Abstract
Patients with a chronic hepatitis B virus (HBV) infection who are treated with nucleos(t)ide analogues (NAs) are still at risk for hepatocellular carcinoma (HCC), and it has been clinically questioned whether patients with a high risk of HCC can be identified efficiently. We aimed to clarify the risk factors associated with the development of HCC during NA therapies. A total of 611 chronically HBV-infected patients without a history of HCC, who were treated with NAs for more than 6 months (median 72 months), from 2000 to 2021, were included from 16 hospitals in the Tohoku district in Japan. Incidences of HCC occurrence were analyzed with clinical factors, including on-treatment responses. Alanine aminotransferase (ALT) normalization, based on the criteria of three guidelines, was analyzed with other parameters, including the age–male–ALBI–platelets (aMAP) risk score. During the observation period, 48 patients developed HCC, and the cumulative HCC incidence was 10.6% at 10 years. Non-achievement of ALT normalization at 1 year of therapy was mostly associated with HCC development when ALT ≤ 30 U/L was used as the cut-off (cumulative incidence, 19.9% vs. 5.3% at 10 years, p < 0.001). The effectiveness of the aMAP risk score at the start of treatment was validated in this cohort. A combination of an aMAP risk score ≥ 50 and non-achievement of ALT normalization could stratify the risk of HCC significantly, and notably, there was no HCC development in 103 patients without these 2 factors. In conclusion, non-achievement of ALT normalization (≤30 U/L) at 1 year might be useful in predicting HCC during NA therapies and, in combination with the aMAP risk score, could stratify the risk more precisely.
1. Introduction
Hepatitis B virus (HBV) infection is a major health problem worldwide, and the World Health Organization (WHO) estimates that 296 million people have a chronic HBV infection [1]. They are at risk of liver cirrhosis and hepatocellular carcinoma (HCC), but antiviral therapies can reduce such life-threatening diseases [2,3,4]. Nucleos(t)ide analogues (NAs) effectively suppress HBV replication and are widely used because they can be administered orally, and usually safely, for a long time [5]. Although NAs inhibit the reverse transcription of the HBV genome and rapidly suppress HBV DNA in the serum during therapy, the effect on the reduction in hepatitis B surface antigens (HBsAg) is limited [6]. When viral suppression is not optimal, hepatitis relapses frequently after the cessation of NAs [7]. The reason for the difficulty of HBV eradication is that HBV forms covalently closed circular DNA (cccDNA) in the nucleus after viral entry into hepatocytes [8,9]. HCC rarely occurs when HBsAg is cleared [10], but the rate of HBsAg loss by NAs is low and on-treatment patients still have a risk for HCC [11,12]. Therefore, it has been clinically questioned whether patients with a high HCC risk can be efficiently identified.
Thus far, several risk factors associated with HCC development during NA treatments have been reported [13], and risk scores have been developed [14,15]. Recently, an age–male–ALBI–platelets (aMAP) risk score that estimates HCC risk universally, including patients with HBV, hepatitis C virus (HCV) and non-viral hepatitis, was developed [16]. This score involves only age, sex, albumin (Alb), total bilirubin (T-Bil) and platelet counts (PLT). Basically, old age, male gender and advanced fibrosis of the liver are associated with a high risk of HCC [13]; however, as a feature of HBV-associated HCC, it can sometimes be found in the liver without advanced fibrosis [17]. Pathogeneses such as an integration of the HBV genome into hepatocytes [18] and the accumulation of mutant HBV proteins [19] have been proposed, but they are hard to evaluate in clinical settings. In some recent studies, normalization of alanine aminotransferase (ALT) during NA therapy was reported as being associated with a low risk of HCC [20,21,22], but the cut-off of ALT normalization and the timing of judgment has not been sufficiently evaluated. In this study, we aimed to find clinically available risk factors, including responses to therapies that can be widely obtained by most physicians in a multicenter retrospective cohort.
2. Materials and Methods
2.1. Study Design
This is a retrospective, multicentric, observational study. We followed up with patients from the start of NAs (lamivudine (LAM), entecavir (ETV), tenofovir disoproxil fumarate (TDF) or tenofovir alafenamide fumarate (TAF)) until the cessation of follow-ups. The primary endpoint was the incidence of HCC.
2.2. Patients
A total of 856 patients with HBV chronic infection who were treated with NAs for more than 6 months from 2000 to 2021 were registered from 16 hospitals in Miyagi, Iwate, Akita, Yamagata and Fukushima prefectures in the Tohoku Hepatology Research Meeting (THERME) Study Group [11]. They were consecutive in each hospital, and after the exclusion of patients with hepatitis C virus (HCV) infection or a history of HCC development before the start of NAs and those without sufficient clinical data, a total of 611 patients were analyzed.
2.3. Assays of Serological Tests and HBV Genotypes
HBsAg was tested by a chemiluminescent immunoassay (CLIA) with ARCHITECT (Abbott Japan, Tokyo, Japan) or a chemiluminescent enzyme immunoassay (CLEIA) with LUMIPULSE HBsAg-HQ (Fujirebio, Tokyo, Japan). Hepatitis B e antigen (HBeAg) was tested by CLIA with ARCHITECT. HBV DNA levels were quantified by a real-time PCR assay using Cobas TaqMan HBV Auto (Roche Diagnostics, Tokyo, Japan). HBcrAg was tested by CLEIA with LUMIPULSE (Fujirebio). HBV genotypes were determined with an IMMUNIS HBV genotype EIA kit (Institute of Immunology, Tokyo, Japan). As a score for evaluating liver fibrosis, the FIB-4 index was calculated as follows: FIB-4 = age (years) × aspartate aminotransferase (AST, U/L)/[PLT (103/μL) × √alanine aminotransferase (ALT, U/L)] [23]. As a risk score for HCC, the aMAP risk score was calculated as follows: aMAP risk score = [(0.06 × age + 0.89 × sex (Male: 1, Female: 0) + 0.48 × {log10 [T-bil (mg/dL) × 17.1] × 0.66 + Alb (g/dL) × −0.85} − 0.01 × PLT) + 7.4]/14.77 × 100 [16].
2.4. HCC Surveillance
For HCC surveillance, ultrasonography was basically performed at least twice a year. Additionally, computed tomography or magnetic resonance imaging was performed at least once every year if the patient was considered to have liver cirrhosis. Serum alpha-fetoprotein (AFP) was assayed at every visit at least once every 3 months, and if the AFP level was increased, imaging tests were performed more intensively [11].
2.5. Statistical Analysis
Statistical analyses were performed using the chi-squared test for a comparison of the proportions between the two groups and a Wilcoxon rank sum test for a comparison of continuous variables between the two groups. Cumulative incidences were estimated using the Kaplan–Meier method and were compared using a log-rank test. A Cox proportional hazards model was used to identify risk factors, and variables with p values less than 0.05 in the univariate analysis were input into the multivariate analysis. Propensity scores were calculated using logistic regression with 5 variables (age, sex, ALT, PLT and HBV DNA), and 1:1 propensity score matching was performed [24]. Differences with p values less than 0.05 were considered statistically significant. All statistical analyses were performed using JMP version 16.0 (SAS Institute Inc., Cary, NC, USA). Data were plotted and graphed using GraphPad Prism version 9.3.0 (GraphPad Software Inc., La Jolla, CA, USA).
3. Results
3.1. Patient Characteristics and Validation of the aMAP Risk Score
Of 611 patients, 390 (63.8%) were male and the median age was 53 (interquartile range, 42–62). Clinical characteristics are shown in Table 1. Among 528 patients whose HBV genotypes were determined, 35.4% and 63.3% were genotypes B and C, respectively. ETV was used as the initial NA in 71.0%. Some of the patients were switched to or supplemented with other NAs (data not shown). Additionally, we evaluated the aMAP risk score (low risk: <50, medium risk: 50–60, high risk: >60) [16]. A total of 48 patients developed HCC during the observation period (median 72 months). When characteristics were compared of patients with HCC and those without, age, sex, the presence of diabetes mellitus (DM), T-Bil, Alb, PLT, AFP, FIB-4 index, aMAP risk and initial NA were significantly different (Table 1). The proportion of patients who were treated with LAM as the initial NA was higher in those with HCC than in those without (31.3% vs. 14.2%). The cumulative incidences of HCC occurrence were 7.4%, 10.6% and 15.0% at 5, 10 and 15 years of NA therapies, respectively (Figure 1a). When the FIB-4 index was analyzed with a cut-off value of 2.5 [11], patients with a higher FIB-4 index showed a significantly higher incidence of HCC (Figure 1b). The aMAP risk score was significantly higher in patients who developed HCC than in those who did not (median 64.2 vs. 54.5, p < 0.001) (Figure 1c). The cumulative incidences of HCC at 10 years were 21.4%, 7.6% and 3.5% in the high-risk group, the medium-risk group and the low-risk group, respectively (Figure 1d).

Table 1.
Baseline clinical characteristics of patients with chronic HBV infection at the start of NA therapies.
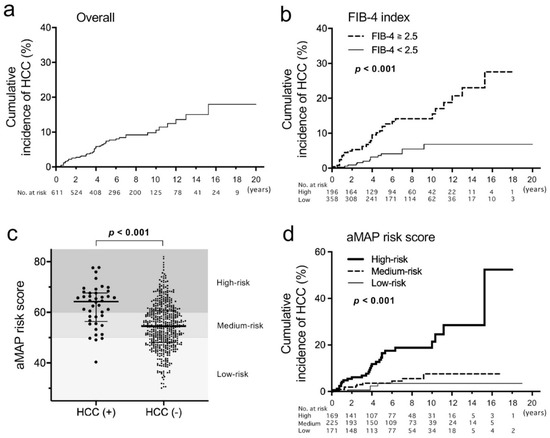
Figure 1.
Cumulative incidences of hepatocellular carcinoma (HCC) and age–male–ALBI–platelets (aMAP) risk score in this study. (a,b) The incidences were estimated using the Kaplan–Meier method in an overall analysis (a) and patients were compared with a FIB-4 index ≥2.5 vs. <2.5. (b,c) Comparison of the aMAP risk score distribution between patients who developed HCC during the observation period and those who did not. Thick lines indicate medians and thin lines indicate interquartile ranges. (d) Cumulative incidences of HCC compared among the high-risk group of the aMAP risk score (>60), the medium-risk group (50–60) and the low-risk group (<50). A log-rank test was used to compare cumulative incidences.
3.2. Factors Associated with HCC Incidence
We performed univariate and multivariate analyses of risk factors for the occurrence of HCC. The cut-off values of age, Alb, PLT, AFP and FIB-4 index were determined from the receiver operating characteristic curves for HCC occurrence at 5 years after NAs in our previous study [11]. In the univariate analysis, male gender, age (≥48 years), Alb (<4.0 g/dL), PLT (<15 × 104/μL), AFP (≥5.5 ng/mL) and FIB-4 index (≥2.5) were significantly associated with HCC occurrence (Table 2). Additionally, we evaluated responses to NA therapies using HBV DNA undetectability (including positive but under the detection limit) and ALT normalization 1 year after the start of NAs. For the evaluation, three types of criteria for ALT normalization were used: the WHO criteria (male, ≤30; female, ≤19), the American Association for the Study of Liver Diseases (AASLD) criteria (male, ≤35; female, ≤25) and the Japan Society of Hepatology (JSH) criteria (male and female, ≤30). The response rates for NA therapy based on these criteria and undetectable HBV DNA after 1 to 5 years are shown in Figure S1. Whereas HBV DNA positivity at 1 year was not associated with the occurrence of HCC, abnormal ALT of the WHO criteria and JSH criteria at 1 year was significantly associated with HCC occurrence, and the JSH criteria showed the highest hazard ratio among the three criteria (Table 2). In a multivariate analysis, significant factors in the univariate analysis were included, but the FIB-4 index and aMAP risk score were excluded because these parameters are calculated using other significant factors. As for abnormal ALT at 1 year, only the JSH criteria were included. After the multivariate analysis, male gender, age, PLT, AFP and abnormal ALT in the JSH criteria at 1 year were extracted as significant factors for the occurrence of HCC.

Table 2.
Univariate and multivariate analysis of risk factors for the occurrence of HCC during NA therapies.
3.3. Evaluation of ALT Normalization after NAs
Cumulative incidences of HCC were compared between patients with and without ALT normalization of the three types of criteria at 1 year of treatment (Figure 2a–c). The differences were significant when the WHO criteria and JSH criteria were used, and the p-value was lower when the JSH criteria were applied. Then we compared the baseline clinical characteristics between patients with and without ALT normalization in the JSH criteria (Table 3). In patients without ALT normalization at 1 year of treatment, the percentage of males was higher (77.6% vs. 58.5%), PLT was lower (16.2 vs. 18.4 ×104/μL) and HBV DNA was significantly lower (5.4 vs. 5.8 log IU/mL). Creatinine (Cr) was higher in patients without ALT normalization, which is probably associated with the higher frequency of males. Of 156 patients with abnormal ALT at 1 year of therapy, a virological breakthrough at that time was found in six patients (LAM, 5 patients; ETV 1 patient).
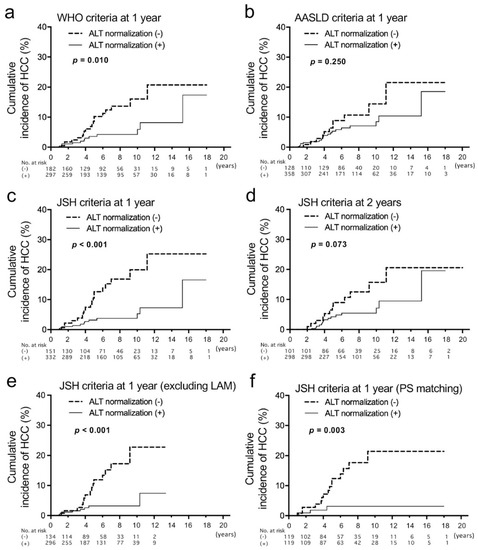
Figure 2.
Analyses of cumulative incidences of HCC according to the achievement of alanine aminotransferase (ALT) normalization 1 year after the start of nucleos(t)ide analog therapies that were evaluated with three types of criteria. (a–c) Comparison of cumulative incidences of HCC between patients who achieved ALT normalization based on the criteria of the World Health Organization (WHO: male, ≤30; female, ≤19) (a), American Association for the Study of Liver Diseases (AASLD: male, ≤35; female, ≤25) (b) and the Japan Society of Hepatology (JSH: ≤30) (c). In these analyses, patients who developed HCC before 12 months and those whose observation periods were less than 12 months were excluded. (d) Comparison of cumulative incidences of HCC based on the ALT normalization criteria of JSH at 2 years of therapy. (e) Comparison of cumulative incidences of HCC based on the ALT normalization criteria of JSH at 1 year, excluding patients who were treated with lamivudine (LAM). (f) Comparison of cumulative incidences of HCC between patients with and without ALT normalization at 1 year based on JSH criteria after propensity score (PS) matching.

Table 3.
Comparison of baseline characteristics between patients who achieved ALT normalization (JSH criteria, ≤30) and those who did not at 1 year of NA therapies.
We also analyzed ALT normalization at 2 years using the three types of criteria. The tendency of HCC incidence with and without ALT normalization was similar with no significant difference (Figure 2d and Figure S2a,b). Therefore, we considered that the evaluation of ALT normalization at 1 year is more useful than that at 2 years for the prediction of HCC occurrence. There was no difference in HCC incidence between patients with positive HBV DNA and those without at 1 year and 2 years (Figure S2c,d). Additionally, we analyzed the HCC incidence of patients whose ALT was abnormal at 1 year but normalized at 2 years. Of 112 patients whose ALT was abnormal at 1 year, 43 patients achieved ALT normalization at 2 years (Figure S3a). When cumulative HCC incidences were compared between the 43 patients and those whose ALT was persistently abnormal until 2 years, no difference was found (Figure S3b). Therefore, early ALT normalization might be important for the prediction of HCC occurrence. Furthermore, univariate and multivariate logistic regression analyses were performed to find baseline factors associated with the non-achievement of ALT normalization (Table 4). Then, only male gender and a lower HBV DNA level were extracted as independent factors.

Table 4.
Univariate and multivariate logistic regression analysis of baseline factors associated with non-achievement of ALT normalization (JSH criteria) at 1 year.
Because LAM is associated with virological breakthroughs leading to an elevation of ALT, we compared HCC incidences excluding patients who were treated with LAM (Figure 2e). The result was similar to that from patients, including LAM-treated patients. Subsequently, propensity score matching for the group with ALT normalization (JSH criteria) at 1 year and without was carried out. After the matching, no baseline factors were significantly different (Table S1). The cumulative HCC incidence of patients without ALT normalization at 1 year was still higher than those with it (Figure 2f).
3.4. Combination of aMAP Risk Score and ALT Normalization
Finally, we analyzed HCC incidence in combination with the aMAP risk score and the achievement/non-achievement of ALT normalization (≤30) at 1 year. To judge the risk simply, we divided patients into three groups: risk-0 group (n = 103) with aMAP < 50 and ALT normalization; risk-1 group (n = 260) with aMAP ≥ 50 or non-achievement of ALT normalization; risk-2 group (n = 106) with aMAP ≥ 50 and non-achievement of ALT normalization. The cumulative incidence of HCC was significantly different among the three groups (p < 0.001) (Figure 3). The cumulative incidences of HCC at 10 years were 25.6%, 8.0% and 0% in the risk-2 group, the risk-1 group and the risk-0 group, respectively. The distribution of the aMAP risk score in patients with and without HCC development and ALT normalization at 1 year is shown in Figure 3b. Regardless of ALT normalization, the aMAP risk scores were significantly higher in patients with HCC development, and notably, there was no HCC development in patients with both low-risk aMAP scores and ALT normalization.
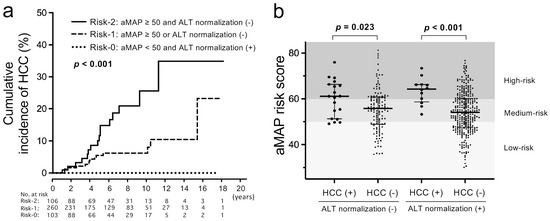
Figure 3.
Analyses of cumulative incidences of HCC according to both baseline aMAP risk scores and 1-year ALT normalization of JSH criteria. (a) Comparison of cumulative incidences of HCC between the risk-0 group (aMAP risk score < 50 and ALT normalization), the risk-1 group (aMAP risk score ≥ 50 or no ALT normalization) and the risk-2 group (aMAP risk score ≥ 50 and no ALT normalization). (b) Distribution of aMAP risk scores in patients with and without ALT normalization at 1 year of NA therapies and with and without HCC development.
4. Discussion
In this study, in addition to the international standards of ALT normalization (the WHO and AASLD criteria), the JSH criteria were also evaluated, and it was demonstrated that ALT normalization in the JSH criteria at 1 year after the start of NA therapies was associated with HCC development. In addition, the combination with the aMAP risk score could precisely stratify HCC risk. The finding that there were no patients with HCC development in the risk-0 group with both aMAP < 50 and ALT normalization suggests that the frequency of HCC surveillance with imaging tests such as ultrasonography may be reduced, but further replication studies will be necessary.
The association between HCC development and ALT normalization during NA treatment was recently reported among some cohorts [20,21,22]. However, there are various criteria for the ALT cut-off, and it is unknown which criteria are the most useful for predicting HCC development during NA treatment. The present study shows that the JSH criteria were better than the WHO and AASLD criteria in the Japanese cohort. The difference between these criteria is that the cut-off of ALT in females was lower than that in males in the WHO and AASLD criteria. The WHO guidelines were based on a study using samples from blood donors in Italy who were at low risk for non-alcoholic liver diseases and without HBV or HCV [25]. The JSH criteria, in which the ALT cut-off in females was higher than other criteria, might be useful for the prediction of HCC because the risk for HCC was lower in females. The slightly high ALT in female patients with HBV infection might not affect the development of HCC. However, ethnic differences and the timing of evaluation may affect the appropriate cut-off value of ALT.
Although the reason why an abnormal ALT during NA treatment is associated with HCC development has not yet been determined, the influence of metabolic-associated liver diseases (MAFLD) has been suggested [20,22]. A recent report showed that a high body mass index and high cholesterol were associated with the absence of ALT normalization [22]. Metabolic syndrome was reported to increase the risk of liver fibrosis progression in chronic hepatitis B patients [26,27] and liver-related clinical events and death [28]. Therefore, the presence of MAFLD should be evaluated; however, a limitation of the present study was that data relating to the presence of fatty liver, body mass index and amount of alcohol consumption were not available. However, another recent study showed that regardless of fatty liver, an association between early ALT normalization and lower HCC risk was observed [21]. It was also reported that fatty liver was associated with lower cirrhosis and HCC risk [29] and higher HBsAg seroclearance [30]. Therefore, mechanisms other than MAFLD might be present.
It was reported that mutated large hepatitis B surface (LHBs) proteins accumulate in the endoplasmic reticulum and cause ER stress and mitochondrial dysfunction, which may lead to the development of HCC [31,32]. NAs inhibit reverse transcription but do not suppress the expression of viral proteins. Therefore, if mutations or deletions are present in the preS region of the HBV genome, mutated LHBs that continue to be expressed long after the start of NAs might be a cause of hepatocellular injury, leading to sustained abnormal ALT. In the present study, a delayed response of ALT at 2 years was not associated with a lower risk of HCC (Figure S3). Prolonged treatment might gradually reduce the accumulation of mutated proteins, but a delayed response of ALT indicates that the accumulated amount of LHBs might be high enough to trigger carcinogenesis. This point needs to be clarified in a future study. Inhibiting the accumulation of LHBs in ER might be a therapeutic target for preventing HCC [19].
A recent report showed that the gamma-glutamyl transferase (GGT) level at 6 months after the initiation of NAs was associated with HCC development [33]. The GGT levels reflect pro-oxidant activity and are used as a marker of liver injury. Moreover, GGT was reported to be involved in many diseases such as cardiovascular diseases and cancer [34]. The elevated ALT might be associated with the GGT level, but the data on GGT were not obtained in this cohort. Its association should be analyzed in a future study.
Interestingly, this study showed that lower HBV DNA was independently associated with non-achievement of ALT normalization (Table 4). An inverse association between the presence of fatty liver and HBsAg positivity was reported [35,36] and it may partly explain the result. Consistent with these results, a study using a transgenic mouse model showed that fatty liver reduced HBV replication [37]. Another possible reason is that the retention of mutated LHBs might inhibit the release of HBV particles. An in vitro study showed that an increase in the ratio of preS2 deletion mutant to wild-type LHBs led to a decrease in the release of extracellular HBV DNA [31].
In this study, HBV genotype C was predominant (63%), followed by genotype B (35%). The frequency of genotype B was higher than in other areas of Japan [38] and our previous report showed that patients with genotype B HBV had a lower risk of HCC than those with genotype C in an age and sex-matched analysis [11]. Moreover, patients with genotype B HBV showed a higher probability of HBsAg loss [11], which is a treatment endpoint with a reduced risk of HCC [39]. As for ALT normalization in this study, the frequency of genotype C tended to be higher in patients without ALT normalization (66.2% vs. 59.2%), but not significantly. ALT normalization at 1 year seemed to be useful for HCC prediction in both genotypes C and B, although the difference in the cumulative HCC incidence in genotype B patients was not statistically significant (genotype C, p < 0.001; genotype B, p = 0.081) because of the low number of HCC patients. Larger numbers of HCC patients with genotype B HBV will be required to clarify this point.
There are limitations in the present study in addition to those mentioned above. First, there is a possibility that HBcrAg might not have been adequately evaluated because the baseline data in many patients were missing. The serum level of HBcrAg is known to be associated with the amount of cccDNA in the liver tissue and has been reported to be a predictive marker for HCC development in several cohorts [40,41,42]. Recently, the usefulness of an ultrasensitive method for HBcrAg, even after HBsAg seroclearance, was reported [43]. Second, the data were obtained only annually, and ALT normalization before 1 year was not evaluated.
In conclusion, the non-achievement of ALT normalization 1 year after the start of NA therapy in patients with chronic HBV infection was associated with HCC development. The combination with the aMAP risk score stratified the HCC risk, and HCC development was rare in patients with both low-risk aMAP and ALT normalization. Further validation studies in other cohorts are required to confirm these results.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm11092354/s1, Figure S1: Response rates for NA therapies based on ALT normalization and HBV DNA undetectability at 1 to 5 years of therapy; Figure S2: Cumulative incidences of HCC according to the achievement of ALT normalization 2 years after the start of NA therapy that were evaluated with different criteria, and those according to the viral response; Figure S3: Analysis of patients whose ALT was abnormal (>30) at 1 year of therapy but was normalized at 2 years; Table S1: Comparison of baseline characteristics between patients with ALT normalization (JSH criteria, ≤30) and those without after propensity score matching at 1 year of NA therapy.
Author Contributions
Concept and design, J.I., T.K., T.A. and O.K.; data acquisition: T.K., T.A., O.K., K.S., M.N., T.I., S.T., N.K., T.S., F.N., M.M., T.N., T.U., A.S., M.T., M.O. and H.N.; manuscript writing, J.I.; statistical analysis: J.I. and M.N.; supervision, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted in accordance with the ethical principles of the Declaration of Helsinki and has been approved by the medical ethics committee of Tohoku University (approval number 2021-1-533).
Informed Consent Statement
Patients were given the opportunity to opt out of this study.
Data Availability Statement
All data used in this study are available from the corresponding author upon reasonable request.
Acknowledgments
The authors thank other members of the THERME Study Group.
Conflicts of Interest
Jun Inoue received research funding from AbbVie. The other authors declare that they have no conflict of interest.
References
- World Health Organization. Hepatitis B. Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b (accessed on 12 March 2022).
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Seko, Y.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Suzuki, Y.; Saitoh, S.; Arase, Y.; et al. Long-term entecavir treatment reduces hepatocellular carcinoma incidence in patients with hepatitis B virus infection. Hepatology 2013, 58, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Su, T.H.; Hu, T.H.; Chen, C.Y.; Huang, Y.H.; Chuang, W.L.; Lin, C.C.; Wang, C.C.; Su, W.W.; Chen, M.Y.; Peng, C.Y.; et al. Four-year entecavir therapy reduces hepatocellular carcinoma, cirrhotic events and mortality in chronic hepatitis B patients. Liver Int. 2016, 36, 1755–1764. [Google Scholar] [CrossRef] [PubMed]
- Yip, T.C.; Lai, J.C.; Wong, G.L. Secondary prevention for hepatocellular carcinoma in patients with chronic hepatitis B: Are all the nucleos(t)ide analogues the same? J. Gastroenterol. 2020, 55, 1023–1036. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.J.; Tonthat, A.; Janssen, H.L.A.; Terrault, N.A. Aiming for Functional Cure with Established and Novel Therapies for Chronic Hepatitis B. Hepatol. Commun. 2021. [Google Scholar] [CrossRef]
- Inoue, J.; Akahane, T.; Kobayashi, T.; Obara, N.; Umetsu, T.; Kakazu, E.; Ninomiya, M.; Iwata, T.; Sano, A.; Tsuruoka, M.; et al. Switching to tenofovir disoproxil fumarate in entecavir-treated chronic hepatitis B patients: A pilot randomized controlled study. Biomed. Rep. 2021, 14, 20. [Google Scholar] [CrossRef]
- Kao, J.H.; Jeng, W.J.; Ning, Q.; Su, T.H.; Tseng, T.C.; Ueno, Y.; Yuen, M.F. APASL guidance on stopping nucleos(t)ide analogues in chronic hepatitis B patients. Hepatol. Int. 2021, 15, 833–851. [Google Scholar] [CrossRef]
- Inoue, J.; Ninomiya, M.; Shimosegawa, T.; McNiven, M.A. Cellular membrane trafficking machineries used by the hepatitis viruses. Hepatology 2018, 68, 751–762. [Google Scholar] [CrossRef]
- Martinez, M.G.; Testoni, B.; Zoulim, F. Biological basis for functional cure of chronic hepatitis B. J. Viral Hepat. 2019, 26, 786–794. [Google Scholar] [CrossRef]
- Vittal, A.; Sharma, D.; Hu, A.; Majeed, N.A.; Terry, N.; Auh, S.; Ghany, M.G. Systematic review with meta-analysis: The impact of functional cure on clinical outcomes in patients with chronic hepatitis B. Aliment. Pharmacol. Ther. 2022, 55, 8–25. [Google Scholar] [CrossRef]
- Inoue, J.; Akahane, T.; Nakayama, H.; Kimura, O.; Kobayashi, T.; Kisara, N.; Sato, T.; Morosawa, T.; Izuma, M.; Kakazu, E.; et al. Comparison of hepatitis B virus genotypes B and C among chronically hepatitis B virus-infected patients who received nucleos(t)ide analogs: A multicenter retrospective study. Hepatol. Res. 2019, 49, 1263–1274. [Google Scholar] [CrossRef]
- Orito, E.; Hasebe, C.; Kurosaki, M.; Osaki, Y.; Joko, K.; Watanabe, H.; Kimura, H.; Nishijima, N.; Kusakabe, A.; Izumi, N.; et al. Risk of hepatocellular carcinoma in cirrhotic hepatitis B virus patients during nucleoside/nucleotide analog therapy. Hepatol. Res. 2015, 45, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Pandyarajan, V.; Govalan, R.; Yang, J.D. Risk Factors and Biomarkers for Chronic Hepatitis B Associated Hepatocellular Carcinoma. Int. J. Mol. Sci. 2021, 22, 479. [Google Scholar] [CrossRef] [PubMed]
- Wong, V.W.; Janssen, H.L. Can we use HCC risk scores to individualize surveillance in chronic hepatitis B infection? J. Hepatol. 2015, 63, 722–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Kim, Y.D.; Lee, M.; Jun, B.G.; Kim, T.S.; Suk, K.T.; Kang, S.H.; Kim, M.Y.; Cheon, G.J.; Kim, D.J.; et al. Modified PAGE-B score predicts the risk of hepatocellular carcinoma in Asians with chronic hepatitis B on antiviral therapy. J. Hepatol. 2018, 69, 1066–1073. [Google Scholar] [CrossRef]
- Fan, R.; Papatheodoridis, G.; Sun, J.; Innes, H.; Toyoda, H.; Xie, Q.; Mo, S.; Sypsa, V.; Guha, I.N.; Kumada, T.; et al. aMAP risk score predicts hepatocellular carcinoma development in patients with chronic hepatitis. J. Hepatol. 2020, 73, 1368–1378. [Google Scholar] [CrossRef]
- Yotsuyanagi, H.; Takano, T.; Tanaka, M.; Amano, K.; Imamura, M.; Ogawa, K.; Yasunaka, T.; Yasui, Y.; Hayashi, K.; Tanaka, Y.; et al. Hepatitis B virus-related hepatocellular carcinoma in young adults: Efficacy of nationwide selective vaccination. Hepatol. Res. 2020, 50, 182–189. [Google Scholar] [CrossRef]
- Kawai-Kitahata, F.; Asahina, Y.; Tanaka, S.; Kakinuma, S.; Murakawa, M.; Nitta, S.; Watanabe, T.; Otani, S.; Taniguchi, M.; Goto, F.; et al. Comprehensive analyses of mutations and hepatitis B virus integration in hepatocellular carcinoma with clinicopathological features. J. Gastroenterol. 2016, 51, 473–486. [Google Scholar] [CrossRef]
- Inoue, J.; Sato, K.; Ninomiya, M.; Masamune, A. Envelope Proteins of Hepatitis B Virus: Molecular Biology and Involvement in Carcinogenesis. Viruses 2021, 13, 1124. [Google Scholar] [CrossRef]
- Wong, G.L.; Chan, H.L.; Tse, Y.K.; Yip, T.C.; Lam, K.L.; Lui, G.C.; Wong, V.W. Normal on-treatment ALT during antiviral treatment is associated with a lower risk of hepatic events in patients with chronic hepatitis B. J. Hepatol. 2018, 69, 793–802. [Google Scholar] [CrossRef]
- Choi, J.; Kim, G.A.; Han, S.; Lim, Y.S. Earlier Alanine Aminotransferase Normalization During Antiviral Treatment Is Independently Associated With Lower Risk of Hepatocellular Carcinoma in Chronic Hepatitis B. Am. J. Gastroenterol. 2020, 115, 406–414. [Google Scholar] [CrossRef]
- Kim, S.; Lee, Y.; Bang, S.M.; Bak, H.; Yim, S.Y.; Lee, Y.S.; Yoo, Y.J.; Jung, Y.K.; Kim, J.H.; Seo, Y.S.; et al. Early Normalization of Alanine Aminotransferase during Antiviral Therapy Reduces Risk of Hepatocellular Carcinoma in HBV Patients. J. Clin. Med. 2021, 10, 1840. [Google Scholar] [CrossRef] [PubMed]
- Vallet-Pichard, A.; Mallet, V.; Nalpas, B.; Verkarre, V.; Nalpas, A.; Dhalluin-Venier, V.; Fontaine, H.; Pol, S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology 2007, 46, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.Q.; Hoang, J.K.; Leong, J.; Riveiro-Barciela, M.; Maeda, M.; Yang, J.D.; Accarino, E.V.; Thin, K.; Trinh, L.; Cheung, R.C.; et al. Differential characteristics and outcomes of Asian and non-Asian patients with HBV-related hepatocellular carcinoma. Liver Int. 2021, 41, 1922–1932. [Google Scholar] [CrossRef] [PubMed]
- Prati, D.; Taioli, E.; Zanella, A.; Della Torre, E.; Butelli, S.; Del Vecchio, E.; Vianello, L.; Zanuso, F.; Mozzi, F.; Milani, S.; et al. Updated definitions of healthy ranges for serum alanine aminotransferase levels. Ann. Intern. Med. 2002, 137, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wong, G.L.; Chan, H.L.; Yu, Z.; Chan, A.W.; Choi, P.C.; Chim, A.M.; Chan, H.Y.; Tse, C.H.; Wong, V.W. Coincidental metabolic syndrome increases the risk of liver fibrosis progression in patients with chronic hepatitis B--a prospective cohort study with paired transient elastography examinations. Aliment. Pharmacol. Ther. 2014, 39, 883–893. [Google Scholar] [CrossRef]
- Van Kleef, L.A.; Ayada, I.; Alferink, L.J.M.; Pan, Q.; de Knegt, R.J. Metabolic dysfunction-associated fatty liver disease improves detection of high liver stiffness: The Rotterdam Study. Hepatology 2022, 75, 419–429. [Google Scholar] [CrossRef]
- Van Kleef, L.A.; Choi, H.S.J.; Brouwer, W.P.; Hansen, B.E.; Patel, K.; de Man, R.A.; Janssen, H.L.A.; de Knegt, R.J.; Sonneveld, M.J. Metabolic dysfunction-associated fatty liver disease increases risk of adverse outcomes in patients with chronic hepatitis B. JHEP Rep. 2021, 3, 100350. [Google Scholar] [CrossRef]
- Li, J.; Yang, H.I.; Yeh, M.L.; Le, M.H.; Le, A.K.; Yeo, Y.H.; Dai, C.Y.; Barnett, S.; Zhang, J.Q.; Huang, J.F.; et al. Association Between Fatty Liver and Cirrhosis, Hepatocellular Carcinoma, and Hepatitis B Surface Antigen Seroclearance in Chronic Hepatitis B. J. Infect. Dis. 2021, 224, 294–302. [Google Scholar] [CrossRef]
- Mak, L.Y.; Hui, R.W.; Fung, J.; Liu, F.; Wong, D.K.; Cheung, K.S.; Yuen, M.F.; Seto, W.K. Diverse effects of hepatic steatosis on fibrosis progression and functional cure in virologically quiescent chronic hepatitis B. J. Hepatol. 2020, 73, 800–806. [Google Scholar] [CrossRef]
- Liang, Y.J.; Teng, W.; Chen, C.L.; Sun, C.P.; Teng, R.D.; Huang, Y.H.; Liang, K.H.; Chen, Y.W.; Lin, C.C.; Su, C.W.; et al. Clinical Implications of HBV PreS/S Mutations and the Effects of PreS2 Deletion on Mitochondria, Liver Fibrosis, and Cancer Development. Hepatology 2021, 74, 641–655. [Google Scholar] [CrossRef]
- Lin, Y.T.; Jeng, L.B.; Chan, W.L.; Su, I.J.; Teng, C.F. Hepatitis B Virus Pre-S Gene Deletions and Pre-S Deleted Proteins: Clinical and Molecular Implications in Hepatocellular Carcinoma. Viruses 2021, 13, 862. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.F.; Jang, T.Y.; Jun, D.W.; Ahn, S.B.; An, J.; Enomoto, M.; Takahashi, H.; Ogawa, E.; Yoon, E.; Jeong, S.W.; et al. On-treatment gamma-glutamyl transferase predicts the development of hepatocellular carcinoma in chronic hepatitis B patients. Liver Int. 2022, 42, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Corti, A.; Belcastro, E.; Dominici, S.; Maellaro, E.; Pompella, A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an ‘antioxidant’ enzyme. Free Radic. Biol. Med. 2020, 160, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.L.; Wang, Y.J.; Kao, W.Y.; Chen, P.H.; Huo, T.I.; Huang, Y.H.; Lan, K.H.; Su, C.W.; Chan, W.L.; Lin, H.C.; et al. Inverse association between hepatitis B virus infection and fatty liver disease: A large-scale study in populations seeking for check-up. PLoS ONE 2013, 8, e72049. [Google Scholar] [CrossRef]
- Joo, E.J.; Chang, Y.; Yeom, J.S.; Ryu, S. Hepatitis B virus infection and decreased risk of nonalcoholic fatty liver disease: A cohort study. Hepatology 2017, 65, 828–835. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Pan, Q.; Duan, X.Y.; Liu, Q.; Mo, G.Y.; Rao, G.R.; Fan, J.G. Fatty liver reduces hepatitis B virus replication in a genotype B hepatitis B virus transgenic mice model. J. Gastroenterol. Hepatol. 2012, 27, 1858–1864. [Google Scholar] [CrossRef]
- Ito, K.; Yotsuyanagi, H.; Sugiyama, M.; Yatsuhashi, H.; Karino, Y.; Takikawa, Y.; Saito, T.; Arase, Y.; Imazeki, F.; Kurosaki, M.; et al. Geographic distribution and characteristics of genotype A hepatitis B virus infection in acute and chronic hepatitis B patients in Japan. J. Gastroenterol. Hepatol. 2016, 31, 180–189. [Google Scholar] [CrossRef]
- Moini, M.; Fung, S. HBsAg Loss as a Treatment Endpoint for Chronic HBV Infection: HBV Cure. Viruses 2022, 14, 657. [Google Scholar] [CrossRef]
- Liang, L.Y.; Wong, V.W.; Toyoda, H.; Tse, Y.K.; Yip, T.C.; Yuen, B.W.; Tada, T.; Kumada, T.; Lee, H.W.; Lui, G.C.; et al. Serum hepatitis B core-related antigen predicts hepatocellular carcinoma in hepatitis B e antigen-negative patients. J. Gastroenterol. 2020, 55, 899–908. [Google Scholar] [CrossRef]
- Kaneko, S.; Kurosaki, M.; Inada, K.; Kirino, S.; Hayakawa, Y.; Yamashita, K.; Osawa, L.; Sekiguchi, S.; Higuchi, M.; Takaura, K.; et al. Hepatitis B core-related antigen predicts disease progression and hepatocellular carcinoma in hepatitis B e antigen-negative chronic hepatitis B patients. J. Gastroenterol. Hepatol. 2021, 36, 2943–2951. [Google Scholar] [CrossRef]
- Hosaka, T.; Suzuki, F.; Kobayashi, M.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Akuta, N.; Kobayashi, M.; Suzuki, Y.; Saitoh, S.; et al. Ultrasensitive Assay for Hepatitis B Core-Related Antigen Predicts Hepatocellular Carcinoma Incidences during Entecavir. Hepatol. Commun. 2022, 6, 36–49. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, F.; Hosaka, T.; Imaizumi, M.; Kobayashi, M.; Ohue, C.; Suzuki, Y.; Fujiyama, S.; Kawamura, Y.; Sezaki, H.; Akuta, N.; et al. Potential of ultra-highly sensitive immunoassays for hepatitis B surface and core-related antigens in patients with or without development of hepatocellular carcinoma after hepatitis B surface antigen seroclearance. Hepatol. Res. 2021, 51, 426–435. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).