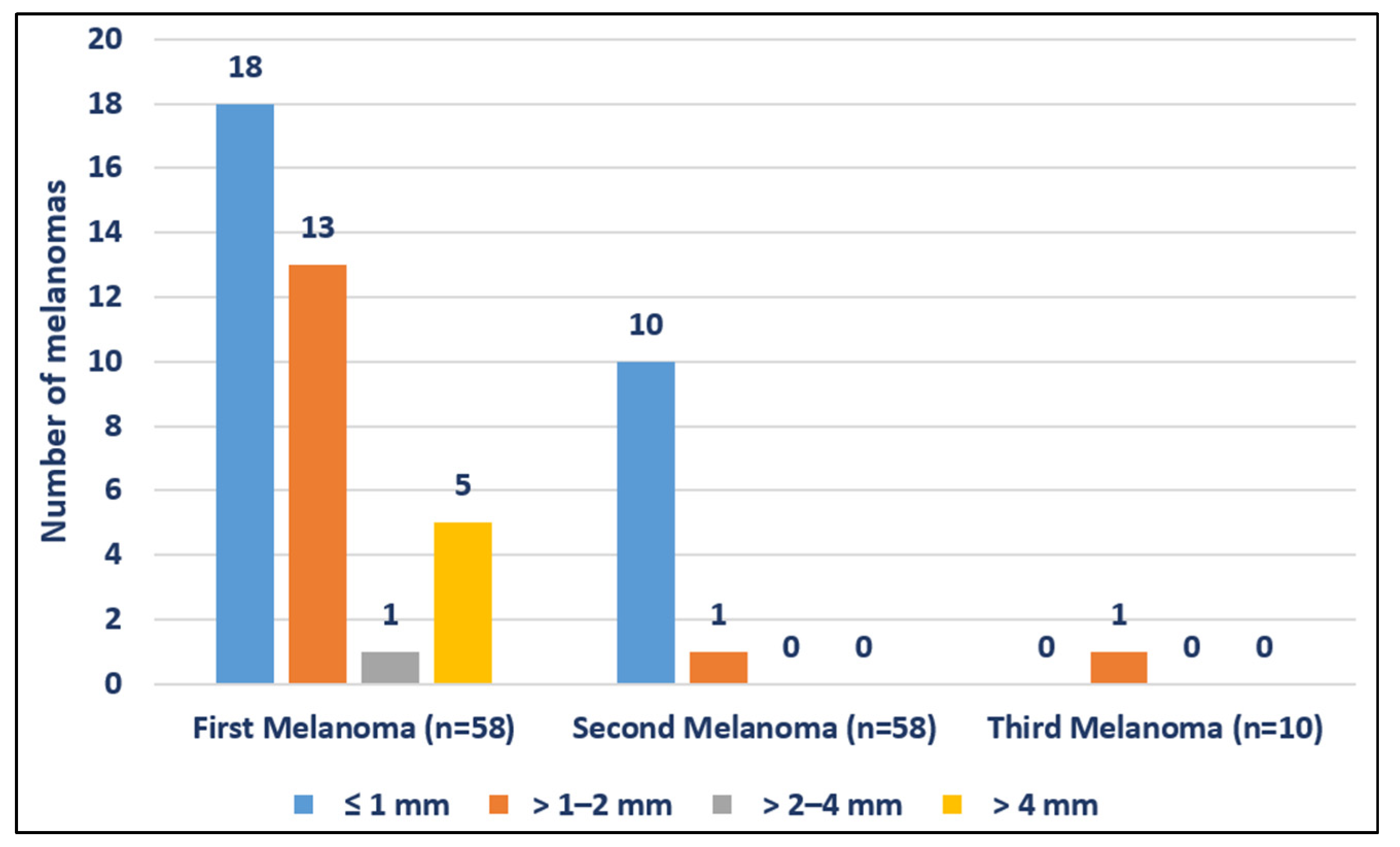
| Breslow (mm) | (n = 37) | (n = 11) | (n = 1) | ||
| Mean (SD) | 1.54 (1.67) | 0.65 (0.49) | 1.4 | ||
| ≤1 mm | 18 (48.65%) | 10 (90.91%) | 0 (0%) | ||
| >1–2 mm | 13 (35.14%) | 1 (9.09%) | 1 (100%) | ||
| >2–4 mm | 1 (2.70%) | 0 (0%) | 0 (0%) | ||
| >4 mm | 5 (13.51%) | 0 (0%) | 0 (0%) | ||
| Clark level | (n = 56) | (n = 56) | |||
| I | 21 (37.50%) | 46 (82.14%) | 9 (90%) | 2 (100%) | 1 (100%) |
| II | 14 (25%) | 6 (10.71%) | 0 (0%) | ||
| III | 14 (25%) | 2 (3.57%) | 0 (0%) | ||
| IV | 7 (12.50%) | 2 (3.57%) | 1 (10%) | ||
| Ulceration | 8 (13.79%) | 1 (1.72%) | 1 (10%) | 0 (0%) | 0 (0%) |
| Lymphocyte infiltration | |||||
| Peritumoral | 24 (41.38%) | 18 (31.03%) | 1 (10%) | 1 (50%) | 0 (0%) |
| Intratumoral | 12 (20.69%) | 12 (20.69%) | 1 (10%) | 1 (50%) | 0 (0%) |
| Tumor mitotic rate (mitosis/mm2) | |||||
| Mean | 1.32 | 0.81 | 1 | ||
| <1 | 22 (59.46%) | 7 (63.64%) | 0 (0%) | ||
| ≥1 | 15 (40.54%) | 4 (36.36%) | 1 (100%) | ||
| Regression | |||||
| None | 23 (39.65%) | 17 (29.31%) | 2 (20%) | 0 (0%) | 100 (100%) |
| <50% | 28 (48.28%) | 30 (51.72%) | 4 (40%) | 2 (100%) | 0 (0%) |
| >50% | 7 (12.07%) | 11 (18.97%) | 4 (40%) | 0 (0%) | 0 (0%) |
| Vascular invasion | 1 (1.72%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Underlying histologic lesion | 11 (18.97%) | 18 (31.03%) | 3 (30%) | 1 (50%) | 0 (0%) |
| Common nevus | 9 (15.52%) | 17 (29.31%) | 3 (30%) | 1 (100%) | |
| Dysplastic nevus | 2 (3.45%) | 1 (1.72%) | |||
| Sentinel lymph node biopsy | |||||
| Done | 18 (31.03%) | 1 (1.72%) | 1 (10%) | 0 (0%) | 0 (0%) |
| Positive | 1 (5.56%) | 0 (0%) | 0 (0%) |
| Authors | Year | MPM/Total n (%) | Age (Mean) | N° of Primary Tumors (n/MPM) | History of Dysplastic Nevi n (%) | Family History of Melanoma n (%) | Synchronous n (%) | Most Frequent Location | |
| 1° Melanoma | 2° Melanoma | ||||||||
| Ferrone C et al. [3] | 2005 | 385/4484 (8.6) | 55 | 866 (2.3) | 101 (41) c | 53 (20) | 139 (36) f | Trunk | Extremities g |
| Moore M et al. [4] | 2015 | 1122/16,570 (6.8) | 64.4 | NA | NA | NA | NA | H&N | H&N |
| Hwa C et al. [6] | 2012 | 61/788 (7.7) | 63.7 | 155 (2.5) | NA | 13 (21) | NA | Trunk | Trunk |
| Ungureanu L et al. [8] | 2021 | 26/699 (3.7) | 55.3 | 59 (2.3) | NA | NA | 13 (45.5) | Trunk | Trunk |
| Salgüero-Fernandez I et al. [9] | 2021 | 31 | 67 b | 84 (2.7) | 10 (31) d | 6 (19) | 39% | Trunk | Trunk |
| Müller C et al. [21] | 2019 | 299/1648 (18.1) | 62 | NA | NA | 16 (15.4) | NA | NA | NA |
| Menzies S et al. [22] | 2017 | 99/2057 (4.8) a | 66 | 114 (2.5) | NA | NA | NA | NA | NA |
| Palacios-Diaz R.D. et al. | 2022 | 58/646 (8.9) | 69.1 | 129 (2.2) | 4 (7) e | 4 (7) | 20 (34.5) | Trunk | Trunk |
| Authors | In-Situ Melanoma; n (%) | Breslow Mean | Histological Subtype | Histological Regression Rate | |||||
| 1° Melanoma | 2° Melanoma | 1° Melanoma | 2° Melanoma | 1° Melanoma | 2° Melanoma | 1° Melanoma | 2° Melanoma | ||
| Ferrone C et al. [3] | 76 (21) | 186 (50) | 1.2 | 0.4 | NA | NA | NA | NA | |
| Moore M et al. [4] | 476 (42.4) | 599 (53.4) | 1.05 | 0.83 | NA | NA | NA | NA | |
| Hwa C et al. [6] | NA | NA | 0.96 | NA | SSM | NA | NA | NA | |
| Ungureanu L et al. [8] | 2 (7.7) | 17 (51.5) | NA | NA | SSM | SSM | NA | NA | |
| Salgüero-Fernandez I et al. [9] | 39% | 58% | 0.8 | 0.47 | SSM | SSM | 32 | 32 | |
| Müller C et al. [21] | NA | NA | NA | NA | NA | NA | NA | NA | |
| Menzies S et al. [22] | 24% | 52% | 1.21 | 0.36 | SSM | LM/LMM | 26 | 17 | |
| Palacios-Diaz R.D. et al. | 21 (36.2) | 46 (80.7) | 1.5 | 0.7 | SSM | SSM | 60.4 | 70.7 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palacios-Diaz, R.D.; de Unamuno-Bustos, B.; Abril-Pérez, C.; Pozuelo-Ruiz, M.; Sánchez-Arraez, J.; Torres-Navarro, I.; Botella-Estrada, R. Multiple Primary Melanomas: Retrospective Review in a Tertiary Care Hospital. J. Clin. Med. 2022, 11, 2355. https://doi.org/10.3390/jcm11092355
Palacios-Diaz RD, de Unamuno-Bustos B, Abril-Pérez C, Pozuelo-Ruiz M, Sánchez-Arraez J, Torres-Navarro I, Botella-Estrada R. Multiple Primary Melanomas: Retrospective Review in a Tertiary Care Hospital. Journal of Clinical Medicine. 2022; 11(9):2355. https://doi.org/10.3390/jcm11092355
Chicago/Turabian StylePalacios-Diaz, Rodolfo David, Blanca de Unamuno-Bustos, Carlos Abril-Pérez, Mónica Pozuelo-Ruiz, Javier Sánchez-Arraez, Ignacio Torres-Navarro, and Rafael Botella-Estrada. 2022. "Multiple Primary Melanomas: Retrospective Review in a Tertiary Care Hospital" Journal of Clinical Medicine 11, no. 9: 2355. https://doi.org/10.3390/jcm11092355
APA StylePalacios-Diaz, R. D., de Unamuno-Bustos, B., Abril-Pérez, C., Pozuelo-Ruiz, M., Sánchez-Arraez, J., Torres-Navarro, I., & Botella-Estrada, R. (2022). Multiple Primary Melanomas: Retrospective Review in a Tertiary Care Hospital. Journal of Clinical Medicine, 11(9), 2355. https://doi.org/10.3390/jcm11092355






