Ultrasound of the Heel Improves Diagnosis—Tender Entheses in the Heel Region Rarely Corresponds to Inflammatory Enthesitis in Patients with Peripheral Spondyloarthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Clinical Evaluation
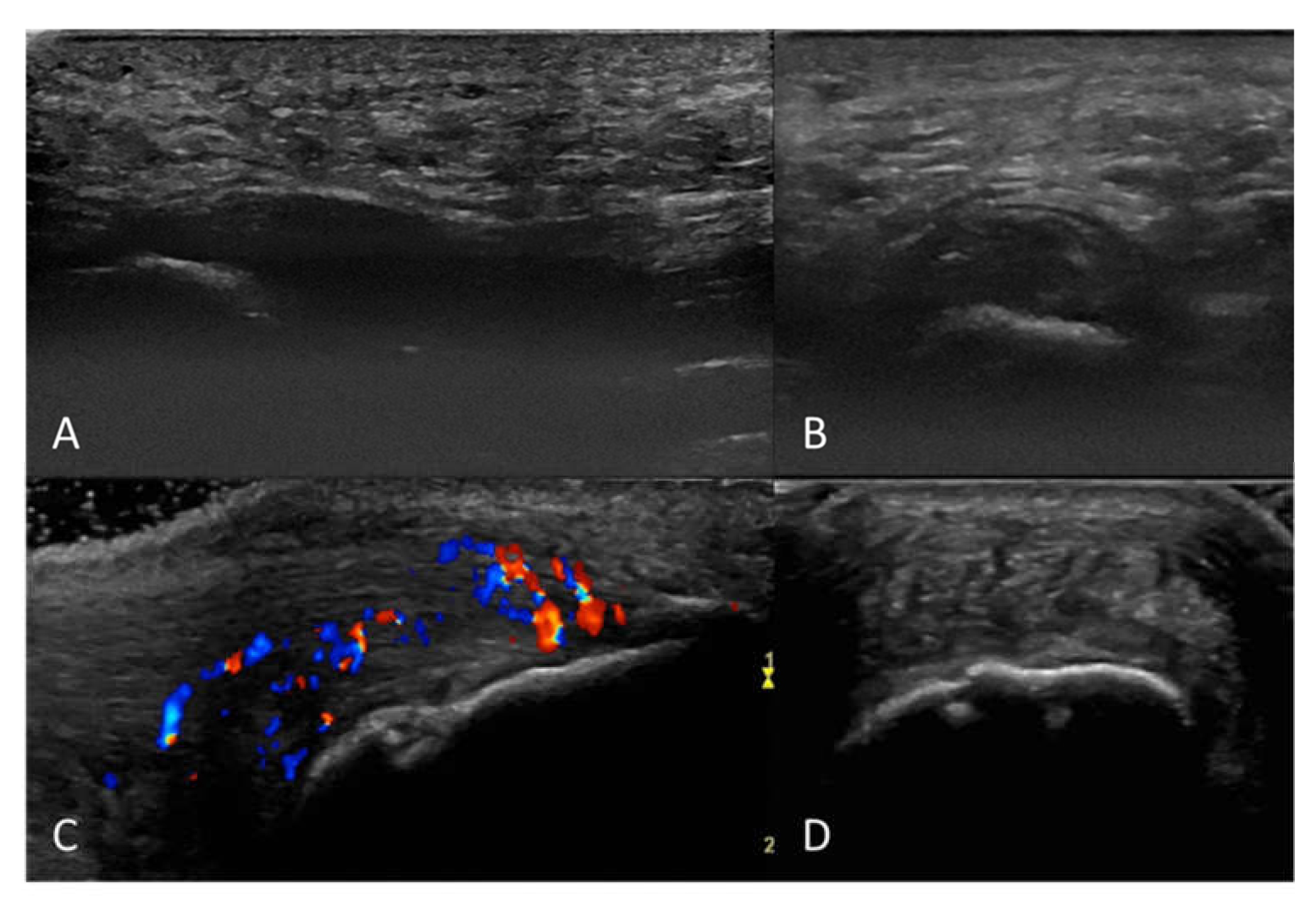
2.2. Ultrasound Examination and Scoring
2.3. Statistics
3. Results
3.1. Population Characteristics
3.2. Ultrasound Findings and Agreement
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Terslev, L.; Naredo, E.; Iagnocco, A.; Balint, P.V.; Wakefield, R.J.; Aegerter, P.; Aydin, S.Z.; Bachta, A.; Hammer, H.B.; Bruyn, G.A.; et al. Defining enthesitis in spondyloarthritis by ultrasound: Results of a Delphi process and of a reliability reading exercise. Arthritis Care Res. (Hoboken) 2014, 66, 741–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balint, P.V.; Terslev, L.; Aegerter, P.; Bruyn, G.A.W.; Chary-Valckenaere, I.; Gandjbakhch, F.; Iagnocco, A.; Jousse-Joulin, S.; Moller, I.; Naredo, E.; et al. Reliability of a consensus-based ultrasound definition and scoring for enthesitis in spondyloarthritis and psoriatic arthritis: An OMERACT US initiative. Ann. Rheum. Dis. 2018, 77, 1730–1735. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, M.-A.; Said-Nahal, R.; Hacquard-Bouder, C.; Brasseur, J.-L.; Dougados, M.; Breban, M. Assessment of peripheral enthesitis in the spondylarthropathies by ultrasonography combined with power Doppler: A cross-sectional study. Arthritis Rheum. 2003, 48, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Lehtinen, A.; Taavitsainen, M.; Leirisalo-Repo, M. Sonographic analysis of enthesopathy in the lower extremities of patients with spondylarthropathy. Clin. Exp. Rheumatol. 1994, 12, 143–148. [Google Scholar] [PubMed]
- Balint, P.V.; Kane, D.; Wilson, H.; McInnes, I.B.; Sturrock, R.D. Ultrasonography of entheseal insertions in the lower limb in spondyloarthropathy. Ann. Rheum. Dis. 2002, 61, 905–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandl, P.; Navarro-Compan, V.; Terslev, L.; Aegerter, P.; van der Heijde, D.; D’Agostino, M.A.; Baraliakos, X.; Pedersen, S.J.; Jurik, A.G.; Naredo, E.; et al. EULAR recommendations for the use of imaging in the diagnosis and management of spondyloarthritis in clinical practice. Ann. Rheum. Dis. 2015, 74, 1327–1339. [Google Scholar] [CrossRef]
- Seven, S.; Pedersen, S.J.; Østergaard, M.; Felbo, S.K.; Sørensen, I.J.; Døhn, U.M.; Terslev, L. Peripheral Enthesitis Detected by Ultrasonography in Patients with Axial Spondyloarthritis-Anatomical Distribution, Morphology, and Response to Tumor Necrosis Factor-Inhibitor Therapy. Front. Med. (Lausanne) 2020, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Naredo, E.; Batlle-Gualda, E.; Garcia-Vivar, M.L.; Garcia-Aparicio, A.M.; Fernandez-Sueiro, J.L.; Fernandez-Prada, M.; Giner, E.; Rodriguez-Gomez, M.; Pina, M.F.; Medina-Luezas, J.A.; et al. Power Doppler ultrasonography assessment of entheses in spondyloarthropathies: Response to therapy of entheseal abnormalities. J. Rheumatol. 2010, 37, 2110–2117. [Google Scholar] [CrossRef]
- Felbo, S.K.; Wiell, C.; Ostergaard, M.; Poggenborg, R.P.; Boyesen, P.; Hammer, H.B.; Boonen, A.; Pedersen, S.J.; Juul Sorensen, I.; Madsen, O.R.; et al. Do tender joints in active psoriatic arthritis reflect inflammation assessed by ultrasound and magnetic resonance imaging? Rheumatology 2021. [Google Scholar] [CrossRef]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Akkoc, N.; Brandt, J.; Chou, C.T.; Dougados, M.; Huang, F.; Gu, J.; Kirazli, Y.; et al. The Assessment of SpondyloArthritis International Society classification criteria for peripheral spondyloarthritis and for spondyloarthritis in general. Ann. Rheum. Dis. 2011, 70, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Torp-Pedersen, S.T.; Terslev, L. Settings and artefacts relevant in colour/power Doppler ultrasound in rheumatology. Ann. Rheum. Dis. 2008, 67, 143–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, I.; Janta, I.; Backhaus, M.; Ohrndorf, S.; Bong, D.A.; Martinoli, C.; Filippucci, E.; Sconfienza, L.M.; Terslev, L.; Damjanov, N.; et al. The 2017 EULAR standardised procedures for ultrasound imaging in rheumatology. Ann. Rheum. Dis. 2017, 76, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Bruyn, G.A.; Iagnocco, A.; Naredo, E.; Balint, P.V.; Gutierrez, M.; Hammer, H.B.; Collado, P.; Filippou, G.; Schmidt, W.A.; Jousse-Joulin, S.; et al. OMERACT Definitions for Ultrasonographic Pathologies and Elementary Lesions of Rheumatic Disorders 15 Years on. J. Rheumatol. 2019, 46, 1388–1393. [Google Scholar] [CrossRef] [PubMed]
- Byrt, T.; Bishop, J.; Carlin, J.B. Bias, prevalence and kappa. J. Clin. Epidemiol. 1993, 46, 423–429. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelsen, B.; Diamantopoulos, A.P.; Soldal, D.M.; Hammer, H.B.; Kavanaugh, A.; Haugeberg, G. Achilles enthesitis defined by ultrasound is not associated with clinical enthesitis in patients with psoriatic arthritis. RMD Open 2017, 3, e000486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guldberg-Moller, J.; Terslev, L.; Nielsen, S.M.; Konig, M.J.; Torp-Pedersen, S.T.; Torp-Pedersen, A.; Christensen, R.; Bliddal, H.; Ellegaard, K. Ultrasound pathology of the entheses in an age and gender stratified sample of healthy adult subjects: A prospective cross-sectional frequency study. Clin. Exp. Rheumatol. 2019, 37, 408–413. [Google Scholar] [PubMed]

| All n = 27 | Achilles n = 14 | Fascia Plantaris n = 13 | Difference | ||
|---|---|---|---|---|---|
| No/Median (%/IQR) | No/Median (%/IQR) | No/Median (%/IQR) | OR (95% CI)/ Difference in Medians (95% CI) 1 | p | |
| Age (years) | 49 (38–56) | 50 (39–57) | 44 (37–52) | 3 (−9–14) | 0.56 |
| Sex (male) | 16 (59) | 8 (57) | 8 (62) | 1.2 (0.2–7.3) | 1 |
| PsA | 15 (56) | 8 (57) | 7 (54) | 1.1 (0.2–6.7) | 1 |
| Enthesis—Achilles | 14 (52) | 14 (100) | 0 (0) | - | - |
| Enthesis—Plantar fascia | 13 (48) | 0 (0) | 13 (100) | - | - |
| Disease duration (years) | 2 (0.25–6) | 1 (0–6) | 2 (1–6) | −1 (−4–3) | 0.42 |
| CRP (mg/L) | 3.5 (1.5–6.8) | 4.4 (1.6–7.8) | 2.8 (1.8–4.7) | 1.0 (−1.8–4.5) | 0.54 |
| TJC (0–68) | 1 (0–11) | 2 (0–11) | 1 (0–8) | 0 (−2–4) | 0.75 |
| SJC (0–66) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0 (0–0) | 0.64 |
| SPARCC (0–16) | 2 (1–4) | 3 (1–4) | 2 (1–2) | 0 (−1–2) | 0.40 |
| DAS28-CRP | 2.5 (2.1–3.0) | 2.3 (2–2.8) | 2.6 (2.3–3.3) | −0.3 (−0.8–0.3) | 0.18 |
| Physician global VAS (0–100) | 27 (14–42) | 30 (23–70) | 17 (12–36) | 13 (−5–36) | 0.15 |
| HAQ (0–3) | 0.88 (0.50–1.20) | 0.75 (0.38–1.38) | 0.88 (0.75–1.13) | −0.25 (−0.75–0.38) | 0.38 |
| Pt. global VAS (0–100) | 72 (52–78) | 71 (19–78) | 72 (53–78) | −4 (−33–13) | 0.68 |
| Pt. pain VAS (0–100) | 63 (41–73) | 65 (38–79) | 59 (47–67) | 5 (−25–21) | 0.70 |
| All n = 27 | Achilles n = 14 | Fascia Plantaris n = 13 | Difference | ||
|---|---|---|---|---|---|
| No/Median (%/IQR) | No/Median (%/IQR) | No/Median (%/IQR) | OR (95% CI)/ Difference in Medians (95% CI) 1 | p | |
| Elementary lesions | |||||
| Thickening | 13 (48) | 6 (43) | 7 (54) | 1.5 (0.3–9.2) | 0.71 |
| Hypoechogenicity | 12 (44) | 6 (43) | 6 (46) | 1.1 (0.2–6.7) | 1 |
| Calcifications/Enthesophytes | 12 (44) | 11 (79) | 1 (8) | 0.0 (0.0–0.3) | <0.001 |
| Erosions | 4 (15) | 4 (29) | 0 (0) | 0.0 (0.0–1.5) | 0.10 |
| CD (presence) | 5 (19) | 5 (36) | 0 (0) | 0.0 (0.0–1.0) | 0.04 |
| CD grade (positive only) | 2 (2–2) | 2 (2-2) | NA | - | - |
| Combined lesions | |||||
| Any inflammatory lesion 2 | 13 (48) | 6 (43) | 7 (54) | 1.5 (0.3–9.2) | 0.71 |
| Any structural lesion 2 | 12 (44) | 11 (79) | 1 (8) | 0.0 (0.0–0.3) | <0.001 |
| Any inflammatory AND any structural lesion 2 | 6 (22) | 5 (36) | 1 (8) | 0.2 (0.0–1.8) | 0.17 |
| Any inflammatory OR any structural lesion 2 | 19 (70) | 12 (86) | 7 (54) | 0.2 (0.0–1.6) | 0.10 |
| Sum-score | |||||
| Sum-score (0–7) 3 | 1 (0.0–2.5) | 1.5 (1–4) | 1 (0–2) | 1.0 (0.0–3.0) | 0.09 |
| PEA | κ | PABAK | |
|---|---|---|---|
| Tenderness vs. any US sign of enthesitis (inflammatory 1 or structural 2) | 70 | 0 | 0.41 |
| Tenderness vs. any US inflammatory sign of enthesitis 1 | 48 | 0 | −0.04 |
| Tenderness vs. US inflammatory enthesitis 1 with Doppler activity | 19 | 0 | −0.63 |
| Tenderness vs. any US inflammatory signs of enthesitis 1 OR other explanatory pathology | 70 | 0 | 0.41 |
| Intrareader (n = 27) | Interreader (n = 10) | |||||||
|---|---|---|---|---|---|---|---|---|
| Prev. (%) | PEA (%) | κ | PABAK | Prev. (%) | PEA (%) | κ | PABAK | |
| Thickened | 48 | 100 | 1 | 1 | 20 | 100 | 1 | 1 |
| Hypoechogenicity | 46 | 96 | 0.93 | 0.93 | 5 | 90 | 0 | 0.8 |
| Erosions | 15 | 100 | 1 | 1 | 20 | 100 | 1 | 1 |
| Enthesophytes/Calcifications | 44 | 100 | 1 | 1 | 60 | 100 | 1 | 1 |
| Color Doppler presence | 19 | 100 | 1 | 1 | 10 | 100 | 1 | 1 |
| Color Doppler grade (0–3) | NA | 96 | 0.97 | NA | NA | 100 | 1 | NA |
| Inflammation 1 yes/no | 48 | 100 | 1 | 1 | 20 | 100 | 1 | 1 |
| Structural 2 yes/no | 44 | 100 | 1 | 1 | 60 | 100 | 1 | 1 |
| ICC (95% CI) | ICC (95% CI) | |||||||
| Ultrasound lesion Sum-score 3 | 0.99 (0.98–1.00) | 0.98 (0.93–0.99) | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felbo, S.K.; Østergaard, M.; Sørensen, I.J.; Terslev, L. Ultrasound of the Heel Improves Diagnosis—Tender Entheses in the Heel Region Rarely Corresponds to Inflammatory Enthesitis in Patients with Peripheral Spondyloarthritis. J. Clin. Med. 2022, 11, 2325. https://doi.org/10.3390/jcm11092325
Felbo SK, Østergaard M, Sørensen IJ, Terslev L. Ultrasound of the Heel Improves Diagnosis—Tender Entheses in the Heel Region Rarely Corresponds to Inflammatory Enthesitis in Patients with Peripheral Spondyloarthritis. Journal of Clinical Medicine. 2022; 11(9):2325. https://doi.org/10.3390/jcm11092325
Chicago/Turabian StyleFelbo, Sara Kamp, Mikkel Østergaard, Inge Juul Sørensen, and Lene Terslev. 2022. "Ultrasound of the Heel Improves Diagnosis—Tender Entheses in the Heel Region Rarely Corresponds to Inflammatory Enthesitis in Patients with Peripheral Spondyloarthritis" Journal of Clinical Medicine 11, no. 9: 2325. https://doi.org/10.3390/jcm11092325
APA StyleFelbo, S. K., Østergaard, M., Sørensen, I. J., & Terslev, L. (2022). Ultrasound of the Heel Improves Diagnosis—Tender Entheses in the Heel Region Rarely Corresponds to Inflammatory Enthesitis in Patients with Peripheral Spondyloarthritis. Journal of Clinical Medicine, 11(9), 2325. https://doi.org/10.3390/jcm11092325







