Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used
Abstract
:1. Introduction
2. Aim
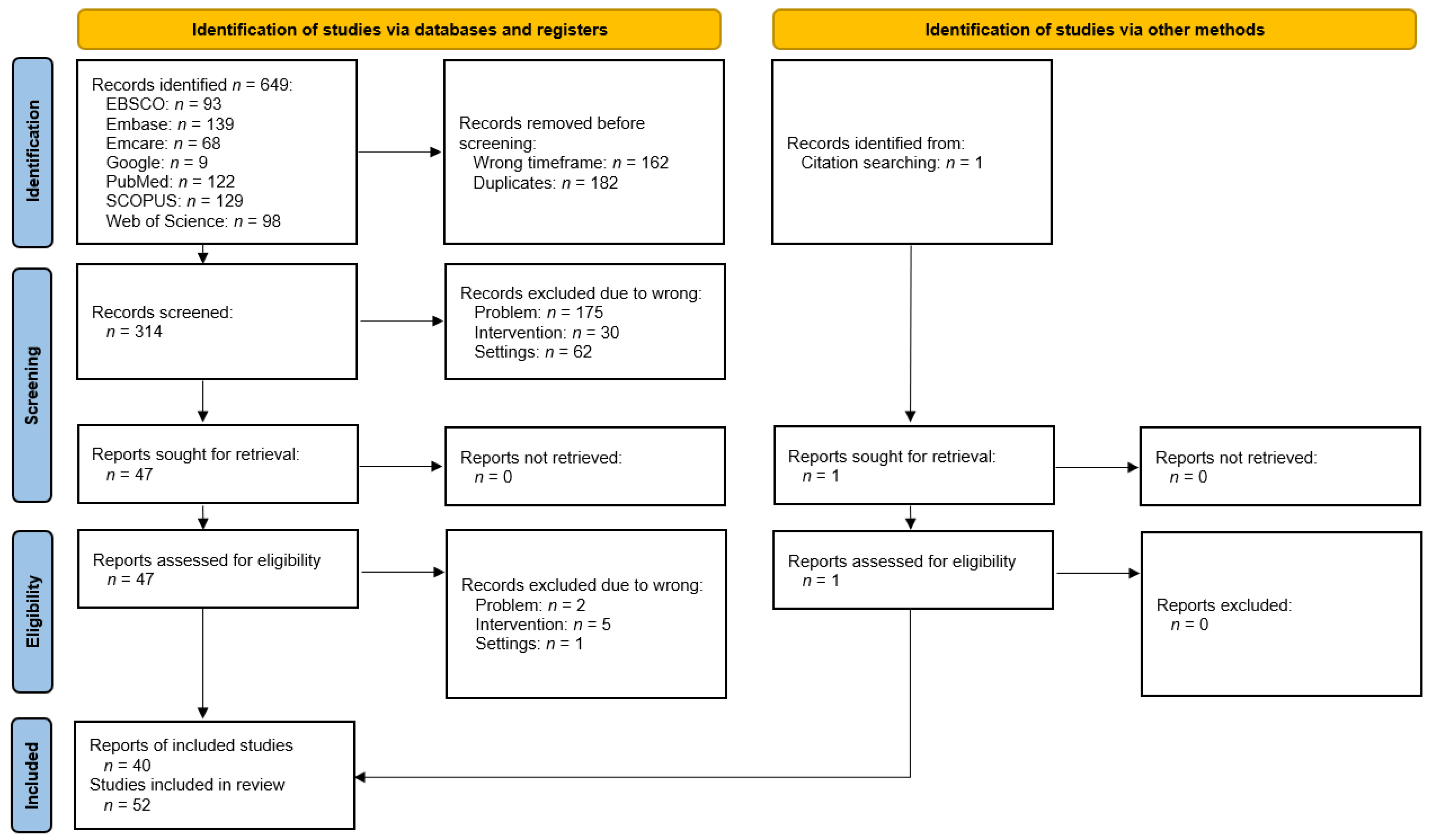
3. Materials and Methods
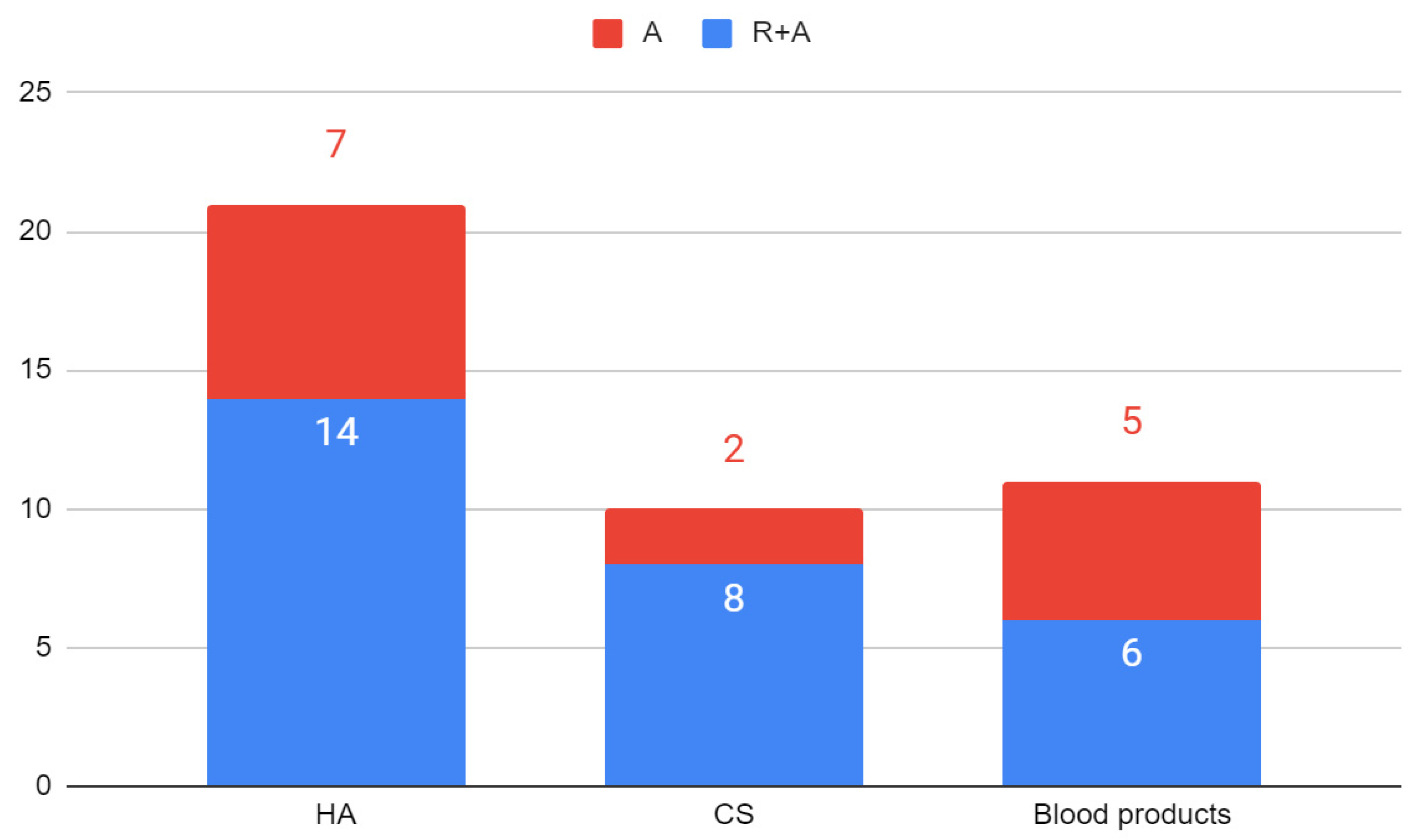
4. Results
5. Discussion
5.1. Hyaluronic Acid
5.2. Corticosteroids
5.3. Blood Products
5.4. Analgesics
5.5. Dextrose
5.6. Transplants
5.7. Ozone Gas
5.8. Differential Diagnosis
5.9. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, S.H.; Park, H.; Yoo, D.S.; Joo, W.; Rhoton, A. Microsurgical anatomy of the facial nerve. Clin. Anat. 2021, 34, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Chęciński, M.; Chlubek, D. Retro-Auricular Approach to the Fractures of the Mandibular Condyle: A Systematic Review. J. Clin. Med. 2021, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Chęciński, M.; Nowak, Z.; Chlubek, D. Variants and Modifications of the Retroauricular Approach Using in Temporomandibular Joint Surgery: A Systematic Review. J. Clin. Med. 2021, 10, 2049. [Google Scholar] [CrossRef] [PubMed]
- Bengtsson, M.; Fransson, P. Do patient-reported outcome measures correlate with clinical follow-up after arthroscopic treatment of internal derangement of the temporomandibular joint? J. Stomatol. Oral Maxillofac. Surg. 2021, 122, e21–e26. [Google Scholar] [CrossRef]
- Kumar, A.; Gupta, A.; Ghosh, R.; Pandey, R.; Kumar, S. A Comparative Study between Concentric Single-Needle Puncture Technique and Conventional 2-Needle Technique for Temporomandibular Joint Arthrocentesis Plus Corticosteroid Injections. Craniomaxillofac. Trauma Reconstr. 2020, 13, 99–104. [Google Scholar] [CrossRef]
- Sikora, M.; Czerwińska-Niezabitowska, B.; Chęciński, M.A.; Sielski, M.; Chlubek, D. Short-Term Effects of Intra-Articular Hyaluronic Acid Administration in Patients with Temporomandibular Joint Disorders. J. Clin. Med. 2020, 9, 1749. [Google Scholar] [CrossRef]
- Liapaki, A.; Thamm, J.R.; Ha, S.; Monteiro, J.L.G.C.; McCain, J.P.; Troulis, M.J.; Guastaldi, F.P.S. Is there a difference in treatment effect of different intra-articular drugs for temporomandibular joint osteoarthritis? A systematic review of randomized controlled trials. Int. J. Oral Maxillofac. Surg. 2021, 50, 1233–1243. [Google Scholar] [CrossRef]
- Nagori, S.A.; Bansal, A.; Jose, A.; Roychoudhury, A. Comparison of outcomes with the single-puncture and double-puncture techniques of arthrocentesis of the temporomandibular joint: An updated systematic review and meta-analysis. J. Oral Rehabil. 2021, 48, 1056–1065. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; De Almeida, A.M.; Manfredini, D. Arthrocentesis of the Temporomandibular Joint: Systematic Review and Clinical Implications of Research Findings. J. Oral Facial Pain Headache 2021, 35, 17–29. [Google Scholar] [CrossRef]
- Li, D.T.S.; Wong, N.S.M.; Li, S.K.Y.; McGrath, C.P.; Leung, Y.Y. Timing of arthrocentesis in the management of temporomandibular disorders: An integrative review and meta-analysis. Int. J. Oral Maxillofac. Surg. 2021, 50, 1078–1088. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Mechanisms of Action and Efficacy of Hyaluronic Acid, Corticosteroids and Platelet-Rich Plasma in the Treatment of Temporomandibular Joint Osteoarthritis—A Systematic Review. Int. J. Mol. Sci. 2021, 22, 7405. [Google Scholar] [CrossRef] [PubMed]
- Chęciński, M.; Sikora, M.; Chęcińska, K.; Nowak, Z.; Chlubek, D. The Administration of Hyaluronic Acid into the Temporomandibular Joints’ Cavities Increases the Mandible’s Mobility: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1901. [Google Scholar] [CrossRef] [PubMed]
- Goker, F.; Russillo, A.; Taschieri, S.; Giannì, A.B.; Mortellaro, C.; Colletti, L.; Manfredi, B.; Rovati, M.; Biagi, R.; Del Fabbro, M. Evaluation of Arthrocentesis with hyaluronic acid injections for management of temporomandibular disorders: A systematic review and case series. J. Biol. Regul. Homeost. Agents 2021, 35, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Sàbado-Bundó, H.; Sánchez-Garcés, M.; Camps-Font, O.; Gay-Escoda, C. Intraarticular injections of hyaluronic acid in arthrocentesis and arthroscopy as a treatment of temporomandibular joint disorders: A systematic review. CRANIO 2021, 1–10. [Google Scholar] [CrossRef]
- Stoustrup, P.; Kristensen, K.D.; Verna, C.; Küseler, A.; Pedersen, T.K.; Herlin, T. Intra-articular steroid injection for temporomandibular joint arthritis in juvenile idiopathic arthritis: A systematic review on efficacy and safety. Semin. Arthritis Rheum. 2013, 43, 63–70. [Google Scholar] [CrossRef]
- Parra, D.A.; Chan, M.; Krishnamurthy, G.; Spiegel, L.; Amaral, J.G.; Temple, M.J.; John, P.R.; Connolly, B.L. Use and accuracy of US guidance for image-guided injections of the temporomandibular joints in children with arthritis. Pediatr. Radiol. 2010, 40, 1498–1504. [Google Scholar] [CrossRef]
- Skármeta, N.P.; Hormazábal, F.A.; Alvarado, J.; Rodriguez, A.M. Subcutaneous Lipoatrophy and Skin Depigmentation Secondary to TMJ Intra-Articular Corticosteroid Injection. J. Oral Maxillofac. Surg. 2017, 75, 2540.e1–2540.e5. [Google Scholar] [CrossRef]
- Al-Hamed, F.S.; Hijazi, A.; Gao, Q.; Badran, Z.; Tamimi, F. Platelet Concentrate Treatments for Temporomandibular Disorders: A Systematic Review and Meta-analysis. JDR Clin. Transl. Res. 2020, 6, 174–183. [Google Scholar] [CrossRef]
- Gutiérrez, I.Q.; Sábado-Bundó, H.; Gay-Escoda, C. Intraarticular Injections of Platelet Rich Plasma and Plasma Rich in Growth Factors with Arthrocenthesis or Arthroscopy in the Treatment of Temporomandibular Joint Disorders: A Systematic Review. J. Stomatol. Oral Maxillofac. Surg. 2021. [Google Scholar] [CrossRef]
- Haigler, M.C.; Abdulrehman, E.; Siddappa, S.; Kishore, R.; Padilla, M.; Enciso, R. Use of platelet-rich plasma, platelet-rich growth factor with arthrocentesis or arthroscopy to treat temporomandibular joint osteoarthritis. J. Am. Dent. Assoc. 2018, 149, 940–952.e2. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Lin, M.-T.; Chang, H.-P. Effectiveness of platelet-rich plasma injection in patients with temporomandibular joint osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2019, 127, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Bousnaki, M.; Bakopoulou, A.; Koidis, P. Platelet-rich plasma for the therapeutic management of temporomandibular joint disorders: A systematic review. Int. J. Oral Maxillofac. Surg. 2018, 47, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Albilia, J.; Herrera-Vizcaíno, C.; Weisleder, H.; Choukroun, J.; Ghanaati, S. Liquid platelet-rich fibrin injections as a treatment adjunct for painful temporomandibular joints: Preliminary results. CRANIO 2020, 38, 292–304. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Y.; Zhang, X. Do intra-articular injections of analgesics improve outcomes after temporomandibular joint arthrocentesis?: A systematic review and meta-analysis. J. Oral Rehabil. 2020, 48, 95–105. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 105906. [Google Scholar] [CrossRef]
- Page, M.J.; Shamseer, L.; Tricco, A.C. Registration of systematic reviews in PROSPERO: 30,000 records and counting. Syst. Rev. 2018, 7, 32. [Google Scholar] [CrossRef]
- Chiappelli, F.; Kasar, V.R.; Balenton, N.; Khakshooy, A. Quantitative Consensus in Systematic Reviews: Current and Future Challenges in Translational Science. Bioinformation 2018, 14, 86–92. [Google Scholar] [CrossRef] [Green Version]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef] [Green Version]
- Rodhen, R.M.; de Holanda, T.A.; Barbon, F.J.; de Oliveira da Rosa, W.L.; Boscato, N. Invasive surgical procedures for the management of internal derangement of the temporomandibular joint: A systematic review and meta-analysis regarding the effects on pain and jaw mobility. Clin. Oral Investig. 2022, 26, 3429–3446. [Google Scholar] [CrossRef]
- Woodford, S.C.; Robinson, D.L.; Mehl, A.; Lee, P.V.S.; Ackland, D.C. Measurement of normal and pathological mandibular and temporomandibular joint kinematics: A systematic review. J. Biomech. 2020, 111, 109994. [Google Scholar] [CrossRef]
- Pinheiro, P.F., Jr.; Da Cunha, D.A.; Filho, M.G.D.; Caldas, A.S.; Melo, T.M.A.; Da Silva, H.J. The Use of Electrognathography in Jaw Movement Research: A Literature Review. CRANIO 2012, 30, 293–303. [Google Scholar] [CrossRef] [PubMed]
- Nitecka-Buchta, A.; Proba, T.; Proba, P.; Stefanski, K.; Baron, S. Functional Assessment of the Stomatognathic System, after the Treatment of Edentulous Patients, with Different Methods of Establishing the Centric Relation. Pain Res. Manag. 2018, 2018, 1572037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, l4898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrette, L.-X.; Connolly, J.; Romeo, D.; Ng, J.; Moreira, A.G.; Rajasekaran, K. Quality appraisal of clinical practice guidelines for temporomandibular joint disorders using the AGREE II instrument. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2021, 133, 402–411. [Google Scholar] [CrossRef]
- Warburton, G.; Patel, N.; Anchlia, S. Current Treatment Strategies for the Management of the Internal Derangements of the Temporomandibular Joint: A Global Perspective. J. Maxillofac. Oral Surg. 2021, 21, 1–13. [Google Scholar] [CrossRef]
- International Classification of Orofacial Pain, 1st edition (ICOP). Cephalalgia 2020, 40, 129–221. [CrossRef] [Green Version]
- Schiffman, E.; Ohrbach, R.; Truelove, E.; Look, J.; Anderson, G.; Goulet, J.-P.; List, T.; Svensson, P.; Gonzalez, Y.; Lobbezoo, F.; et al. Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for Clinical and Research Applications: Recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J. Oral Facial Pain Headache 2014, 28, 6–27. [Google Scholar] [CrossRef]
- Ghoneim, N.I.; Mansour, N.A.; Elmaghraby, S.A.; Abdelsameaa, S.E. Treatment of temporomandibular joint disc displacement using arthrocentesis combined with injectable platelet rich fibrin versus arthrocentesis alone. J. Dent. Sci. 2021, 17, 468–475. [Google Scholar] [CrossRef]
- Sembronio, S.; Tel, A.; Tremolada, C.; Lazzarotto, A.; Isola, M.; Robiony, M. Temporomandibular Joint Arthrocentesis and Microfragmented Adipose Tissue Injection for the Treatment of Internal Derangement and Osteoarthritis: A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2021, 79, 1447–1456. [Google Scholar] [CrossRef]
- Karadayi, U.; Gursoytrak, B. Randomised controlled trial of arthrocentesis with or without PRF for internal derangement of the TMJ. J. Cranio-Maxillofac. Surg. 2021, 49, 362–367. [Google Scholar] [CrossRef]
- Jacob, S.M.; Bandyopadhyay, T.K.; Chattopadhyay, P.K.; Parihar, V.S. Efficacy of Platelet-Rich Plasma Versus Hyaluronic Acid Following Arthrocentesis for Temporomandibular Joint Disc Disorders: A Randomized Controlled Trial. J. Maxillofac. Oral Surg. 2021, 20, 1–6. [Google Scholar] [CrossRef]
- Singh, A.K.; Sharma, N.K.; Kumar, P.G.N.; Singh, S.; Mishra, N.; Bera, R.N. Evaluation of Arthrocentesis with and Without Platelet-Rich Plasma in the Management of Internal Derangement of Temporomandibular Joint: A Randomized Controlled Trial. J. Maxillofac. Oral Surg. 2019, 20, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Dolwick, M.F.; Diaz, D.; Freburg-Hoffmeister, D.L.; Widmer, C.G. A Randomized, Double-Blind, Placebo-Controlled Study of the Efficacy of Steroid Supplementation After Temporomandibular Joint Arthrocentesis. J. Oral Maxillofac. Surg. 2020, 78, 1088–1099. [Google Scholar] [CrossRef] [PubMed]
- Zarate, M.A.; Frusso, R.D.; Reeves, K.D.; Cheng, A.-L.; Rabago, D. Dextrose Prolotherapy Versus Lidocaine Injection for Temporomandibular Dysfunction: A Pragmatic Randomized Controlled Trial. J. Altern. Complement. Med. 2020, 26, 1064–1073. [Google Scholar] [CrossRef]
- De Riu, G.; Vaira, L.A.; Carta, E.; Meloni, S.M.; Sembronio, S.; Robiony, M. Bone marrow nucleated cell concentrate autograft in temporomandibular joint degenerative disorders: 1-year results of a randomized clinical trial. J. Cranio-Maxillofac. Surg. 2018, 47, 1728–1738. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, O.; Korkmaz, Y.T.; Tuzuner, T. Comparison of treatment efficacy between hyaluronic acid and arthrocentesis plus hyaluronic acid in internal derangements of temporomandibular joint. J. Cranio-Maxillofac. Surg. 2019, 47, 1720–1727. [Google Scholar] [CrossRef]
- Bergstrand, S.; Ingstad, H.K.; Møystad, A.; Bjørnland, T. Long-term effectiveness of arthrocentesis with and without hyaluronic acid injection for treatment of temporomandibular joint osteoarthritis. J. Oral Sci. 2019, 61, 82–88. [Google Scholar] [CrossRef] [Green Version]
- Isacsson, G.; Schumann, M.; Nohlert, E.; Mejersjö, C.; Tegelberg, Å. Pain relief following a single-dose intra-articular injection of methylprednisolone in the temporomandibular joint arthralgia—A multicentre randomised controlled trial. J. Oral Rehabil. 2018, 46, 5–13. [Google Scholar] [CrossRef]
- Louw, W.F.; Reeves, K.D.; Lam, S.K.; Cheng, A.-L.; Rabago, D. Treatment of Temporomandibular Dysfunction with Hypertonic Dextrose Injection (Prolotherapy): A Randomized Controlled Trial with Long-term Partial Crossover. Mayo Clin. Proc. 2019, 94, 820–832. [Google Scholar] [CrossRef] [Green Version]
- Kutuk, S.G.; Gökçe, G.; Arslan, M.; Özkan, Y.; Kütük, M.; Arikan, O.K. Clinical and Radiological Comparison of Effects of Platelet-Rich Plasma, Hyaluronic Acid, and Corticosteroid Injections on Temporomandibular Joint Osteoarthritis. J. Craniofacial Surg. 2019, 30, 1144–1148. [Google Scholar] [CrossRef]
- Diaz, D.; Dolwick, M.; Freburg-Hoffmeister, D.; Widmer, C. Double-Blind, Randomized, Placebo Controlled Clinical Trial Examining the Efficacy of Steroid Supplementation after TMJ Arthrocentesis. J. Oral Maxillofac. Surg. 2019, 77, e50–e51. [Google Scholar] [CrossRef]
- Yapici-Yavuz, G.; Simsek-Kaya, G.; Ogul, H. A comparison of the effects of Methylprednisolone Acetate, Sodium Hyaluronate and Tenoxicam in the treatment of non-reducing disc displacement of the temporomandibular joint. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e351–e358. [Google Scholar] [CrossRef] [PubMed]
- Ozdamar, S.M.; Alev, B.; Yarat, A. The impact of arthrocentesis with and without hyaluronic acid injection in the prognosis and synovial fluid myeloperoxidase levels of patients with painful symptomatic internal derangement of temporomandibular joint: A randomised controlled clinical trial. J. Oral Rehabil. 2017, 44, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Gorrela, H.; Prameela, J.; Srinivas, G.; Reddy, B.V.B.; Sudhir, M.; Arakeri, G. Efficacy of Temporomandibular Joint Arthrocentesis with Sodium Hyaluronate in the Management of Temporomandibular Joint Disorders: A Prospective Randomized Control Trial. J. Maxillofac. Oral Surg. 2016, 16, 479–484. [Google Scholar] [CrossRef]
- Gurung, T.; Singh, R.K.; Mohammad, S.; Pal, U.S.; Mahdi, A.A.; Kumar, M. Efficacy of arthrocentesis versus arthrocentesis with sodium hyaluronic acid in temporomandibular joint osteoarthritis: A comparison. Natl. J. Maxillofac. Surg. 2017, 8, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiliç, S.C. Does Injection of Corticosteroid After Arthrocentesis Improve Outcomes of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2016, 74, 2151–2158. [Google Scholar] [CrossRef]
- Patel, P.; Idrees, F.; Newaskar, V.; Agrawal, D. Sodium hyaluronate: An effective adjunct in temporomandibular joint arthrocentesis. Oral Maxillofac. Surg. 2016, 20, 405–410. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Chou, J.; Krishnan, D.; Aghaloo, T.; Kahenasa, N.; Smith, J.A.; Giannakopoulos, H. Is Hyaluronic Acid or Corticosteroid Superior to Lactated Ringer Solution in the Short Term for Improving Function and Quality of Life After Arthrocentesis? Part 2. J. Oral Maxillofac. Surg. 2016, 75, 63–72. [Google Scholar] [CrossRef]
- Bouloux, G.F.; Chou, J.; Krishnan, D.; Aghaloo, T.; Kahenasa, N.; Smith, J.A.; Giannakopoulos, H. Is Hyaluronic Acid or Corticosteroid Superior to Lactated Ringer Solution in the Short-Term Reduction of Temporomandibular Joint Pain After Arthrocentesis? Part 1. J. Oral Maxillofac. Surg. 2016, 75, 52–62. [Google Scholar] [CrossRef] [Green Version]
- Kiliç, S.C.; Güngörmüş, M. Is arthrocentesis plus platelet-rich plasma superior to arthrocentesis plus hyaluronic acid for the treatment of temporomandibular joint osteoarthritis: A randomized clinical trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 1538–1544. [Google Scholar] [CrossRef]
- Korkmaz, Y.T.; Altıntas, N.Y.; Korkmaz, F.M.; Candırlı, C.; Coskun, U.; Durmuslar, M.C. Is Hyaluronic Acid Injection Effective for the Treatment of Temporomandibular Joint Disc Displacement with Reduction? J. Oral Maxillofac. Surg. 2016, 74, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Reeves, K.D.; Rabago, D. Dextrose Prolotherapy for Chronic Temporomandibular Pain and Dysfunction: Results of a Pilot-Level Randomized Controlled Study. Arch. Phys. Med. Rehabil. 2016, 97, e139. [Google Scholar] [CrossRef]
- Kiliç, S.C.; Güngörmüş, M.; Sümbüllü, M.A. Is Arthrocentesis Plus Platelet-Rich Plasma Superior to Arthrocentesis Alone in the Treatment of Temporomandibular Joint Osteoarthritis? A Randomized Clinical Trial. J. Oral Maxillofac. Surg. 2015, 73, 1473–1483. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.F.; Ali, H.E.; Elmasry, M.; Khallaf, M.G. Platelet-Rich Plasma Injection as an Effective Treatment for Temporomandibular Joint Osteoarthritis. J. Oral Maxillofac. Surg. 2015, 73, 1706–1713. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Rossi, A.; Arboretti, R.; Bonnini, S.; Stellini, E.; Manfredini, D.; Guarda-Nardini, L.; Rossi, A.; Arboretti, R.; Bonnini, S.; et al. Single- or multiple-session viscosupplementation protocols for temporomandibular joint degenerative disorders: A randomized clinical trial. J. Oral Rehabil. 2015, 42, 521–528. [Google Scholar] [CrossRef] [PubMed]
- Sipahi, A.; Satilmis, T.; Basa, S. Comparative study in patients with symptomatic internal derangements of the temporomandibular joint: Analgesic outcomes of arthrocentesis with or without intra-articular morphine and tramadol. Br. J. Oral Maxillofac. Surg. 2015, 53, 316–320. [Google Scholar] [CrossRef]
- Hancı, M.; Karamese, M.; Tosun, Z.; Aktan, T.M.; Duman, S.; Savaci, N. Intra-articular platelet-rich plasma injection for the treatment of temporomandibular disorders and a comparison with arthrocentesis. J. Cranio-Maxillofac. Surg. 2015, 43, 162–166. [Google Scholar] [CrossRef]
- Tabrizi, R.; Karagah, T.; Arabion, H.; Soleimanpour, M.R.; Soleimanpour, M. Outcomes of Arthrocentesis for the Treatment of Internal Derangement Pain: With or without corticosteroids? J. Craniofacial Surg. 2014, 25, e571–e575. [Google Scholar] [CrossRef]
- Bustaman, F.; Torroni, A.; Samman, N. The efficacy of intra-articular hyaluronic acid in chronic symptomatic temporomandibular joints: A randomized controlled trial. Int. J. Oral Maxillofac. Surg. 2013, 42, 1370. [Google Scholar] [CrossRef]
- Guarda-Nardini, L.; Cadorin, C.; Frizziero, A.; Ferronato, G.; Manfredini, D. Comparison of 2 Hyaluronic Acid Drugs for the Treatment of Temporomandibular Joint Osteoarthritis. J. Oral Maxillofac. Surg. 2012, 70, 2522–2530. [Google Scholar] [CrossRef]
- Daif, E.T. Role of intra-articular ozone gas injection in the management of internal derangement of the temporomandibular joint. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 113, e10–e14. [Google Scholar] [CrossRef] [PubMed]
- Guarda-Nardini, L.; Ferronato, G.; Manfredini, D. Two-needle vs. single-needle technique for TMJ arthrocentesis plus hyaluronic acid injections: A comparative trial over a six-month follow up. Int. J. Oral Maxillofac. Surg. 2012, 41, 506–513. [Google Scholar] [CrossRef] [PubMed]
- Manfredini, D.; Rancitelli, D.; Ferronato, G.; Guarda-Nardini, L. Arthrocentesis with or without additional drugs in temporomandibular joint inflammatory-degenerative disease: Comparison of six treatment protocols. J. Oral Rehabil. 2011, 39, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Huddleston Slater, J.J.; Vos, L.M.; Stroy, L.P.; Stegenga, B. Randomized Trial on the Effectiveness of Dexamethasone in TMJ Arthrocentesis. J. Dent. Res. 2011, 91, 173–178. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Bhardwaj, B. Treatment of Temporomandibular Joint Arthritis with Triamcinolone Acetonide and Hyaluronic Acid Injection: An Observational Study. Indian J. Otolaryngol. Head Neck Surg. 2019, 72, 403–410. [Google Scholar] [CrossRef]
- Giacomello, M.; Mortellaro, C.; Viganoni, C.; Crimella, A.; Fossati, J.; Lauritano, D. PRGF® endoret injections for temporomandibular joint osteoarthritis treatment: A one-year follow-up. J. Biol. Regul. Homeost. Agents 2020, 33, 215–222. [Google Scholar]
- Pihut, M.; Szuta, M.; Ferendiuk, E.; Zeńczak-Więckiewicz, D. Evaluation of Pain Regression in Patients with Temporomandibular Dysfunction Treated by Intra-Articular Platelet-Rich Plasma Injections: A Preliminary Report. BioMed Res. Int. 2014, 2014, 132369. [Google Scholar] [CrossRef] [Green Version]
- Fernández Sanromán, J.; Fernández Ferro, M.; Costas López, A.; Arenaz Bua, J.; López, A. Does injection of plasma rich in growth factors after temporomandibular joint arthroscopy improve outcomes in patients with Wilkes stage IV internal derangement? A randomized prospective clinical study. Int. J. Oral Maxillofac. Surg. 2016, 45, 828–835. [Google Scholar] [CrossRef]
- Fernández-Ferro, M.; Fernández-Sanromán, J.; Blanco-Carrión, A.; Costas-López, A.; López-Betancourt, A.; Arenaz-Bua, J.; Stavaru Marinescu, B. Comparison of intra-articular injection of plasma rich in growth factors versus hyaluronic acid following arthroscopy in the treatment of temporomandibular dysfunction: A randomised prospective study. J. Craniomaxillofac. Surg. 2017, 45, 449–454. [Google Scholar] [CrossRef]
- Rajput, A.; Bansal, V.; Dubey, P.; Kapoor, A. A Comparative Analysis of Intra-articular Injection of Platelet-Rich Plasma and Arthrocentesis in Temporomandibular Joint Disorders. J. Maxillofac. Oral Surg. 2022, 21, 168–175. [Google Scholar] [CrossRef]
- Masuki, H.; Okudera, T.; Watanabe, T.; Suzuki, M.; Nishiyama, K.; Okudera, H.; Nakata, K.; Uematsu, K.; Su, C.-Y.; Kawase, T. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int. J. Implant Dent. 2016, 2, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonelli, A.; Giudice, A.; Muraca, D.; Fortunato, L. Usefulness of advanced-platelet rich fibrin (A-PRF) and injectable-platelet rich fibrin (i-PRF) in the management of a massive medication-related osteonecrosis of the jaw (MRONJ): A 5-years follow-up case report. Indian J. Dent. Res. 2020, 31, 813–818. [Google Scholar] [CrossRef] [PubMed]
- Giudice, A.; Esposito, M.; Bennardo, F.; Brancaccio, Y.; Buti, J.; Fortunato, L. Dental extractions for patients on oral antiplatelet: A within-person randomised controlled trial comparing haemostatic plugs, advanced-platelet-rich fibrin (A-PRF+) plugs, leukocyte- and platelet-rich fibrin (L-PRF) plugs and suturing alone. Int. J. Oral Implant. 2019, 12, 77–87. [Google Scholar]
- Koyuncu, B.; Işık, G.; Yüce, M.; Günbay, S.; Günbay, T. Effect of concentrated growth factors on frequency of alveolar Osteitis following partially-erupted mandibular third molar surgery: A randomized controlled clinical study. BMC Oral Health 2020, 20, 222. [Google Scholar] [CrossRef]
- Brancaccio, Y.; Antonelli, A.; Barone, S.; Bennardo, F.; Fortunato, L.; Giudice, A. Evaluation of local hemostatic efficacy after dental extractions in patients taking antiplatelet drugs: A randomized clinical trial. Clin. Oral Investig. 2020, 25, 1159–1167. [Google Scholar] [CrossRef]
- Lin, S.L.; Tsai, C.C.; Wu, S.L.; Ko, S.Y.; Chiang, W.F.; Yang, J.W. Effect of arthrocentesis plus platelet-rich plasma and platelet-rich plasma alone in the treatment of temporomandibular joint osteoarthritis: A retrospective matched cohort study (A STROBE-compliant article). Medicine 2018, 97, e0477. [Google Scholar] [CrossRef]
- Gopalakrishnan, V.; Nagori, S.A.; Roy Chowdhury, S.K.; Saxena, V. The use of intra-articular analgesics to improve outcomes after temporomandibular joint arthrocentesis: A review. Oral Maxillofac. Surg. 2018, 22, 357–364. [Google Scholar] [CrossRef]
- Al-Kibsi, T.A.; Elsharrawy, E.A.; Ghanem, W.A.; El Sholkamy, M.A.; Tawfik, M.K. Clinical assessment of intra-articular injection of butorphanol in management of temporomandibular joint internal derangement. Egypt. J. Oral Maxillofac. Surg. 2017, 8, 83–87. [Google Scholar] [CrossRef]
- Fayed, H.T.A.M.; Elsharrawy, E.A.; Hamed, T.A.; Abd-Allah, A.-E. Clinical assessment of intra-articular fentanyl injection following arthrocentesis for management of temporomandibular joint internal derangement. Future Dent. J. 2016, 2, 86–90. [Google Scholar] [CrossRef]
- El-Gerby, Y.M.; El-Sholkamy, M.A.; El-Sharrawy, E.A. Comparativestudy between Tramadol Hydrochloride and Sodium Hyaloronatefor Management of Tempromandibular Joint Internal Derangement. Adv. Surg. Sci. 2015, 3, 19–26. [Google Scholar] [CrossRef]
- Escoda, C.G.; Hanna, M.; Montero, A.; Dietrich, T.; Milleri, S.; Giergiel, E.; Zoltán, T.B.; Varassi, G. Tramadol/dexketoprofen (TRAM/DKP) compared with tramadol/paracetamol in moderate to severe acute pain: Results of a randomised, double-blind, placebo and active-controlled, parallel group trial in the impacted third molar extraction pain model (DAVID study). BMJ Open 2019, 9, e023715. [Google Scholar] [CrossRef] [Green Version]
- Sit, R.W.-S.; Reeves, K.D.; Zhong, C.C.; Wong, C.H.L.; Wang, B.; Chung, V.C.-H.; Wong, S.Y.-S.; Rabago, D. Efficacy of hypertonic dextrose injection (prolotherapy) in temporomandibular joint dysfunction: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 14638. [Google Scholar] [CrossRef] [PubMed]
- El Qashty, R.M.N.; Mohamed, N.N.; Radwan, L.R.S.; Ibrahim, F.M.M. Effect of bone marrow mesenchymal stem cells on healing of temporomandibular joints in rats with induced rheumatoid arthritis. Eur. J. Oral Sci. 2018, 126, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Carboni, A.; Amodeo, G.; Perugini, M.; Arangio, P.; Orsini, R.; Scopelliti, D. Temporomandibular Disorders Clinical and Anatomical Outcomes After Fat-Derived Stem Cells Injection. J. Craniofacial Surg. 2019, 30, 793–797. [Google Scholar] [CrossRef] [PubMed]
- Behdin, S.; Alqahtani, H.M.; Bissada, N.F. Therapeutic potential of adipose tissue stem cells for periodontal regeneration. J. Periodontol. 2019, 91, 732–733. [Google Scholar] [CrossRef]
- Sen, S.; Sen, S. Ozone therapy a new vista in dentistry: Integrated review. Med. Gas Res. 2020, 10, 189–192. [Google Scholar] [CrossRef]
- Oshaghi, S.; Haghighat, S. Effectiveness of ozone injection therapy in temporomandibular disorders. Adv. Biomed. Res. 2020, 9, 73. [Google Scholar] [CrossRef]
- de Sire, A.; Marotta, N.; Ferrillo, M.; Agostini, F.; Sconza, C.; Lippi, L.; Respizzi, S.; Giudice, A.; Invernizzi, M.; Ammendolia, A. Oxygen-Ozone Therapy for Reducing Pro-Inflammatory Cytokines Serum Levels in Musculoskeletal and Temporomandibular Disorders: A Comprehensive Review. Int. J. Mol. Sci. 2022, 23, 2528. [Google Scholar] [CrossRef]
- Nowak, Z.; Chęciński, M.; Nitecka-Buchta, A.; Bulanda, S.; Ilczuk-Rypuła, D.; Postek-Stefańska, L.; Baron, S. Intramuscular Injections and Dry Needling within Masticatory Muscles in Management of Myofascial Pain. Systematic Review of Clinical Trials. Int. J. Environ. Res. Public Health 2021, 18, 9552. [Google Scholar] [CrossRef]
- Melis, M.; Di Giosia, M.; Zawawi, K.H. Oral myofunctional therapy for the treatment of temporomandibular disorders: A systematic review. CRANIO 2019, 40, 41–47. [Google Scholar] [CrossRef]
- Montinaro, F.; Nucci, L.; D’Apuzzo, F.; Perillo, L.; Chiarenza, M.C.; Grassia, V. Oral nonsteroidal anti-inflammatory drugs as treatment of joint and muscle pain in temporomandibular disorders: A systematic review. CRANIO 2022, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Al-Moraissi, E.A.; El-Sharkawy, T.M.; Mounair, R.M.; El-Ghareeb, T.I. A systematic review and meta-analysis of the clinical outcomes for various surgical modalities in the management of temporomandibular joint ankylosis. Int. J. Oral Maxillofac. Surg. 2015, 44, 470–482. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Chęciński, M.; Chlubek, D. Non-shortening fracture of the mandibular head resulting in severe mouth opening disorder operated on via the retroauricular approach—A case report. Pomeranian J. Life Sci. 2021, 67, 15–20. [Google Scholar] [CrossRef]
- Farronato, M.; Lucchina, A.G.; Mortellaro, C.; Fama, A.; Galbiati, G.; Farronato, G.; Maspero, C. Bilateral Hyperplasia of the Coronoid Process in Pediatric Patients: What is the Gold Standard for Treatment? J. Craniofacial Surg. 2019, 30, 1058–1063. [Google Scholar] [CrossRef]

| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| Patient description | Temporomandibular joint (TMJ) disease | Animal studies |
| Intervention description | TMJ injection with or without arthrocentesis | TMJ injection as part of a more complex treatment; any additional intervention |
| Comparators description | Placebo or other injectable group with a similar size (+/−10%) and assessed for the same outcomes as the study group or no control group | None |
| Outcomes description | Primary outcome: (1) improvement of mandibular abduction; secondary outcomes: (2) improvement of mandibular lateral mobility, (3) improvement of mandibular protrusion, (4) pain relief of TMJ | None |
| Timeline | Papers published from 1 January 2012 to 3 April 2022 | |
| Settings | Clinical trials | No abstract available |
| Report | PICOS Criterion | Reason for Exclusion |
|---|---|---|
| Cömert Kılıç, S. Does glucosamine, chondroitin sulfate, and methylsulfonylmethane supplementation improve the outcome of temporomandibular joint osteoarthritis management with arthrocentesis plus intra-articular hyaluronic acid injection. A randomized clinical trial. J. Craniomaxillofac. Surg. 2021, 49, 711–718. | Intervention | Oral administration |
| Haghighat, S.; Oshaghi, S. Effectiveness of Ozone Injection Therapy in Temporomandibular Disorders. Adv. Biomed. Res. 2020, 28, 73. | Settings | Review article |
| Sakalys, D.; Dvylys, D.; Simuntis, R,.; Leketas, M. Comparison of Different Intraarticular Injection Substances Followed by Temporomandibular Joint Arthroscopy. J. Craniofac. Surg. 2020, 31, 637–641. | Intervention | Additional intervention |
| Özkan, H.S.; Irkören, S.; Karaca, H.; Yıldırım, T.D.; Çiçek, K.; Tataroğlu, C. Effects of Intra-Articular Platelet-Rich Plasma Administration in Temporomandibular Joint Arthritis: An Experimental Study. Meandros Med. Dent. J. 2018, 19, 198–204 | Patient | Animal studies |
| Buendía-López, D.; Medina-Quirós, M.; Fernández-Villacañas Marín, M.Á. Clinical and radiographic comparison of a single LP-PRP injection, a single hyaluronic acid injection and daily NSAID administration with a 52-week follow-up: a randomized controlled trial. J. Orthop. Traumatol. 2018, 19, 3. | Patient | Wrong joint |
| Campbell, B.K.; Fillingim, R.B.; Lee, S.; Brao, R.; Price, D.D.; Neubert, J.K. Effects of High-Dose Capsaicin on TMD Subjects: A Randomized Clinical Study. JDR Clin. Trans. Res. 2017, 2, 58–65. | Intervention | Transdermal administration |
| Baker, Z.; Eriksson, L.; Englesson Sahlström, L.; Ekberg, E. Questionable effect of lavage for treatment of painful jaw movements at disc displacement without reduction: a 3-year randomised controlled follow-up. J. Oral. Rehabil. 2015, 42, 742–750. | Intervention | Extra-articular administration |
| Sahlström, L.E.; Ekberg, E.C.; List, T.; Petersson, A.; Eriksson, L. Lavage treatment of painful jaw movements at disc displacement without reduction. A randomized controlled trial in a short-term perspective. Int. J. Oral Maxillofac Surg. 2013, 42, 356–363. | Intervention | Extra-articular administration |
| Section 1: Comparative Studies | |||||
|---|---|---|---|---|---|
| Publication Year | First Author | Diagnosis | Intervention | Substance | Comparison Group |
| 2022 | Ghoneim [38] | DDwR | R+A | I-PRF | R * |
| 2021 | Sembronio [39] | ID, OA | R+A | Adipose tissue | R+HA * |
| 2021 | Sembronio [39] | ID, OA | R+A | HA | R+Adipose tissue * |
| 2021 | Karadayi [40] | ID | R+A | I-PRF | R * |
| 2021 | Jacob [41] | DDwR, DDwoR | R+A | PRP | R * |
| 2021 | Jacob [41] | DDwR, DDwoR | R+A | HA | R * |
| 2021 | Singh [42] | ID | R+A | PRP | R * |
| 2020 | Dolwick [43] | P | R+A | CS | R+Placebo * |
| 2020 | Zarate [44] | P | A | Dextrose+Lidocaine | Lidocaine * |
| 2019 | De Riu [45] | DD | R+A | HA | R+Bone marrow * |
| 2019 | De Riu [45] | DD | R+A | Bone marrow | R+HA * |
| 2019 | Yilmaz [46] | ID | A | HA | R+HA * |
| 2019 | Yilmaz [46] | ID | R+A | HA | HA * |
| 2019 | Bergstrand [47] | OA | R+A | HA | R * |
| 2019 | Isacsson [48] | P | A | CS | Placebo * |
| 2019 | Louw [49] | P | A | Dextrose+Lidocaine | Lidocaine * |
| 2019 | Gokçe Kutuk [50] | P | A | HA | CS * |
| 2019 | Gokçe Kutuk [50] | P | A | CS | HA * |
| 2019 | Gokçe Kutuk [50] | P | A | PRP | CS * |
| 2019 | Diaz [51] | P | R+A | CS | R+Placebo * |
| 2018 | Yapici-Yavuz [52] | DDwoR | R+A | CS | R * |
| 2018 | Yapici-Yavuz [52] | DDwoR | R+A | HA | R * |
| 2018 | Yapici-Yavuz [52] | DDwoR | R+A | Tenoxicam | R * |
| 2017 | Ozdamar [53] | ID | R+A | HA | R * |
| 2017 | Gorrela [54] | DDwR, DDwoR | R+A | HA | R * |
| 2017 | Gurung [55] | OA | R+A | HA | R * |
| 2016 | Cömert Kiliç [56] | OA | R+A | CS | R * |
| 2016 | Patel [57] | ID | R+A | HA | R * |
| 2016 | Bouloux [58,59] | P | R+A | CS | R * |
| 2016 | Bouloux [58,59] | P | R+A | HA | R * |
| 2016 | Cömert Kiliç [60] | OA | R+A | PRP | R+HA * |
| 2016 | Korkmaz [61] | DDwR | A | HA | Splint therapy * |
| 2016 | Lam [62] | P | A | Dextrose+Lidocaine | Lidocaine * |
| 2015 | Cömert Kiliç [63] | OA | R+A | PRP | R * |
| 2015 | Hegab [64] | OA | A | HA | PRP * |
| 2015 | Hegab [64] | OA | A | PRP | HA * |
| 2015 | Guarda-Nardini [65] | DD | A | HA | R+HA * |
| 2015 | Sipahi [66] | ID | R+A | Morphine | R+Placebo * |
| 2015 | Sipahi [66] | ID | R+A | Tramadol | R+Placebo * |
| 2014 | Hancı [67] | DDwR | A | PRP | R * |
| 2014 | Tabrizi [68] | ID | R+A | CS | R * |
| 2013 | Bustaman [69] | OA | A | HA | Placebo * |
| 2012 | Guarda-Nardini [70] | DD | R+A | HA | HA* |
| 2012 | Daif [71] | ID | A | Ozone gas | Oral drugs * |
| 2012 | Guarda-Nardini [72] | DD | R+A | HA | HA * |
| 2012 | Manfredini [73] | DD | R+A | CS | R * |
| 2012 | Manfredini [73] | DD | R+A | HA | R * |
| 2012 | Huddleston Slater [74] | P | R+A | CS | R * |
| Section 2: before-and-after studies | |||||
| Publication | First author | Diagnosis | Intervention | Substance | |
| 2020 | Singh [75] | OA | A | CS+HA | |
| 2020 | Sikora [6] | P | A | HA | |
| 2019 | Giacomello [76] | OA | A | PRGF | |
| 2014 | Pihut [77] | P | A | PRP | |
| First Author | Domain 1 | Domain 2 | Domain 3 | Domain 4 | Domain 5 | Overall |
|---|---|---|---|---|---|---|
| Sembronio [39] | Low | Moderate | Low | Low | Low | Moderate |
| Zarate [44] | Low | Low | Low | Low | Low | Low |
| De Riu [45] | Low | Moderate | Low | Low | Low | Moderate |
| Louw [49] | Low | Low | Low | Low | Low | Low |
| Yapici-Yavuz [52] | Low | Moderate | Low | Low | Low | Moderate |
| Lam [62] | Low | Low | Low | Low | Low | Low |
| Daif [71] | Low | Moderate | Low | Low | Low | Moderate |
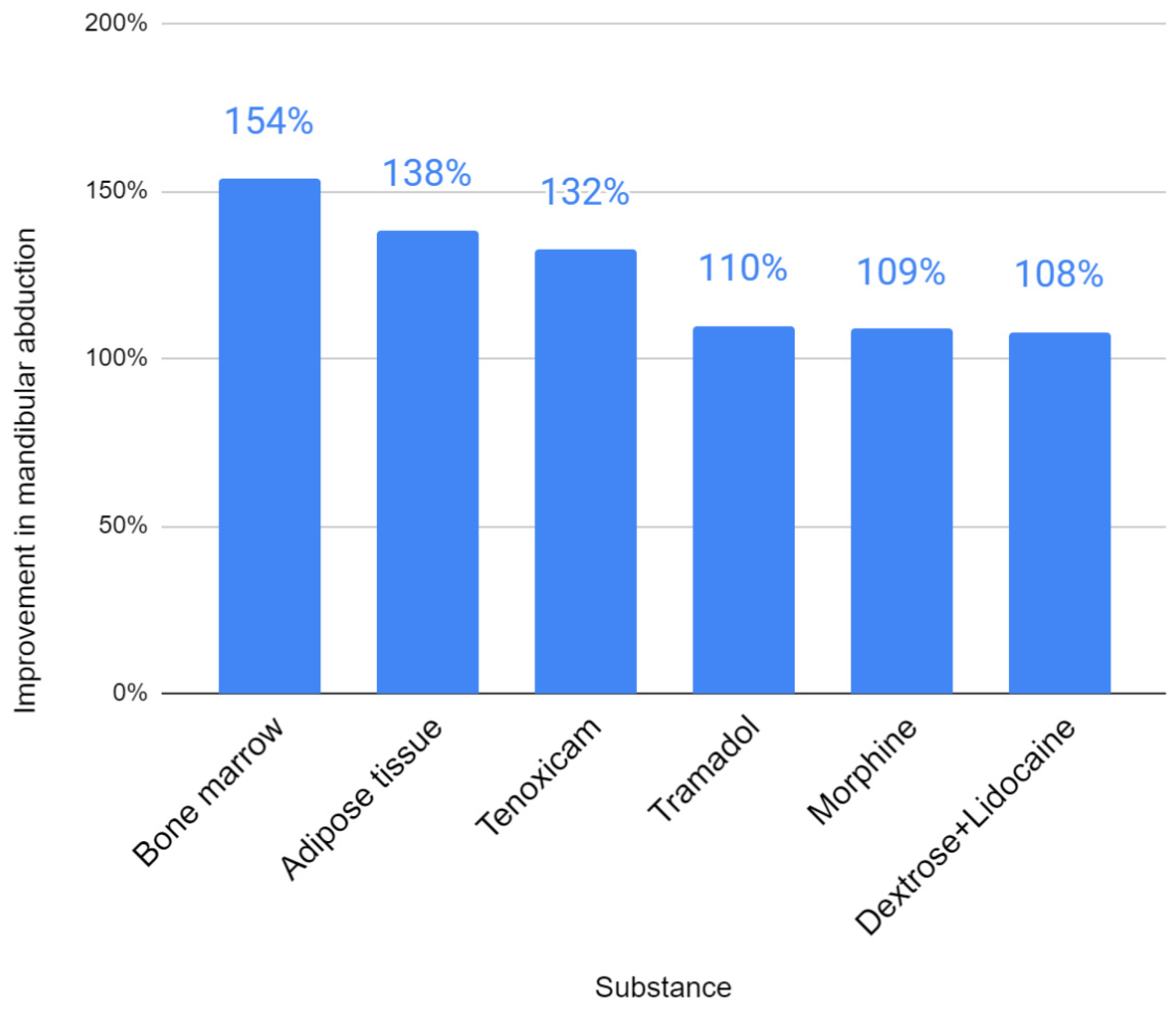
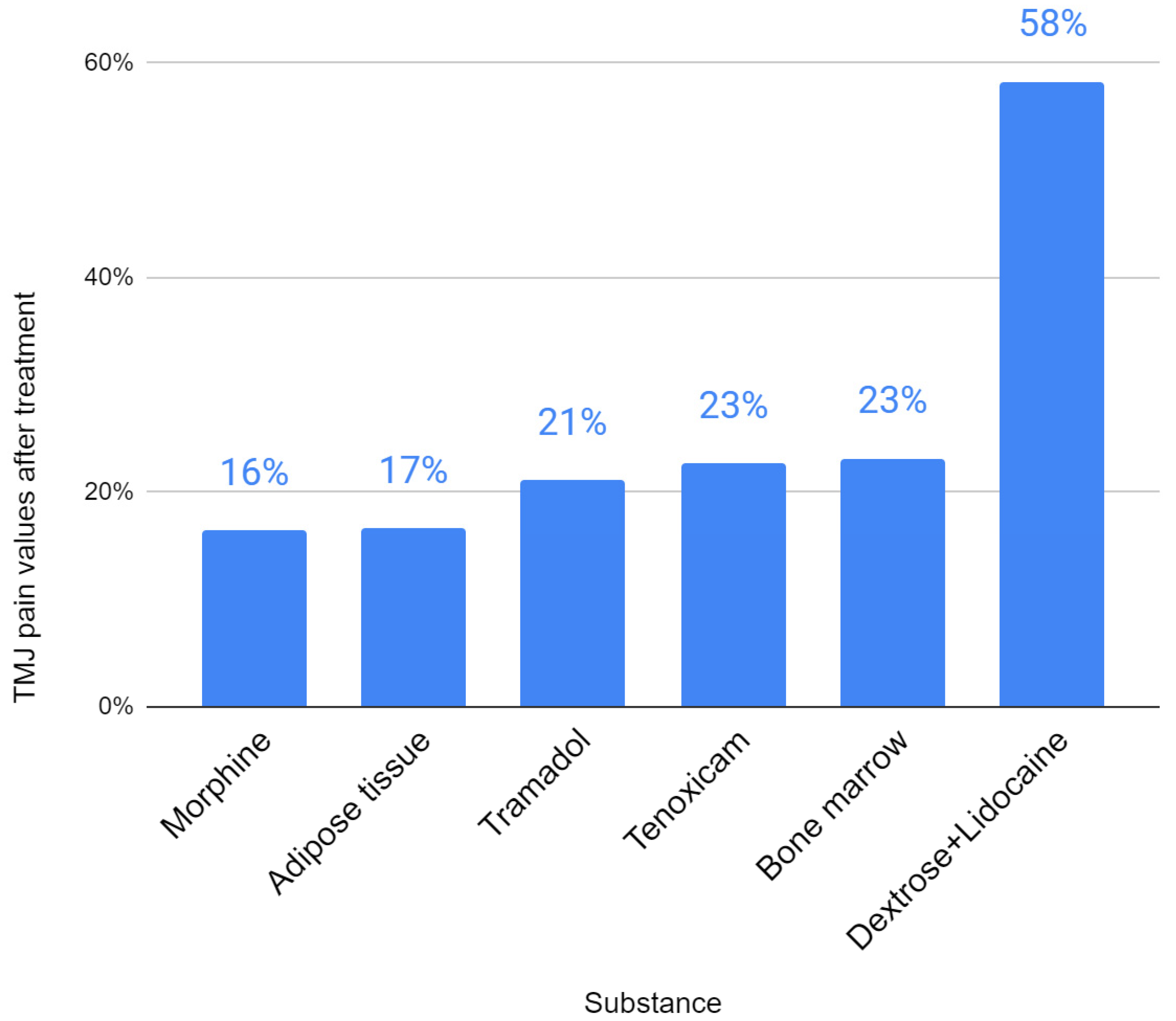
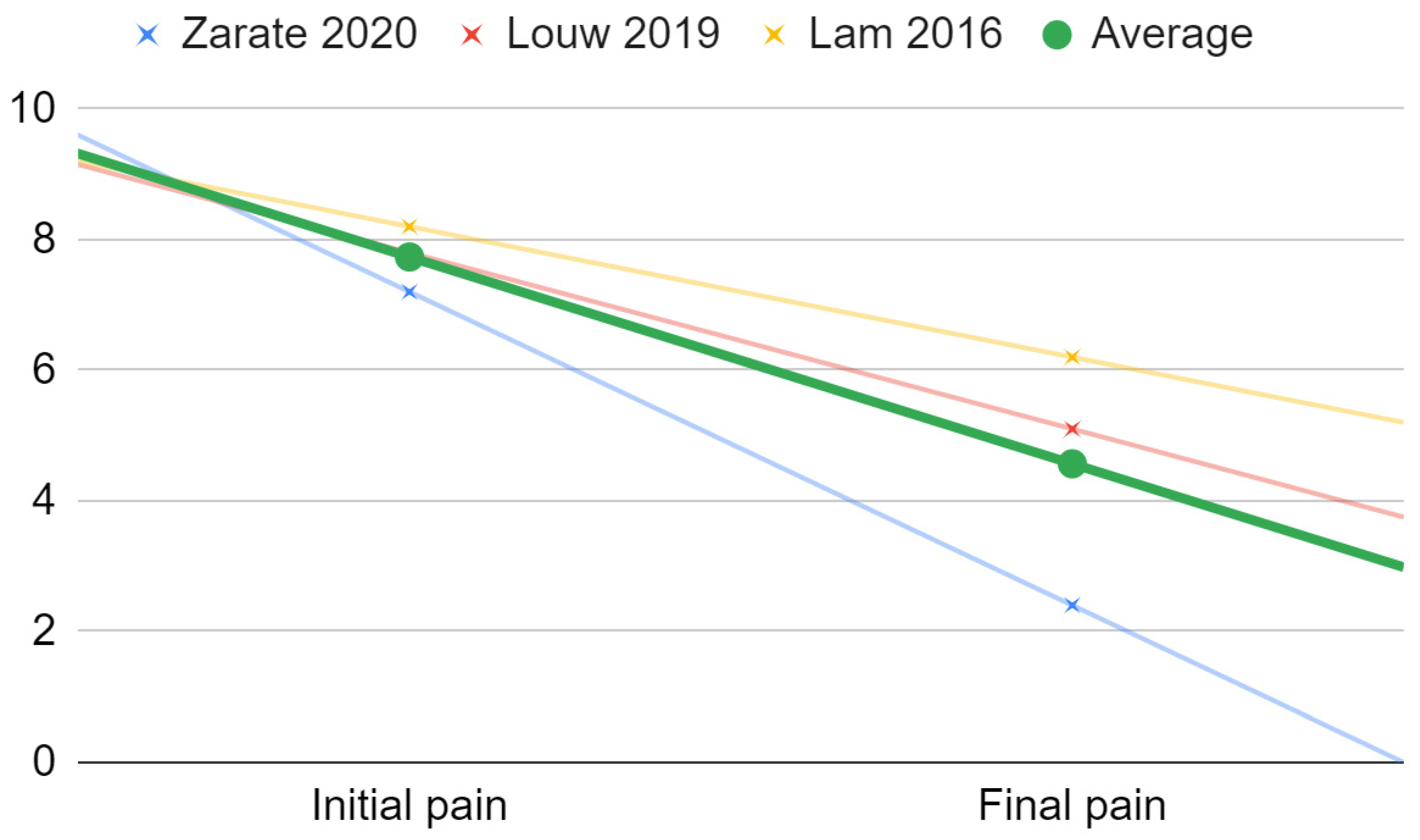
| First Author | Substance | Initial Abduction | Final Abduction | Initial Pain | Final Pain | Abduction Improvement | Pain Improvement |
|---|---|---|---|---|---|---|---|
| Sembronio [39] | Adipose tissue | 30.7 | 42.4 | 7.2 | 1.2 | 138% | 17% |
| Zarate [44] | Dextrose+Lidocaine | 38.7 | 43.4 | 7.2 | 2.4 | 112% | 33% |
| De Riu [45] | Bone marrow | 22 | 33.8 | 8.2 | 1.9 | 154% | 23% |
| Louw [49] | Dextrose+Lidocaine | 43.4 | 45 | 7.8 | 5.1 | 104% | 65% |
| Yapici-Yavuz [52] | Tenoxicam | 25.3 | 33.5 | 7.5 | 1.7 | 132% | 23% |
| Lam [62] | Dextrose+Lidocaine | 8.2 | 6.2 | 76% | |||
| Sipahi [66] | Morphine | 37.7 | 41 | 7.3 | 1.2 | 109% | 16% |
| Sipahi [66] | Tramadol | 34.6 | 38 | 7.1 | 1.5 | 110% | 21% |
| Daif [71] | Ozone gas | No data | No data | No data | No data | No data | No data |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chęciński, M.; Chęcińska, K.; Nowak, Z.; Sikora, M.; Chlubek, D. Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used. J. Clin. Med. 2022, 11, 2305. https://doi.org/10.3390/jcm11092305
Chęciński M, Chęcińska K, Nowak Z, Sikora M, Chlubek D. Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used. Journal of Clinical Medicine. 2022; 11(9):2305. https://doi.org/10.3390/jcm11092305
Chicago/Turabian StyleChęciński, Maciej, Kamila Chęcińska, Zuzanna Nowak, Maciej Sikora, and Dariusz Chlubek. 2022. "Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used" Journal of Clinical Medicine 11, no. 9: 2305. https://doi.org/10.3390/jcm11092305
APA StyleChęciński, M., Chęcińska, K., Nowak, Z., Sikora, M., & Chlubek, D. (2022). Treatment of Mandibular Hypomobility by Injections into the Temporomandibular Joints: A Systematic Review of the Substances Used. Journal of Clinical Medicine, 11(9), 2305. https://doi.org/10.3390/jcm11092305









