The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Group
2.2. Anthropometric Data
2.3. Sleep Examination
2.4. Laboratory Measurements
2.5. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population
3.2. Obstructive Sleep Apnea Assessment
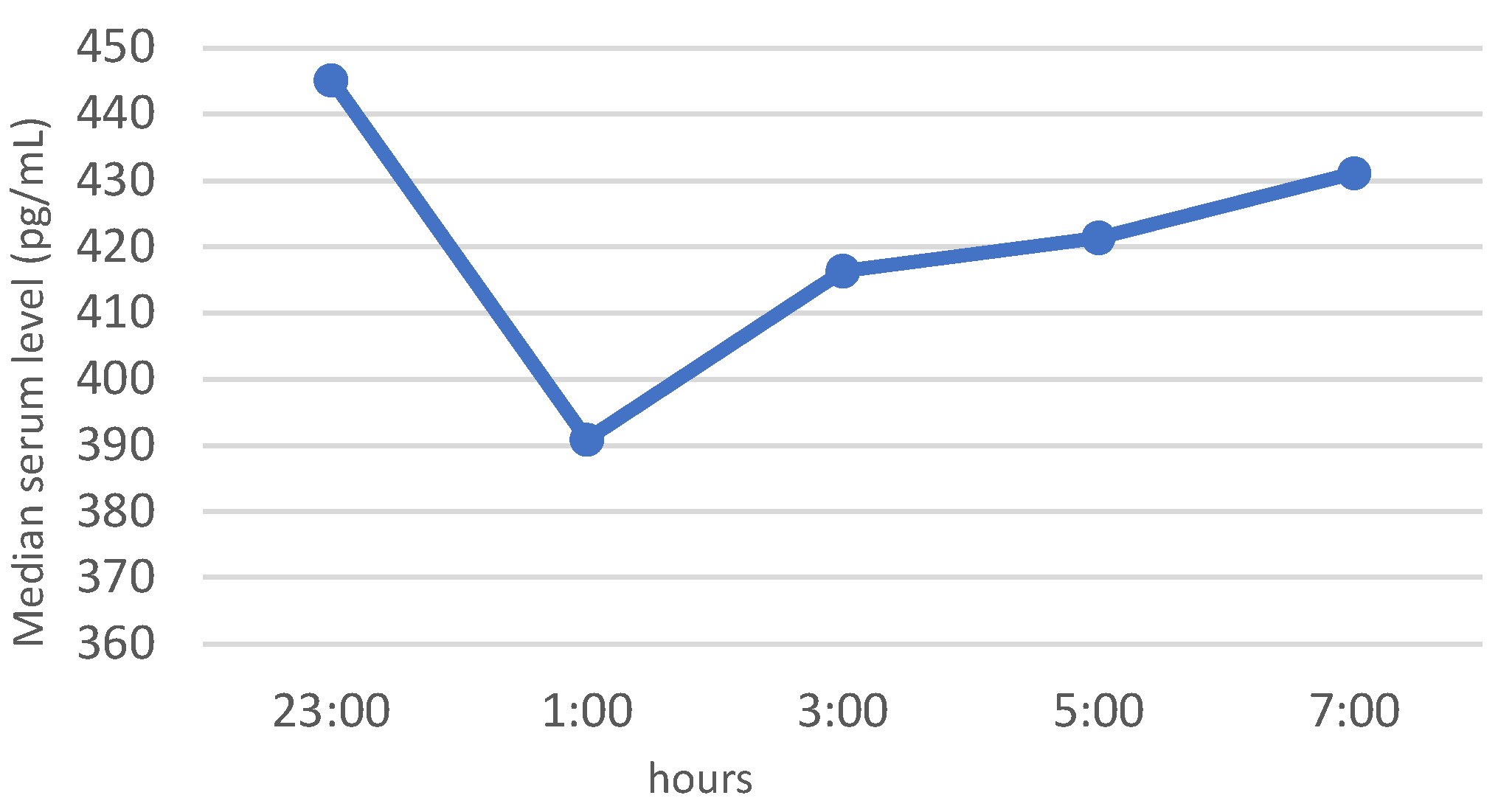
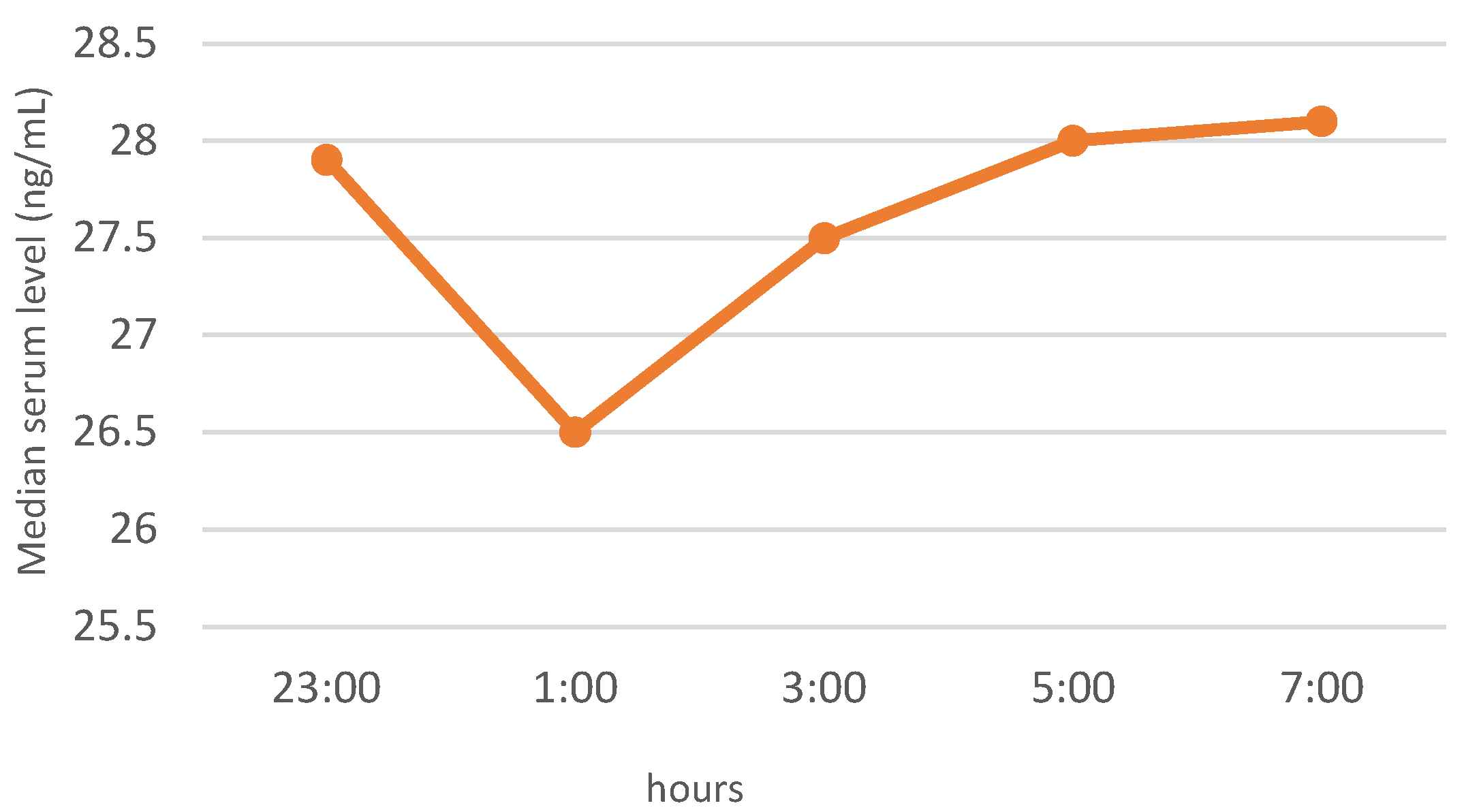
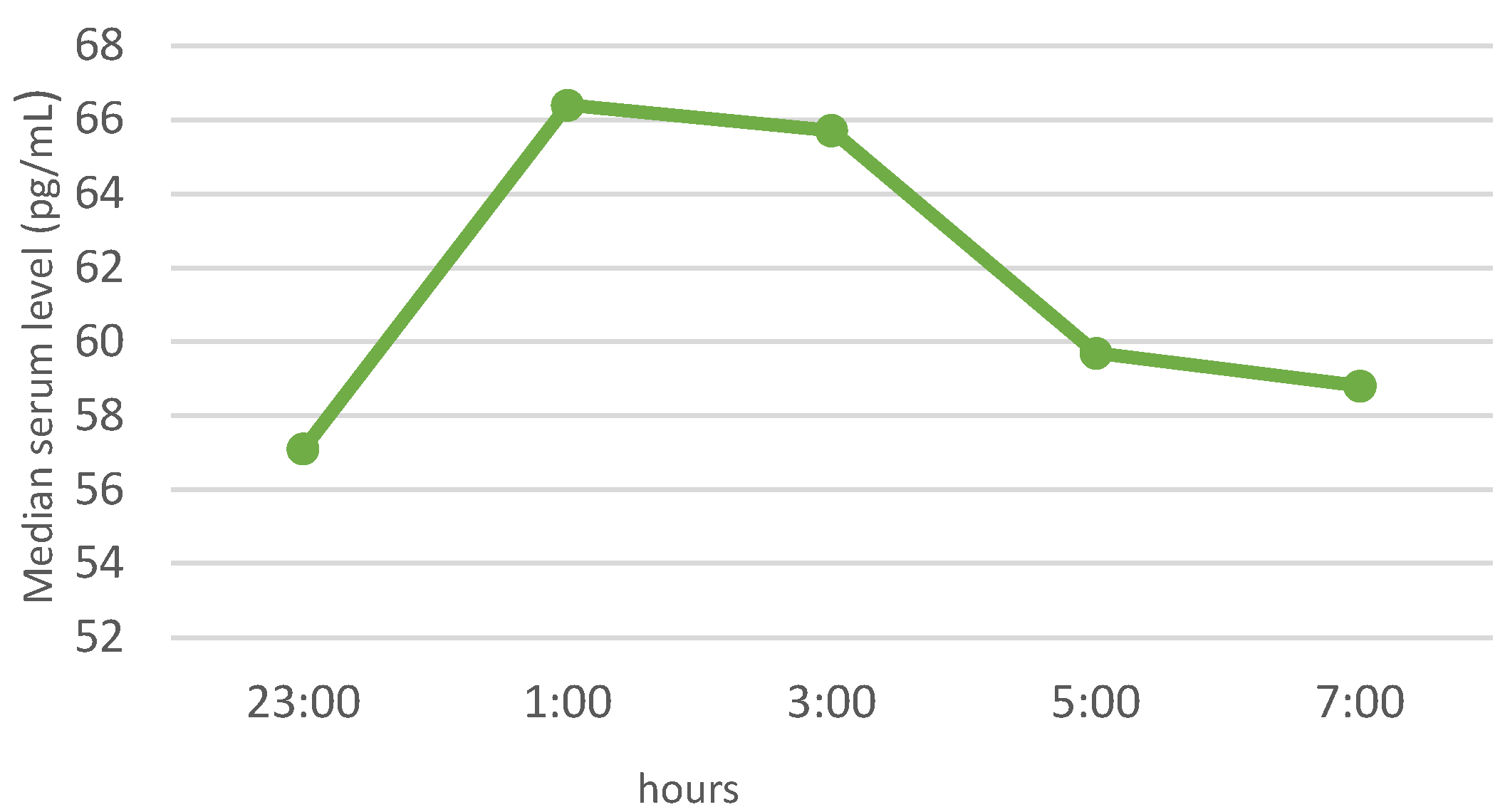
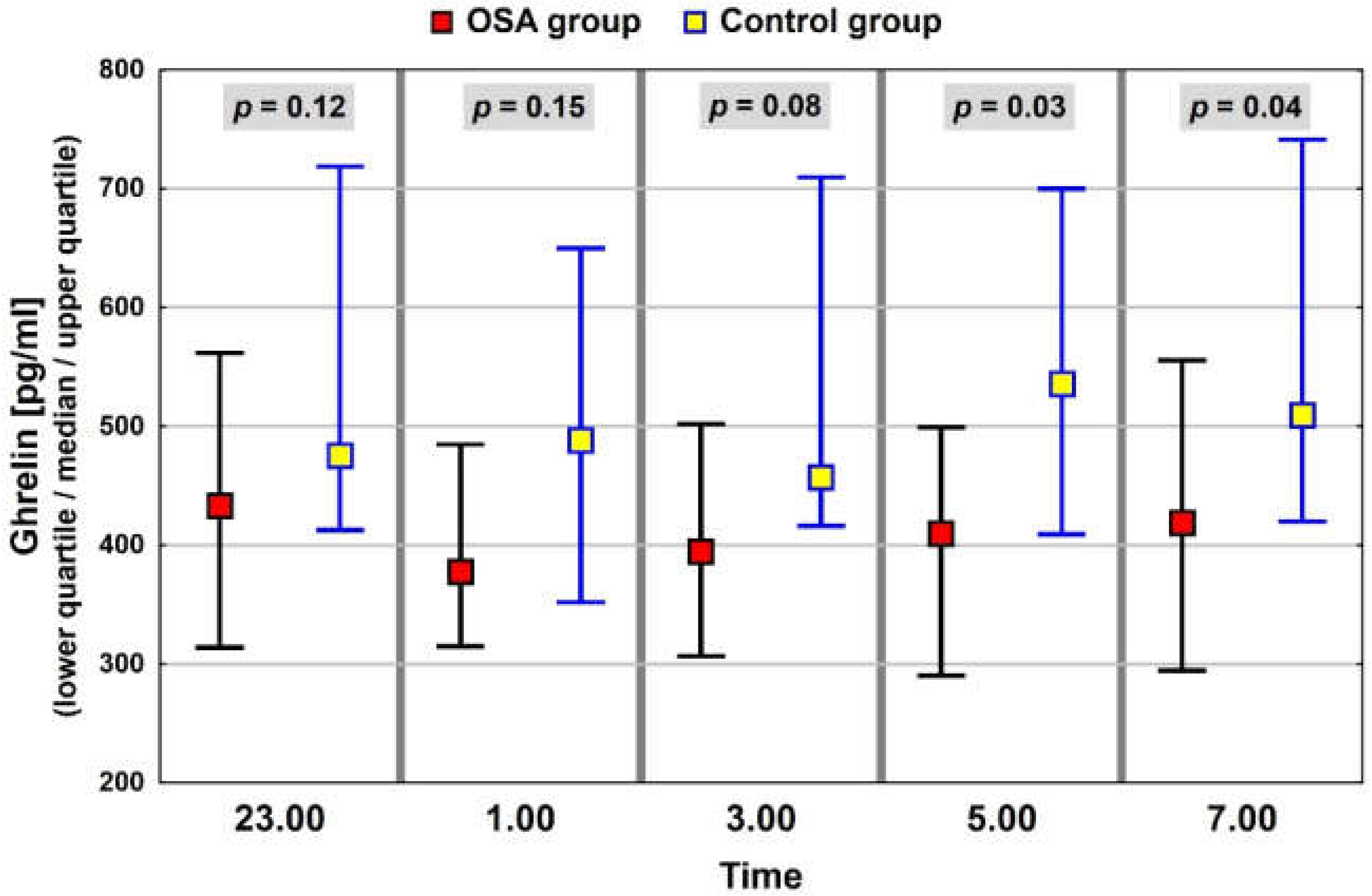
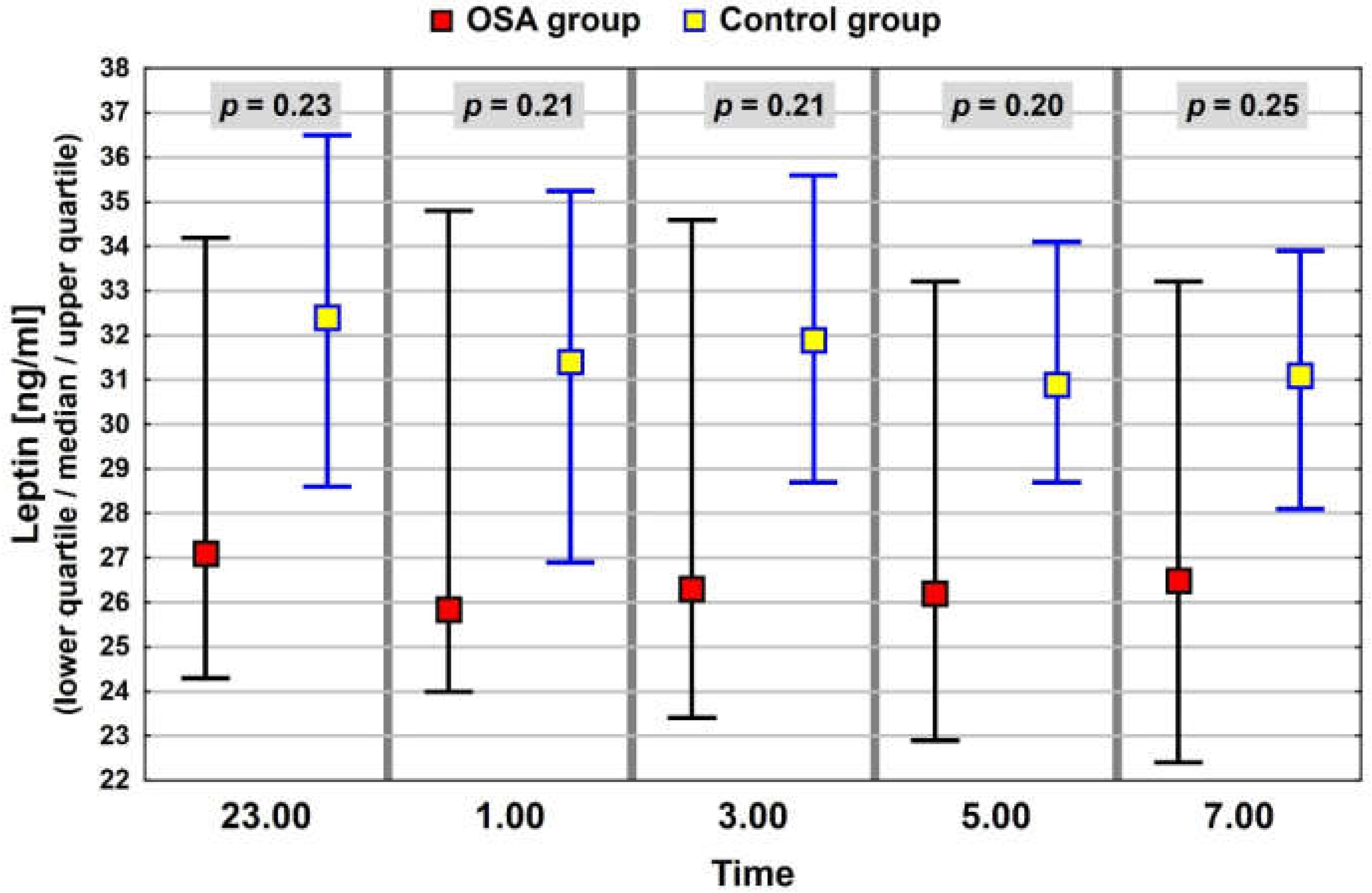
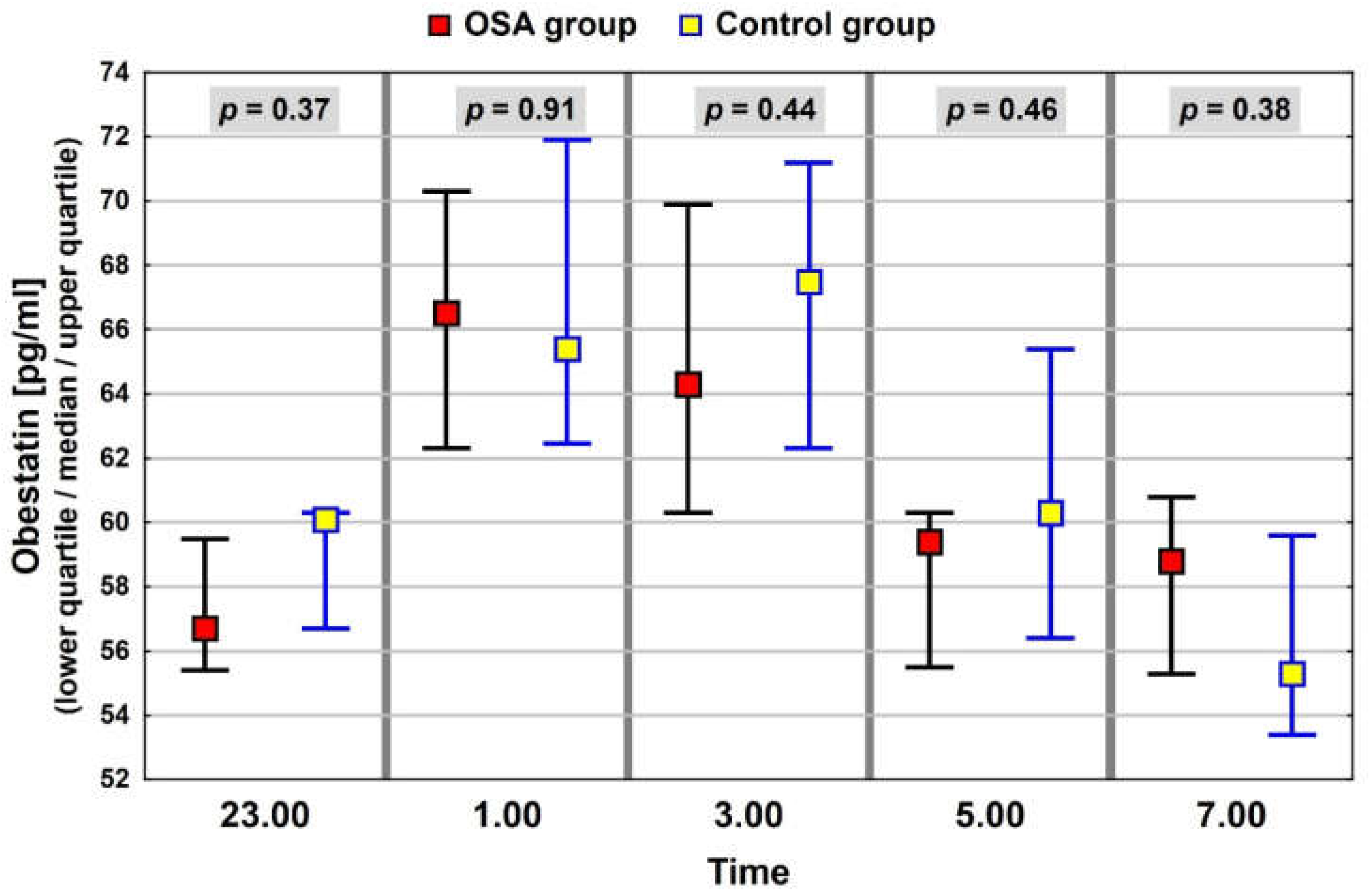
3.3. Neuropeptide Profile Evaluation
3.4. Association of Anthropometric Measurements with Sleep Disorder Parameters
3.5. Association of Gender and Anthropometric Measurements with Neuropeptide Levels
3.6. Association of Sleep Disorder Parameters with Neuropeptide Levels
3.7. Multivariate Analysis of the Association between BMI, Sleep Disorder Parameters, and Ghrelin Levels
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Centers for Disease Control and Prevention. Overweight & Obesity. 2021. Available online: https://www.cdc.gov/obesity/data/adult.html (accessed on 2 March 2022).
- World Health Organization. Obesity and Overweight. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 January 2022).
- Olszanecka-Glinianowicz, M.; Ostrowska, L.; Płaczkiewicz-Jankowska, E. Otyłość. In Interna Szczeklika; Medycyna Praktyczna; Polski Instytut Evidence Based Medicine: Kraków, Poland, 2021; pp. 2745–2762. [Google Scholar]
- Buysse, D.J. Sleep Health: Can We Define It? Does It Matter? Sleep 2014, 37, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Cain, S.W.; Filtness, A.J.; Phillips, C.L.; Anderson, C. Enhanced preference for high-fat foods following a simulated night shift. Scand. J. Work. Environ. Health 2015, 41, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Salzano, G.; Maglitto, F.; Bisogno, A.; Vaira, L.A.; De Riu, G.; Cavaliere, M.; di Stadio, A.; Mesolella, M.; Motta, G.; Ionna, F.; et al. Obstructive sleep apnoea/hypopnoea syndrome: Relationship with obesity and management in obese patients. Acta Otorhinolaryngol. Ital. 2021, 41, 120–130. [Google Scholar] [CrossRef] [PubMed]
- St-Onge, M.-P.; McReynolds, A.; Trivedi, Z.B.; Roberts, A.L.; Sy, M.; Hirsch, J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am. J. Clin. Nutr. 2012, 95, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Ralls, F.M.; Grigg-Damberger, M. Roles of gender, age, race/ethnicity, and residential socioeconomics in obstructive sleep apnea syndromes. Curr. Opin. Pulm. Med. 2012, 18, 568–573. [Google Scholar] [CrossRef]
- Rodrigues, G.D.; Fiorelli, E.M.; Furlan, L.; Montano, N.; Tobaldini, E. Obesity and sleep disturbances: The “chicken or the egg” question. Eur. J. Intern. Med. 2021, 92, 11–16. [Google Scholar] [CrossRef]
- Senaratna, C.V.; Perret, J.L.; Lodge, C.J.; Lowe, A.J.; Campbell, B.E.; Matheson, M.C.; Hamilton, G.S.; Dharmage, S.C. Prevalence of obstructive sleep apnea in the general population: A systematic review. Sleep Med. Rev. 2017, 34, 70–81. [Google Scholar] [CrossRef]
- Young, T.; Peppard, P.E.; Taheri, S. Excess weight and sleep-disordered breathing. J. Appl. Physiol. 2005, 99, 1592–1599. [Google Scholar] [CrossRef]
- Drager, L.F.; Lopes, H.; Maki-Nunes, C.; Trombetta, I.C.; Toschi-Dias, E.; Alves, M.J.N.N.; Fraga, R.F.; Jun, J.C.; Negrão, C.E.; Krieger, E.M.; et al. The Impact of Obstructive Sleep Apnea on Metabolic and Inflammatory Markers in Consecutive Patients with Metabolic Syndrome. PLoS ONE 2010, 5, e12065. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Dempsey, J.; Skatrud, J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA 2000, 284, 3015–3021. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A Preprandial Rise in Plasma Ghrelin Levels Suggests a Role in Meal Initiation in Humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Puzianowska-Kuznicka, M. Neuropeptydy regulujące homeostazę energetyczną. In Endokrynologia kliniczna (Red. Milewicz A.); Polskie Towarzystwo Endokrynologiczne: Wroclaw, Poland, 2012; Volume III. [Google Scholar]
- le Roux, C.W.; Patterson, M.; Vincent, R.P.; Hunt, C.; Ghatei, M.A.; Bloom, S.R. Postprandial Plasma Ghrelin Is Suppressed Proportional to Meal Calorie Content in Normal-Weight but Not Obese Subjects. J. Clin. Endocrinol. Metab. 2005, 90, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating Ghrelin Levels Are Decreased in Human Obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-De-La-Torre, M.; Mediano, O.; Barceló, A.; Piérola, J.; De La Peña, M.; Esquinas, C.; Miro, A.; Durán-Cantolla, J.; Agusti, A.G.; Capote, F.; et al. The influence of obesity and obstructive sleep apnea on metabolic hormones. Sleep Breath. 2012, 16, 649–656. [Google Scholar] [CrossRef]
- Mashaqi, S.; Badr, M.S. The Impact of Obstructive Sleep Apnea and Positive Airway Pressure Therapy on Metabolic Peptides Regulating Appetite, Food Intake, Energy Homeostasis, and Systemic Inflammation: A Literature Review. J. Clin. Sleep Med. 2019, 15, 1037–1050. [Google Scholar] [CrossRef]
- Ursavas, A.; Ilcol, Y.O.; Nalci, N.; Karadag, M.; Ege, E. Ghrelin, leptin, adiponectin, and resistin levels in sleep apnea syndrome: Role of obesity. Ann. Thorac. Med. 2010, 5, 161–165. [Google Scholar] [CrossRef]
- Garcia, J.M.; Sharafkhaneh, H.; Hirshkowitz, M.; Elkhatib, R.; Sharafkhaneh, A. Weight and metabolic effects of cpap in obstructive sleep apnea patients with obesity. Respir. Res. 2011, 12, 80. [Google Scholar] [CrossRef]
- Chihara, Y.; Akamizu, T.; Azuma, M.; Murase, K.; Harada, Y.; Tanizawa, K.; Handa, T.; Oga, T.; Mishima, M.; Chin, K. Among Metabolic Factors, Significance of Fasting and Postprandial Increases in Acyl and Desacyl Ghrelin and the Acyl/Desacyl Ratio in Obstructive Sleep Apnea before and after Treatment. J. Clin. Sleep Med. 2015, 11, 895–905. [Google Scholar] [CrossRef]
- Weber, S.; Pissulin, F.D.; Beneti, R.; Pacagnelli, F.L. Is OSA an important risk factor for metabolic dysregulation in obese COPD patients. Eur. Respir. J. 2018, 52, PA2501. [Google Scholar]
- Liu, W.; Yue, H.; Zhang, J.; Pu, J.; Yu, Q. Effects of plasma ghrelin, obestatin, and ghrelin/obestatin ratio on blood pressure circadian rhythms in patients with obstructive sleep apnea syndrome. Chin. Med. J. 2014, 127, 850–855. [Google Scholar]
- Obradovic, M.; Sudar-Milovanovic, E.; Soskic, S.; Essack, M.; Arya, S.; Stewart, A.J.; Gojobori, T.; Isenovic, E.R. Leptin and Obesity: Role and Clinical Implication. Front. Endocrinol. 2021, 12, 585–887. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Polotsky, V.Y. Leptin and Leptin Resistance in the Pathogenesis of Obstructive Sleep Apnea: A Possible Link to Oxidative Stress and Cardiovascular Complications. Oxidative Med. Cell. Longev. 2018, 2018, 5137947. [Google Scholar] [CrossRef] [PubMed]
- Zirlik, S.; Hauck, T.; Fuchs, F.S.; Neurath, M.F.; Konturek, P.C.; Harsch, I.A. Leptin, Obestatin and Apelin levels in patients with obstructive sleep apnoea syndrome. Med. Sci. Monit. 2011, 17, CR159–CR164. [Google Scholar] [CrossRef] [PubMed]
- De Santis, S.; Cambi, J.; Bellussi, L.; Tatti, P.; Passali, D. Changes in ghrelin, leptin and pro-inflammatory cytokines after therapy in Obstructive Sleep Apnea Syndrome (OSAS) patients. Otolaryngol. Polska 2015, 69, 1–8. [Google Scholar] [CrossRef]
- Martins, C.; Kjelstrup, L.; Mostad, I.L.; Kulseng, B. Impact of Sustained Weight Loss Achieved through Roux-en-Y Gastric Bypass or a Lifestyle Intervention on Ghrelin, Obestatin, and Ghrelin/Obestatin Ratio in Morbidly Obese Patients. Obes. Surg. 2011, 21, 751–758. [Google Scholar] [CrossRef][Green Version]
- Pan, W.; Tu, H.; Kastin, A.J. Differential BBB interactions of three ingestive peptides: Obestatin, ghrelin, and adiponectin. Peptides 2006, 27, 911–916. [Google Scholar] [CrossRef]
- Lacquaniti, A.; Donato, V.; Chirico, V.; Buemi, A.; Buemi, M. Obestatin: An Interesting but Controversial Gut Hormone. Ann. Nutr. Metab. 2011, 59, 193–199. [Google Scholar] [CrossRef]
- Pardak, P.; Filip, R.; Woliński, J.; Krzaczek, M. Associations of Obstructive Sleep Apnea, Obestatin, Leptin, and Ghrelin with Gastroesophageal Reflux. J. Clin. Med. 2021, 10, 5195. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep Med. 2017, 13, 479–504. [Google Scholar] [CrossRef]
- Park, J.G.; Ramar, K.; Olson, E.J. Updates on Definition, Consequences, and Management of Obstructive Sleep Apnea. Mayo Clin. Proc. 2011, 86, 549–555. [Google Scholar] [CrossRef]
- Palla, A.; Digiorgio, M.; Carpenè, N.; Rossi, G.; D’Amico, I.; Santini, F.; Pinchera, A. Sleep Apnea in Morbidly Obese Patients: Prevalence and Clinical Predictivity. Respiration 2009, 78, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Peppard, P.E.; Young, T.; Barnet, J.H.; Palta, M.; Hagen, E.W.; Hla, K.M. Increased Prevalence of Sleep-Disordered Breathing in Adults. Am. J. Epidemiol. 2013, 177, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Lin, L.; Austin, D.; Young, T.; Mignot, E. Short Sleep Duration Is Associated with Reduced Leptin, Elevated Ghrelin, and Increased Body Mass Index. PLoS Med. 2004, 1, e62. [Google Scholar] [CrossRef] [PubMed]
- Pardak, P.; Paprzycki, P.; Panasiuk, L. Ocena przydatności wybranych elementów badania lekarskiego w prognozowaniu stopnia ciężkości obturacyjnego bezdechu sennego. Med. Ogólna I Nauk. O Zdrowiu 2014, 20, 282–286. [Google Scholar] [CrossRef]
- Leibel, R.L. The Role of Leptin in the Control of Body Weight. Nutr. Rev. 2002, 60, S15–S19. [Google Scholar] [CrossRef]
- Dashti, H.S.; Scheer, F.A.; Jacques, P.F.; Lamon-Fava, S.; Ordovás, J.M. Short Sleep Duration and Dietary Intake: Epidemiologic Evidence, Mechanisms, and Health Implications. Adv. Nutr. 2015, 6, 648–659. [Google Scholar] [CrossRef]
- Van der Lely, A.J.; Tschöp, M.; Heiman, M.L.; Ghigo, E. Biological, Physiological, Pathophysiological, and Pharmacological Aspects of Ghrelin. Endocr. Rev. 2004, 25, 426–457. [Google Scholar] [CrossRef]
- Shiiya, T.; Nakazato, M.; Mizuta, M.; Date, Y.; Mondal, M.S.; Tanaka, M.; Nozoe, S.I.; Hosoda, H.; Kangawa, K.; Matsukura, S. Plasma Ghrelin Levels in Lean and Obese Humans and the Effect of Glucose on Ghrelin Secretion. J. Clin. Endocrinol. Metab. 2002, 87, 240–244. [Google Scholar] [CrossRef]
- Spiegel, K.; Tasali, E.; Leproult, R.; Scherberg, N.; Van Cauter, E. Twenty-Four-Hour Profiles of Acylated and Total Ghrelin: Relationship with Glucose Levels and Impact of Time of Day and Sleep. J. Clin. Endocrinol. Metab. 2011, 96, 486–493. [Google Scholar] [CrossRef]
- Anderwald, C.; Brabant, G.; Bernroider, E.; Horn, R.; Brehm, A.; Waldhaüsl, W.; Roden, M. Insulin-Dependent Modulation of Plasma Ghrelin and Leptin Concentrations Is Less Pronounced in Type 2 Diabetic Patients. Diabetes 2003, 52, 1792–1798. [Google Scholar] [CrossRef]
- Yildiz, B.O.; Suchard, M.A.; Wong, M.-L.; McCann, S.M.; Licinio, J. Alterations in the dynamics of circulating ghrelin, adiponectin, and leptin in human obesity. Proc. Natl. Acad. Sci. USA 2004, 101, 10434–10439. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.C.; Pizzolanti, G.; Torregrossa, V.; Misiano, G.; Milano, S.; Giordano, C. Visceral Adiposity Index (VAI) Is Predictive of an Altered Adipokine Profile in Patients with Type 2 Diabetes. PLoS ONE 2014, 9, e91969. [Google Scholar]
- Korek, E.; Krauss, H.; Gibas-Dorna, M.; Kupsz, J.; Piątek, M.; Piątek, J. Fasting and postprandial levels of ghrelin, leptin and insulin in lean, obese and anorexic subjects. Gastroenterol. Rev. 2013, 6, 383–389. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Jiang, Y.; Wang, G.; Meng, M.; Zhu, Q.; Mei, H.; Liu, S.; Jiang, F. Associations of short sleep duration with appetite-regulating hormones and adipokines: A systematic review and meta-analysis. Obes. Rev. 2020, 21, e13051. [Google Scholar] [CrossRef] [PubMed]
- Spiegel, K.; Tasali, E.; Penev, P.; Cauter, E.V. Brief Communication: Sleep Curtailment in Healthy Young Men Is Associated with Decreased Leptin Levels, Elevated Ghrelin Levels, and Increased Hunger and Appetite. Ann. Intern. Med. 2004, 141, 846. [Google Scholar] [CrossRef] [PubMed]
- Harsch, I.; Konturek, P.; Koebnick, C.; Kuehnlein, P.; Fuchs, F.; Pour Schahin, S.; Wiest, G.; Hahn, E.; Lohmann, T.; Ficker, J. Leptin and ghrelin levels in patients with obstructive sleep apnoea: Effect of CPAP treatment. Eur. Respir. J. 2003, 22, 251–257. [Google Scholar] [CrossRef]
- Papaioannou, I.; Patterson, M.; Twigg, G.L.; Vazir, A.; Ghatei, M.; Morrell, M.J.; Polkey, M.I. Lack of Association Between Impaired Glucose Tolerance and Appetite Regulating Hormones in Patients with Obstructive Sleep Apnea. J. Clin. Sleep Med. 2011, 07, 486–492. [Google Scholar] [CrossRef]
- Ulukavak Ciftci, T.; Kokturk, O.; Bukan, N.; Bilgihan, A. Leptin and Ghrelin Levels in Patients with Obstructive Sleep Apnea Syndrome. Respiration 2005, 72, 395–401. [Google Scholar] [CrossRef]
- Atalayer, D.; Gibson, C.; Konopacka, A.; Geliebter, A. Ghrelin and eating disorders. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2013, 40, 70–82. [Google Scholar] [CrossRef]
- Hernandez, D.; Mehta, N.; Geliebter, A. Meal-Related Acyl and Des-Acyl Ghrelin and Other Appetite-Related Hormones in People with Obesity and Binge Eating. Obesity 2019, 27, 629–635. [Google Scholar] [CrossRef]
- Arnardottir, E.S.; Maislin, G.; Jackson, N.; Schwab, R.J.; Benediktsdottir, B.; Teff, K.; Juliusson, S.; Pack, A.; Gislason, T. The role of obesity, different fat compartments and sleep apnea severity in circulating leptin levels: The Icelandic Sleep Apnea Cohort study. Int. J. Obes. 2012, 37, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Makinodan, K.; Yoshikawa, M.; Fukuoka, A.; Tamaki, S.; Koyama, N.; Yamauchi, M.; Tomoda, K.; Hamada, K.; Kimura, H. Effect of serum leptin levels on hypercapnic ventilatory response in obstructive sleep apnea. Respiration. 2008, 75, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.D.; Chin, C.H.; Kirkness, J.P.; McGinley, B.M.; Patil, S.P.; Polotsky, V.Y.; Cesare Biselli, P.J.; Smith, P.L.; Schneider, H.; Schwartz, A.R.; et al. Leptin and the control of pharyngeal patency during sleep in severe obesity. J. Appl. Physiol. 2014, 116, 1334–1341. [Google Scholar] [CrossRef] [PubMed]
- Zamrazilová, H.; Hainer, V.; Sedlácková, D.; Papezová, H.; Kunesová, M.; Bellisle, F.; Hill, M.; Nedvídková, J. Plasma Obestatin Levels in Normal Weight, Obese and Anorectic Women. Physiol. Res. 2008, 48, S49–S55. [Google Scholar] [CrossRef]

| Items | Whole study Population (n = 58) | OSA Group (n = 46) | Control Group (n = 12) | |||
|---|---|---|---|---|---|---|
| mean ± SD | min–max | mean ± SD | min–max | mean ± SD | min–max | |
| Gender [M/F] | 48/10 | 41/5 | 7/5 | |||
| Age (years) | 55 ± 11.2 | 34–75 | 55 ± 10.6 | 34–75 | 57 ± 8.7 | 46–72 |
| Weight (kg) | 105 ± 20.8 | 77–161 | 108 ± 21.1 | 78–161 | 93 ± 15.0 | 77–117 |
| BMI (kg/m2) | 35 ± 6.4 | 25–50 | 36 ± 6.5 | 27–50 | 33 ± 5.5 | 25–43 |
| WC (cm) | 116 ± 12.8 | 96–147 | 117 ± 13.4 | 96–147 | 111 ± 9.0 | 99–122 |
| AHI | 38 ± 28.1 | 0.7–119.9 | 46 ± 25.8 | 13.2–119.9 | 6 ± 4.2 | 0.7–12.4 |
| Snoring time (%) | 14 ± 15.9 | 0–71 | 15 ± 16.3 | 0–71 | 8 ± 13.1 | 0–47 |
| SpO2 mean (%) | 91 ± 4.7 | 66–97 | 91 ± 5.2 | 66–97 | 93 ± 1.7 | 91–96 |
| SpO2 lowest (%) *** | 76 ± 11.9 | 49–91 | 74 ± 12.1 | 49–91 | 85 ± 4.9 | 72–90 |
| Sleep time (min.) | 449 ± 80 | 223–557 | 447 ± 84 | 223–557 | 456 ± 65 | 328–528 |
| Sleep test time (min.) | 484 ± 60 | 266–580 | 484 ± 63 | 266–580 | 485 ± 47 | 418–543 |
| Overweight n = 14 (n,%) | Obesity | ||||
|---|---|---|---|---|---|
| Class I n = 17 (n,%) | Class II n = 12 (n,%) | Class III n = 15 (n,%) | Total n = 44 (n,%) | ||
| OSA group n = 46 | 10 (71%) | 13 (76%) | 9 (75%) | 14 (93%) | 36 (82%) |
| Control group n = 12 | 4 (29%) | 4 (24%) | 3 (25%) | 1 (7%) | 8 (18%) |
| OSA Group | Control Group | |||
|---|---|---|---|---|
| BMI | WC | BMI | WC | |
| AHI | 0.21 | 0.19 | −0.56 | −0.04 |
| Snoring time (%) | 0.31 ** | 0.30 ** | −0.13 | 0.30 |
| SpO2 mean (%) | −0.52 ** | −0.53 ** | −0.57 | 0.07 |
| SpO2 lowest (%) | −0.41 ** | −0.39 ** | −0.04 | 0.24 |
| OSA Group | Control Group | |
|---|---|---|
| Ghrelin (pg/mL) p = 0.05 | 426.6 (211.5–1928.0) | 496.2 (324.2–1147.1) |
| Leptin (ng/mL) p = 0.19 | 25.9 (16.5–44.8) | 31.9 (21.5–37.1) |
| Obestatin (pg/mL) p = 0.7 | 61.9 (55.5–98.2) | 61.6 (53.9–71.4) |
| Independent Variable | Logarithm of the Mean Ghrelin Concentration R2 = 9.7%, F = 6.0, p = 0.0175 * | ||
|---|---|---|---|
| B (95% CI) | p | β | |
| BMI | −0.020 (−0.037, −0.004) | 0.0175 * | −0.31 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardak, P.; Filip, R.; Woliński, J. The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight. J. Clin. Med. 2022, 11, 2032. https://doi.org/10.3390/jcm11072032
Pardak P, Filip R, Woliński J. The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight. Journal of Clinical Medicine. 2022; 11(7):2032. https://doi.org/10.3390/jcm11072032
Chicago/Turabian StylePardak, Piotr, Rafał Filip, and Jarosław Woliński. 2022. "The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight" Journal of Clinical Medicine 11, no. 7: 2032. https://doi.org/10.3390/jcm11072032
APA StylePardak, P., Filip, R., & Woliński, J. (2022). The Impact of Sleep-Disordered Breathing on Ghrelin, Obestatin, and Leptin Profiles in Patients with Obesity or Overweight. Journal of Clinical Medicine, 11(7), 2032. https://doi.org/10.3390/jcm11072032






