An Up-to-Date Article Regarding Particularities of Drug Treatment in Patients with Chronic Heart Failure
Abstract
1. Introduction
2. General Considerations Regarding HF Treatment
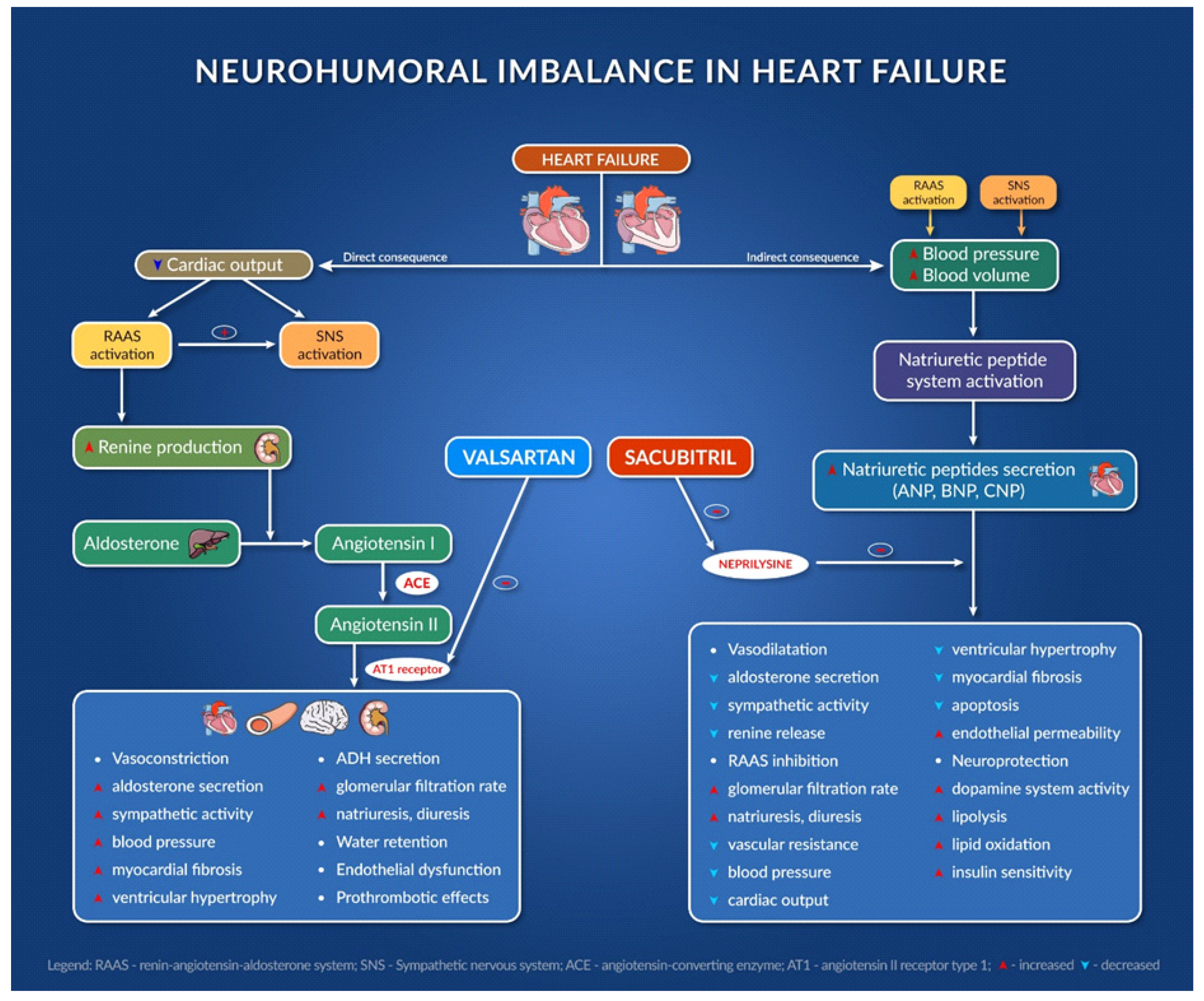
2.1. Mechanisms of Action of the Classical Therapy in Chronic HF Patients
2.2. New Approaches in HF Pharmacological Treatment
2.2.1. Sacubitril/Valsartan
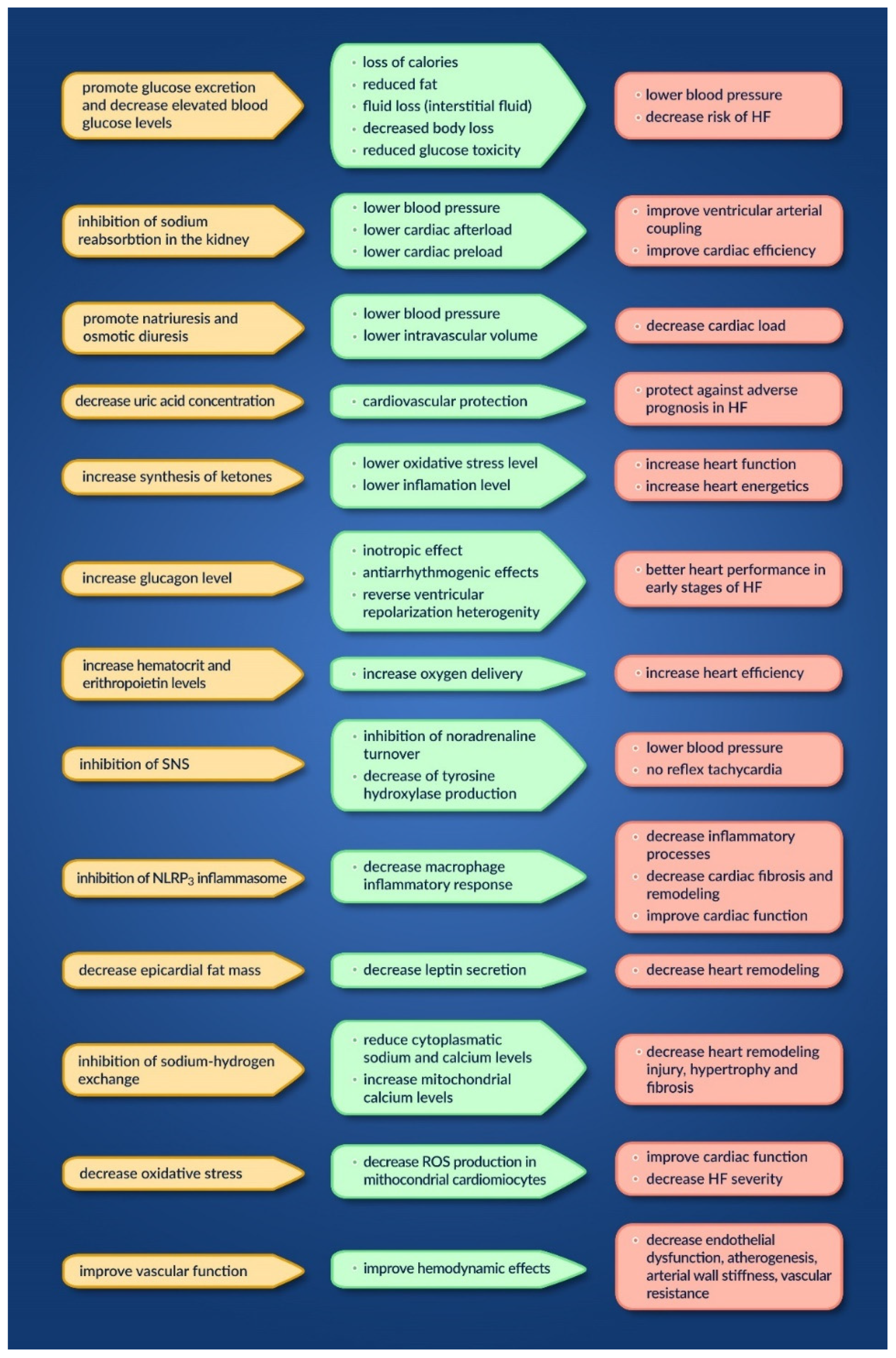
2.2.2. Sodium Glucose Co-Transporter-2 Inhibitors
3. Treatment Strategies in HF Patients
4. Particularities of Patients
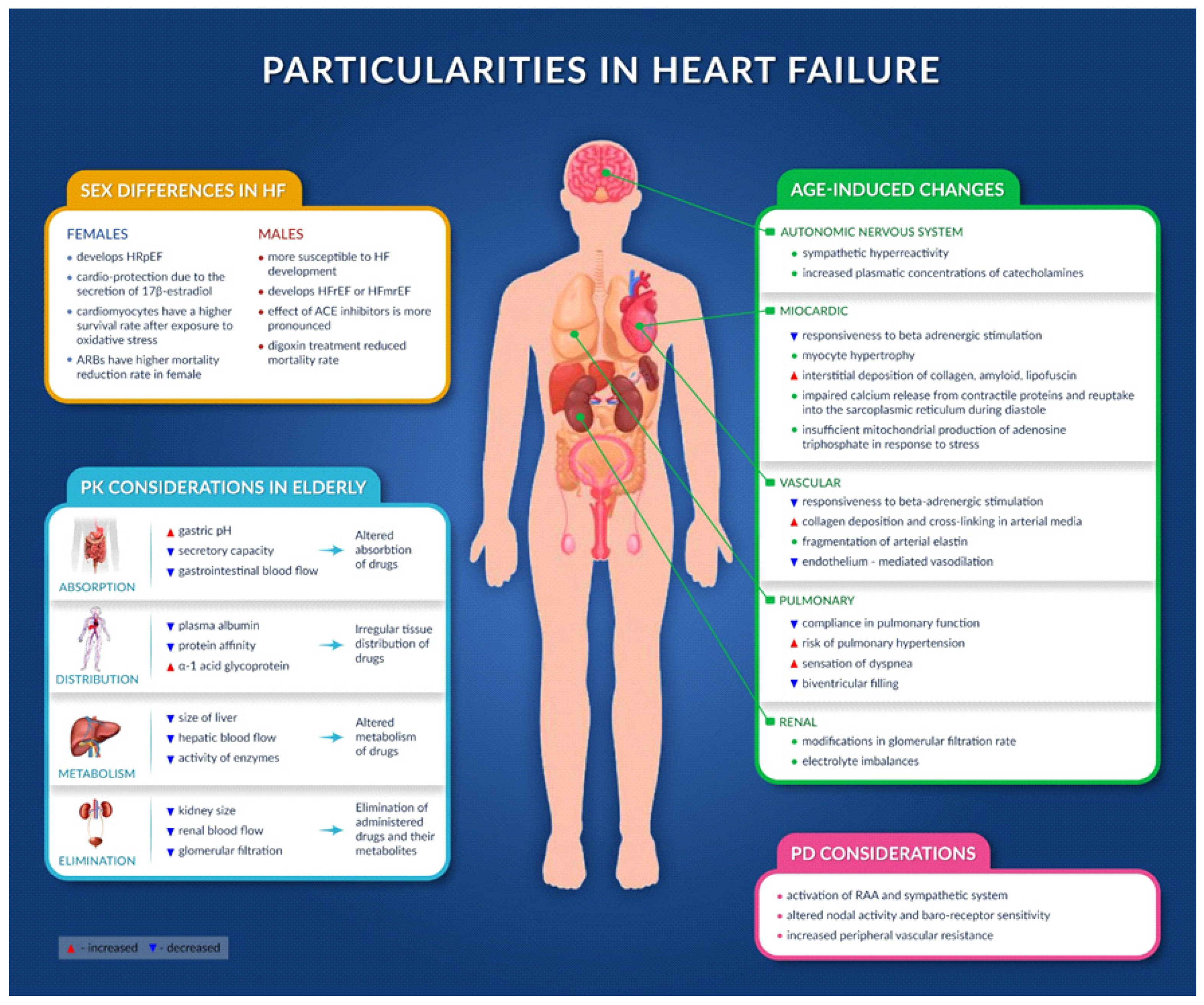
4.1. Age-Induced Changes
4.1.1. Cardiovascular Structure and Function
4.1.2. Other Organs
4.2. Sex Differences in HF
5. Particularities of Treatment
5.1. Pharmacokinetic Considerations and Their Consequences in HF Patients
5.2. Pharmadynamic Considerations
5.3. HF Treatment in Patients with Cardiorenal Syndrome
5.4. HF Treatment in Pregnancy and Lactation
6. Drugs and Food Supplements That Can Aggravate HF
7. Potential Drug–Drug Interactions in HF Patients
8. Adverse Drug Reactions in HF Patients
9. Discontinuation of Drugs in HF Patients
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Drugs [99] | Possible Mechanism Involved | Results | References |
|---|---|---|---|
| NSAIDs | Inhibition of cyclooxygenase enzyme Inhibition of renal prostaglandin synthesis | Sodium and water retention Higher systemic vascular resistance Reduction in renal perfusion, glomerular filtration rate, sodium excretion | [100,101,102,103,104,105,106] |
| Alpha-1 blockers (e.g., doxazosin) | Beta-1 receptor stimulation Stimulation of renin and aldosterone release Chronic alfa1 antagonism Stimulation of heart fibrosis factor galectin-3 expression | Edema Tachyphylaxis Cardiomyocyte apoptosis Myocardial hypertrophy | [107] |
| Calcium channel blockers (e.g., verapamil, diltiazem) | Negative inotrope Calcium channel blockade | Cardiac depression Atrioventricular conduction block | [103,106] |
| Moxonidine (centrally acting α-adrenergic drug) | Possible sympathetic withdrawal | Myocardial depression Hypotension Rebound norepinephrine increase | [108] |
| Class I antiarrhythmic (e.g., flecainide, disopyramide) | Negative inotrope Pro-arrhythmic stimulation | Myocardial infarction Premature ventricular beats Myocardial depressant effects | [106,109] |
| Class III antiarrhythmic (e.g., sotalol) | Beta inhibition Pro-arrhythmic stimulation Potassium channel blockade | Bradycardia Prolonged QT interval Torsades de pointes T-wave abnormalities | [109,110,111] |
| Inhibitors of dipeptidyl peptidase 4 (e.g., sitagliptin, saxagliptin) | Dipeptidyl peptidase 4 enzyme interference Direct interaction in myocytes Calcium channel interference Interference in substance P degradation Sympathetic nervous system stimulation | Myocardial infarction Stroke | [103,112,113,114,115] |
| Thiazolidinediones (e.g., rosiglitazone, pioglitazone) | Possible calcium channel blockade Interference with mitochondrial respiration or oxidative stress | Sodium and water retention Peripheral edema Myocardial infarction Stroke Transient ischemic attacks | [106,115,116,117] |
| Itraconazole | Negative inotropic effect Mitochondrial dysfunction Inhibition of 11 beta-hydroxysteroid dehydrogenase 2 Cytochrome P450 inhibition | Peripheral edema Hypertension Prolonged QT interval Cardiac depression Excess mineralocorticoid Myofibroblast damage | [118,119,120] |
| Amphotericin B | Unknown | Cardiotoxicity Dilated cardiomyopathy | [121] |
| Carbamazepine(overdose) | Negative inotropic and chronotropic effects Depression of phase 2 repolarization Direct toxic effect on myocardial fibers Anticholinergic action Increased automaticity of ectopic pacemakers Sodium channel blockade | Left ventricular dysfunction Suppressed sinus nodal activity Atrioventricular conduction disturbances Hypotension | [122,123,124] |
| Pregabalin | Alterations in cardiac renin angiotensin system (RAS) L-type calcium channel blockade | Peripheral edema Decreased calcium influx in cardiomyocytes Left ventricular deterioration | [125,126,127] |
| Tricyclic antidepressants | Negative inotrope Pro-arrhythmic stimulation Norepinephrine and serotonin reuptake blockade Sodium channel blockade Suppression of potassium channels in myocytes Vasoconstriction of cerebral arteries | Arrhythmias Impaired heart conduction Prolonged intraventricular conduction Prolonged QT interval Hemorrhagic stroke Ischemic stroke | [128,129] |
| Citalopram | Inhibition of depolarizing current mediated by L-type calcium channels Antagonistic effects on myocardial potassium channels | Prolonged QT interval Episodes of torsades de pointes Arrhythmias | [130,131] |
| Pergolide, cabergoline, pramipexole | Potent agonists at cardiac myocyte 5- HT2B serotonin receptors Induction of fibroblast activation | Valvular damage Cardiac valvular regurgitation Pulmonary arterial hypertension Peripheral edema | [132,133,134] |
| Clozapine | Calcium channel blockade Ig-E mediated hypersensitivity Reduced left ventricular function | Myocarditis Cardiomyopathy Prolonged QT interval Elevated troponin | [135,136,137,138] |
| Lithium | Altered acetylcholinesterase activity Direct myofibril degeneration Induction of oxidative stress Interference with calcium ion influx | Cardiac fibrosis Cardiomyocyte apoptosis Rhythm disturbances Edema, ascites Complete heart block and first-degree AV block | [139,140,141,142,143] |
| β2 adrenergic agonists (e.g., salbutamol) | Decreased β-receptor responsiveness Small positive inotropic and chronotropic effects Activation of Gs/cAMP/PKA Inhibition of Gi/PDE | Arrhythmias Prolonged QT interval | [144,145] |
| Tumor necrosis factor-α (TNF-α) inhibitors | Cytokine mediation Sympathetic excitation Inflammation and renin-angiotensin system upregulation | Peripheral inflammation Cardiac dysfunction | [146,147,148] |
| Topical beta-blockers (e.g., timolol) | Hemodynamic effects due to beta blockade | Arrhythmias Myocardial ischemia Hypotension Pulmonary edema | [99] |
| Food supplements [149] | Possible mechanism involved | Results | Reference |
| Aconitum spp. (Monkshood) | Alkaloids block potassium channels | Ventricular fibrillation Bradycardia Hypotension | [150] |
| Aesculus hippocastanum L. (Horse chestnut) | Antiplatelet effect | Increased risk of bleeding when associated with anticoagulant drugs | [151] |
| Allium sativum L. (Garlic) | Inhibition of platelet aggregation (dose-dependent) | Increased risk of bleeding when associated with anti-thrombotic drugs | [152] |
| Aloe barbadensis Mill. (Aloe vera) | Laxative effect | Risk of hypokalemia with increased toxicity of cardiotonic glycosides or antiarrhythmia drugs | [153] |
| Angelica sinensis (Oliv.) Diels (Angelica) | Antiplatelet and anticoagulant effect | Increased anticoagulant effect | [154] |
| Cassia senna L. (Senna) | Laxative effect | Risk of hypokalemia with increased toxicity of digitalis or antiarrhythmia drugs | [153] |
| Citrus paradisi Macfad. (Grapefruit) | Inhibition of CYP3A4 enzyme | Increased effects (therapeutic or toxic) of co-administered drugs (e.g., calcium channel blockers, antiarrhythmia drugs) Inefficacy of pro-drugs metabolized by CYP3A4 | [155,156] |
| Cratageus spp. (Hawthorn) | Increases digitalis toxicity (incompletely elucidated) | Risk of digitalis intoxication if co-administered | [157,158] |
| Ephedra sinica Stapf (Chinese ephera) | Alkaloids stimulate adrenergic receptors Indirect agonist stimulation and noradrenaline release | Tachycardia Hypertension Arrythmias Heart attack Stroke | [159] |
| Ginkgo biloba L. (Ginkgo) | Antiplatelet effect | Increased risk of bleeding when co-administered with antithrombotic drugs | [160,161] |
| Glycyrrhiza glabra L. (Licorice) | Hypokalemia Reduced sodium and water excretion | Increased toxicity of digitalis or antiarrhythmic drugs Decreased effect of diuretics | [153] |
| Harpagophytum procumbens Burch. (Devil’s claw) | Inhibition of CYP1A2 and CYP2D6 | Increased effects of diuretics, antihypertensives, statins, and anticoagulants | [162,163] |
| Hypericum perforatum L. (St. John’s Wort) | Induction of CYP3A4 isoenzyme activity | Decreases plasma levels of co-administered drugs metabolized by this enzyme | [164,165,166] |
| Leonurus cardiaca L. (Motherwort) | Antiplatelet effect | Increased risk of bleeding when co-administered with antithrombotic drugs | [152] |
| Oenothera biennis L. (Evening primrose) | Inhibition of platelet activating factor | Increased risk of bleeding when co-administered with antithrombotic drugs | [167,168] |
| Panax ginseng C.A. Meyer (Asian ginseng) | Decreased prothrombin time | Decreased warfarin effect and increased risk of thrombo-embolic events | [152] |
| Stephania tetrandra S. Moore | Calcium channel blockade | Cardiac depression | [169] |
| Zingiber officinale Roscoe (Ginger) | Thromboxane synthase inhibition Prostacyclin agonist | Increased risk of bleeding when co-administered with antithrombotic drugs Increased effects of antihypertensive drugs | [170] |
Appendix B
| Main Drug for HF | Co-Administered Drugs | Consequences | Recommendations |
|---|---|---|---|
| ACE inhibitors | ARBs/aliskiren (angiotensin II receptor blockers) | Increased risk of impaired renal function, acute renal failure, hyperkalemia, hypotension, syncope and falls, thus increased risk of fractures in the elderly | Avoid association |
| Sacubitril | High risk of angioedema | Avoid association | |
| NSAIDs (nonsteroidal anti-inflammatory drugs) | Risk of acute renal failure due to decreased glomerular filtration rate (decreased synthesis of renal vasodilating prostaglandins), especially if patient is elderly, dehydrated, or under diuretic treatment | If possible, avoid association If association is needed, proper hydration is recommended, monitoring of renal function, administering the lowest therapeutic NSAID dose and for the shortest period of time | |
| Spironolactone, amiloride, triamterene | High risk of hyperkalemia, especially in patients with chronic renal failure | Evaluate renal function before beginning of treatment (determine creatinine clearance), administer in therapeutically effective minimum doses and periodically check potassium | |
| Allopurinol | Higher risk of hypersensitivity reactions (Steven-Johnson syndrome) | If associated, ensure clinical supervision and adjust dose [177] | |
| Gliptins | Increased risk of angioedema through decreased DPPIV by gliptin | Avoid association If associated, ensure clinical supervision and adjust dose | |
| Insulin | High risk of hypoglycemia | Monitor blood glucose and adjust insulin dosage | |
| Hypoglycemic sulfonamides | Hypoglycemic risk through improved glucose tolerance and decreased hypoglycemic sulfonamide dose requirements | Monitor blood glucose and adjust dosage of hypoglycemic sulfonamides | |
| Racecadotril | High risk of allergic side effects (angioneurotic edema) | Avoid association If associated, ensure clinical supervision and adjust dose | |
| Lithium | Increased lithium plasma concentration through decreased elimination | Avoiding association If associated, ensure clinical supervision and adjust lithium dose | |
| ARBs | ACE inhibitors NSAIDs Spironolactone Lithium | Same as for ACE inhibitors | |
| Sacubitril/valsartan | Statins | Increased effects of statins | Adjust statin dose [178] |
| Sildenafil | Additional blood pressure reduction | Use caution when associated and adjust dose of sildenafil [179] | |
| Beta blockers (carvedilol, bisoprolol, metoprolol, nebivolol) | Amiodarone | Cardiac conduction disorders, bradycardia, atrioventricular block | Preferably avoid association, or adapt drug dosages and conduct patient monitoring (ECG, heart rate) |
| Verapamil Diltiazem | Cardiac depression, HF decompensation, AV block | Preferably avoid association | |
| Antidiabetic drugs | Risk of masking signs of hypoglycemia (palpitations, tachycardia, tremor of extremities) | Preferably avoid association or closely monitor dosage of antidiabetic drugs | |
| Digitalis | Automatic disorders (bradycardia, sinus arrest), AV block | Preferably avoid association or adjust dosages | |
| NSAIDs | Decreased antihypertensive effect due to inhibition of renal vasodilating prostaglandin synthesis by NSAIDs | Preferably avoid association or adjust dosages | |
| Mexiletine | Negative inotropic effect Automation disorders Risk of cardiac decompensation | Preferably avoid association | |
| Central antihypertensives | Decreased central sympathetic tone and vasodilating effect of central blood-lowering drugs | Preferably avoid association | |
| Imipramine antidepressants (e.g., amitriptyline) | Intensification of vasodilating effect and risk of orthostatic hypotension | Avoid association or adapt beta blocker dosage | |
| Neuroleptics | Vasodilator effect Risk of orthostatic hypotension | Monitor blood pressure and adapt dosages if needed | |
| Anticholinesterases | Excessive bradycardia | Avoid association or monitor heart rate with adjustment of beta blocker dosage | |
| Diuretics | NSAIDs | Decreased diuretic effect and risk of kidney failure | Avoid association if possible |
| Carbamazepine | Increased risk of hyponatremia | Hydrate patient and correct electrolyte imbalances | |
| Lithium | Decreased renal elimination of lithium with high risk of accumulation | Avoid association if possible or adapt lithium dosage | |
| SGLT2 inhibitors Dapagliflozin Empagliflozin | Thiazide diuretics/loop diuretics | Increased diuretic effect | Adjust dosage Monitor the blood pressure. Hydrate patients and monitor the electrolyte balance |
| Nitrates | Sildenafil | Increased risk of hypotension, blood pressure collapse | Avoid association or adjust dosage |
| Heparins | Increased excretion of heparins | Adjust dosage | |
| Digoxin | Amiodarone Propafenone Quinidine Clarithromycin Hypokalemic diuretics | Digoxin toxicity | Avoid association or adjust dosage |
| Carbamazepine Dronedarone | Decreased plasma concentration of digoxin Cardiac deprivation Increased digoxinemia | Therapeutic supervision Therapeutic supervision (clinical and ECG) Reduce digoxin dosage by half | |
| Amiodarone | Verapamil/ Diltiazem | Cardiac deprivation with high risk of bradycardia and atrioventricular block | Avoid intravenous administration, use ECG surveillance when administered orally |
| Levofloxacin/ moxifloxacin | Ventricular rhythm disorders (risk of torsades des pointes) | Therapeutic supervision (clinical and ECG) | |
| Statins | Increased effects of statins | Adjust statin dose (maximum 20 mg/day for simvastatin) | |
| Ivabradine | Verapamil/ diltiazem | Increased ivabradine plasma concentration with increased risk of side effects Marked bradycardia | Avoid association |
| Azithromycin | Ventricular rhythm disorders (risk of torsades des pointes) | Therapeutic supervision (clinical and ECG) | |
| AVK | Amiodarone | Increased AVK effects Hemorrhagic risk | INR (International Normalized Ratio) control Adjust dosage (up to 4 weeks after stopping amiodarone treatment) |
| Allopurinol | Increased hemorrhagic risk | INR surveillance and adjust AVK dosage up to 8 days after stopping allopurinol treatment [180] | |
| Cefamandole/ cefazolin/ ceftriaxone | Increased AVK plasma concentration with high hemorrhagic risk | INR surveillance and adjust AVK dosage | |
| Fluoroquinolones | Increased AVK plasma concentration with high hemorrhagic risk | INR surveillance and adjust AVK dosage | |
| Fenofibrate | Increased AVK plasma concentration with high hemorrhagic risk | INR surveillance and adjust AVK dosage | |
| Paracetamol | Increased AVK plasma concentration with high hemorrhagic risk when given paracetamol in high dosage (>4 g/day), >4 days | INR surveillance and adjust AVK and paracetamol dosage | |
| Thiamazole (methimazole) | Increased risk of bleeding due to hypoprothrombinemia caused by methimazole | If possible, avoid association or conduct INR surveillance and adjust AVK dosage [181] | |
| NSAIDs | Increased AVK plasmatic concentration with high hemorrhagic risk | If possible, avoid association or conduct INR surveillance and adjust AVK dosage | |
| NOAC (New Oral Anticoagulants) | Rifampicin | Decreased NOAC efficacy and increased thromboembolic risk | Clinical supervision Adjust NOAC dose up to 8 days after stopping rifampicin treatment [180] |
| Itraconazole/ ketoconazole/ voriconazole | Increased NOAC plasma concentration and efficacy with high risk of bleeding | Clinical surveillance and adjust dose of NOAC | |
| Carbamazepine/ levetiracetam/ phenobarbital/ valproic acid | Decreased NOAC efficacy and increased thromboembolic risk | Clinical supervision and adjust NOAC dose | |
| Dabigatran | Amiodarone | High plasma concentration of dabigatran and increased risk of bleeding | Clinical supervision and adjust dabigatran dose (maximum 150 mg/day) [182] |
| Dronedarone | High plasma concentration of dabigatran (also rivaroxaban) with increased risk of bleeding | Clinical supervision and adjust dabigatran/rivaroxaban dose | |
| Quinidine | High plasma concentration of dabigatran with increased risk of bleeding | Avoid association If associated, clinical supervision and adjust dabigatran dose | |
| Fluconazole/ itraconazole/ ketoconazole | High plasma concentration of dabigatran with increased risk of bleeding | Avoid association If associated, clinical supervision and adjust dabigatran dose | |
| Apixaban | Diltiazem | Increased plasma concentration of apixaban with increased risk of bleeding [182,183] | Clinical supervision and adjust apixaban dose |
| Clarithromycin/ Erythromycin | High plasma concentration of apixaban/rivaroxaban with increased risk of bleeding | Clinical supervision and adjust apixaban/rivaroxaban dose | |
| Fluconazole | High plasma concentration of apixaban/rivaroxaban with increased risk of bleeding | Avoid association If associated, clinical supervision and adjust apixaban dose | |
| Antiplatelet agents | NSAIDs | Increased risk of bleeding (especially gastro-intestinal) | Avoid association If associated, clinical supervision and adjust dose |
| Heparins/ oral anticoagulants | Increased risk of bleeding | Avoid association If associated, clinical supervision and adjust dose | |
| Selective serotonin reuptake inhibitors (SSRIs) | Increased risk of bleeding | Avoid association If associated, clinical supervision and adjust dose | |
| Antidepressants with mixed adrenergic–serotoninergic mechanism | Increased risk of bleeding | Avoid association If associated, clinical supervision and adjust dose | |
| Pentoxifylline | Increased risk of bleeding | Clinical supervision and dose adjustments | |
| Clopidogrel | Proton pump inhibitors (PPIs) | High thromboembolic risk | Avoid association [184] |
| Repaglinide | Increased plasma concentration of oral antidiabetic with intensified side effects | Adjust repaglinide dose | |
| Ticagrelor | Dabigatran | High plasma concentration of dabigatran and increased risk of bleeding | Avoid association If associated, clinical supervision and adjust dabigatran dose |
| Diltiazem/ verapamil | High plasma concentration of ticagrelor and increased risk of bleeding | Avoid association If associated, clinical supervision and adjust ticagrelor dose | |
| Atorvastatin | Increased plasma concentration of statin | Adjust statin dosage (maximum 40 mg/day) [178,185] |
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Andronic, A.A.; Mihaila, S.; Cinteza, M. Heart Failure with Mid-Range Ejection Fraction—A New Category of Heart Failure or Still a Gray Zone. Maedica 2016, 11, 320–324. [Google Scholar] [PubMed]
- Delepaul, B.; Robin, G.; Delmas, C.; Moine, T.; Blanc, A.; Fournier, P.; Roger-Rollé, A.; Domain, G.; Delon, C.; Uzan, C.; et al. Who are patients classified within the new terminology of heart failure from the 2016 ESC guidelines? ESC Heart Fail. 2017, 4, 99–104. [Google Scholar] [CrossRef]
- Mosterd, A.; Hoes, A.W. Clinical epidemiology of heart failure. Heart 2007, 93, 1137–1146. [Google Scholar] [CrossRef] [PubMed]
- Dharmarajan, K.; Rich, M.W. Epidemiology, Pathophysiology, and Prognosis of Heart Failure in Older Adults. Heart Fail. Clin. 2017, 13, 417–426. [Google Scholar] [CrossRef]
- Snipelisky, D.; Chaudhry, S.P.; Stewart, G.C. The Many Faces of Heart Failure. Card. Electrophysiol. Clin. 2019, 11, 11–20. [Google Scholar] [CrossRef]
- Crooks, J.; O’Malley, K.; Stevenson, I.H. Pharmacokinetics in the elderly. Clin. Pharmacokinet. 1976, 1, 280–296. [Google Scholar] [CrossRef] [PubMed]
- Turnheim, K. When drug therapy gets old: Pharmacokinetics and pharmacodynamics in the elderly. Exp. Gerontol. 2003, 38, 843–853. [Google Scholar] [CrossRef] [PubMed]
- Salwe, K.J.; Kalyansundaram, D.; Bahurupi, Y. A Study on Polypharmacy and Potential Drug-Drug Interactions among Elderly Patients Admitted in Department of Medicine of a Tertiary Care Hospital in Puducherry. J. Clin. Diagn. Res. 2016, 10, FC06. [Google Scholar] [CrossRef] [PubMed]
- Mastromarino, V.; Casenghi, M.; Testa, M.; Gabriele, E.; Coluccia, R.; Rubattu, S.; Volpe, M. Polypharmacy in heart failure patients. Curr. Heart Fail. Rep. 2014, 11, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; Vitale, C.; Seferovic, P.; Spoletini, I.; Cvan Trobec, K.; Rosano, G.M. Pharmacokinetics and pharmacodynamics of cardiovascular drugs in chronic heart failure. Int. J. Cardiol. 2016, 224, 191–198. [Google Scholar] [CrossRef] [PubMed]
- Lainscak, M.; Vitale, C. Biological and chronological age in heart failure: Role of immunosenescence. J. Cardiovasc. Med. 2016, 17, 857–859. [Google Scholar] [CrossRef]
- United Nations. Population Ageing. 2015. Available online: WPA2015_Report.pdf(un.org) (accessed on 25 January 2022).
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef]
- Writing Committee; Maddox, T.M.; Januzzi, J.L., Jr.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 77, 772–810. [Google Scholar] [CrossRef]
- Oliver, E.; Mayor, F., Jr.; D’Ocon, P. Beta-blockers: Historical Perspective and Mechanisms of Action. Rev. Española Cardiol. 2019, 72, 853–862, (In English and Spanish). [Google Scholar] [CrossRef]
- Bie, P.; Mølstrøm, S.; Wamberg, S. Normotensive sodium loading in conscious dogs: Regulation of renin secretion during beta-receptor blockade. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R428–R435. [Google Scholar] [CrossRef]
- Sayer, G.; Bhat, G. The renin-angiotensin-aldosterone system and heart failure. Cardiol. Clin. 2014, 32, 21–32. [Google Scholar] [CrossRef]
- Bruno, N.; Sinagra, G.; Paolillo, S.; Bonomi, A.; Corrà, U.; Piepoli, M.; Veglia, F.; Salvioni, E.; Lagioia, R.; Metra, M.; et al. Mineralocorticoid receptor antagonists for heart failure: A real-life observational study. ESC Heart Fail. 2018, 5, 267–274. [Google Scholar] [CrossRef]
- Casu, G.; Merella, P. Diuretic Therapy in Heart Failure-Current Approaches. Eur. Cardiol. 2015, 10, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Levine, T.B. Role of vasodilators in the treatment of congestive heart failure. Am. J. Cardiol. 1985, 55, 32A–35A. [Google Scholar] [CrossRef]
- David, M.N.V.; Shetty, M. Digoxin Toxicity. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK556025/ (accessed on 25 January 2022).
- Reed, M.; Kerndt, C.C.; Nicolas, D. Ivabradine. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507783/ (accessed on 25 January 2022).
- Yancy, C.; Jessup, M.; Butler, J.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.S.; Fonarow, G.C.; Givertz, M.M.; et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation 2017, 136, e137–e161. [Google Scholar] [CrossRef] [PubMed]
- Sauer, A.J.; Cole, R.; Jensen, B.C.; Pal, J.; Sharma, N.; Yehya, A.; Vader, J. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail. Rev. 2019, 24, 167–176. [Google Scholar] [CrossRef]
- Chandra, A.; Lewis, E.; Claggertt, B.; Desai, A.S.; Packer, M.; Zile, M.R.; Swedberg, K.; Rouleau, J.L.; Shi, V.C.; Lefkowitz, M.P.; et al. The Effects of Sacubitril/Valsartan on Physical and Social Activity Limitations in Heart Failure Patients: The PARADIGM-HF Trial. JAMA Cardiol. 2018, 3, 498–505. [Google Scholar] [CrossRef]
- Velazquez, E.; Morrow, D.; DeVore, A.; Duffy, C.I.; Ambrosy, A.P.; McCague, K.; Rocha, R.; Braunwald, E.; PIONEER-HF Investigators. Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure. N. Engl. J. Med. 2019, 380, 539–548. [Google Scholar] [CrossRef]
- Myhre, P.L.; Vaduganathan, M.; Claggett, B.; Packer, M.; Desai, A.S.; Rouleau, J.L.; Zile, M.R.; Swedberg, K.; Lefkowitz, M.; Shi, V.; et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: The PARADIGM-HF trial. J. Am. Coll. Cardiol. 2019, 73, 1264–1272. [Google Scholar] [CrossRef]
- Lewis, E.F.; Claggett, B.L.; McMurray, J.J.V.; Packer, M.; Lefkowitz, M.P.; Rouleau, J.L.; Liu, J.; Shi, V.C.; Zile, M.R.; Desai, A.S.; et al. Health-related quality of life outcomes in PARADIGM-HF. Circ. Heart Fail. 2017, 10, e003430. [Google Scholar] [CrossRef]
- Seferovic, P.; Ponikowski, P.; Anker, S.; Bauersachs, J.; Chioncel, O.; Cleland, J.G.F.; de Boer, R.A.; Drexel, H.; Ben Gal, T.; Hill, L.; et al. Clinical practice update on heart failure 2019: Pharmacotherapy, procedures, devices and patient management. An expert consensus meeting report of The Heart Failure Association of the European Society of Cardiology. Eur. J. Heart Fail. 2019, 21, 1169–1186. [Google Scholar] [CrossRef]
- Gillette, M.; Bozkurt, B. Ins and Outs: Perspectives of Inpatient Prescribing for Sacubitril/Valsartan. Ann. Pharmacother. 2020, 55, 805–813. [Google Scholar] [CrossRef]
- Byrne, D.; Fahey, T.; Moriarty, F. Efficacy and safety of sacubitril/valsartan in the treatment of heart failure: Protocol for a systematic review incorporating unpublished clinical study reports. HRB Open Res. 2020, 3, 5. [Google Scholar] [CrossRef]
- Scheen, A.J. Sodium-glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020, 16, 556–577. [Google Scholar] [CrossRef]
- Al Hamed, F.A.; Elewa, H. Potential Therapeutic Effects of Sodium Glucose-linked Cotransporter 2 Inhibitors in Stroke. Clin. Ther. 2020, 42, e242–e249. [Google Scholar] [CrossRef] [PubMed]
- Ling, A.W.; Chan, C.C.; Chen, S.W.; Kao, Y.W.; Huang, C.Y.; Chan, Y.H.; Chu, P.H. The risk of new-onset atrial fibrillation in patients with type 2 diabetes mellitus treated with sodium glucose cotransporter 2 inhibitors versus dipeptidyl peptidase-4 inhibitors. Cardiovasc. Diabetol. 2020, 19, 188. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Shiou, Y.L.; Jhuo, S.J.; Chang, C.Y.; Liu, P.L.; Jhuang, W.J.; Dai, Z.K.; Chen, W.Y.; Chen, Y.F.; Lee, A.S. The sodium-glucose co-transporter 2 inhibitor empagliflozin attenuates cardiac fibrosis and improves ventricular hemodynamics in hypertensive heart failure rats. Cardiovasc. Diabetol. 2019, 18, 45. [Google Scholar] [CrossRef] [PubMed]
- GrubićRotkvić, P.; CigrovskiBerković, M.; Bulj, N.; Rotkvić, L. Minireview: Are SGLT2 inhibitors heart savers in diabetes? Heart Fail. Rev. 2020, 25, 899–905. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Verma, S. Mechanisms of Cardiovascular Benefits of Sodium Glucose Co-Transporter 2 (SGLT2) Inhibitors: A State-of-the-Art Review. JACC Basic Transl. Sci. 2020, 5, 632–644. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Tang, F.; Khariton, Y.; Husain, M.; Inzucchi, S.E.; McGuire, D.K.; Pitt, B.; Scirica, B.M.; Austin, B.; et al. Dapagliflozin Effects on Biomarkers, Symptoms, and Functional Status in Patients with Heart Failure with Reduced Ejection Fraction: The DEFINE-HF Trial. Circulation 2019, 140, 1463–1476. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Shahzeb Khan, M.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; EMPEROR-Preserved Trial Committees and Investigators; et al. Baseline characteristics of patients with heart failure with preserved ejection fraction in the EMPEROR-Preserved trial. Eur. J. Heart Fail. 2020, 22, 2383–2392. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Butler, J.; Zannad, F.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on Worsening Heart Failure Events in Patients with Heart Failure and Preserved Ejection Fraction: EMPEROR-Preserved Trial. Circulation 2021, 144, 1284–1294. [Google Scholar] [CrossRef] [PubMed]
- Gazewood, J.D.; Turner, P.L. Heart failure with preserved ejection fraction: Diagnosis and management. Am. Fam. Physician 2017, 96, 582–588. [Google Scholar]
- Mulder, B.A.; Schnabel, R.B.; Rienstra, M. Predicting the future in patients with atrial fibrillation: Who develops heart failure? Eur. J. Heart Fail. 2013, 15, 366–367. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, A.J.; Lv, Y.N.; Zhong, H.L.; Wen, J.H.; Wei, X.H.; Peng, H.W.; Zhou, J.; Liu, L.L. Meta-analysis of digoxin use and risk of mortality in patients with atrial fibrillation. Am. J. Cardiol. 2015, 115, 901–906. [Google Scholar] [CrossRef] [PubMed]
- Van Gelder, I.C.; Haegeli, L.M.; Brandes, A.; Heidbuchel, H.; Aliot, E.; Kautzner, J.; Szumowski, L.; Mont, L.; Morgan, J.; Willems, S.; et al. Rationale and current perspective for early rhythm control therapy in atrial fibrillation. Europace 2011, 13, 1517–1525. [Google Scholar] [CrossRef] [PubMed]
- Packer, M.; Califf, R.M.; Konstam, M.A.; Krum, H.; McMurray, J.J.; Rouleau, J.L.; Swedberg, K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: The Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002, 106, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Fleg, J.L.; Strait, J. Age-associated changes in cardiovascular structure and function: A fertile milieu for future disease. Heart Fail. Rev. 2012, 17, 545–554. [Google Scholar] [CrossRef]
- Lakatta, E.G. Diminished beta-adrenergic modulation of cardiovascular function in advanced age. Cardiol. Clin. 1986, 4, 185–200. [Google Scholar] [CrossRef]
- Loffredo, F.S.; Nikolova, A.P.; Pancoast, J.R.; Lee, R.T. Heart failure with preserved ejection fraction: Molecular pathways of the aging myocardium. Circ. Res. 2014, 115, 97–107. [Google Scholar] [CrossRef]
- Olivetti, G.; Melissari, M.; Capasso, J.M.; Anversa, P. Cardiomyopathy of the aging human heart. Myocyte loss and reactive cellular hypertrophy. Circ. Res. 1991, 68, 1560–1568. [Google Scholar] [CrossRef] [PubMed]
- Burgess, M.L.; McCrea, J.C.; Hedrick, H.L. Age-associated changes in cardiac matrix and integrins. Mech. Ageing Dev. 2001, 122, 1739–1756. [Google Scholar] [CrossRef]
- Eghbali, M.; Eghbali, M.; Robinson, T.F.; Seifter, S.; Blumenfeld, O.O. Collagen accumulation in heart ventricles as a function of growth and aging. Cardiovasc. Res. 1989, 23, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part I: Aging arteries: A “set up” for vascular disease. Circulation 2003, 107, 139–146. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Levy, D. Arterial and cardiac aging: Major shareholders in cardiovascular disease enterprises: Part II: The aging heart in health: Links to heart disease. Circulation 2003, 107, 346–354. [Google Scholar] [CrossRef]
- Picard, M.; McEwen, B.S. Psychological Stress and Mitochondria: A Conceptual Framework. Psychosom. Med. 2018, 80, 126–140. [Google Scholar] [CrossRef]
- Liamis, G.; Milionis, H.J.; Elisaf, M. A review of drug-induced hypernatraemia. NDT Plus 2009, 2, 339–346. [Google Scholar] [CrossRef]
- Miller, M. Fluid and electrolyte homeostasis in the elderly: Physiological changes of ageing and clinical consequences. Baillière’s Clin. Endocrinol. Metab. 1997, 11, 367–387. [Google Scholar] [CrossRef]
- Peeters, L.E.J.; Kester, M.P.; Feyz, L.; Van Den Bemt, P.M.L.A.; Koch, B.C.P.; Van Gelder, T.; Versmissen, J. Pharmacokinetic and pharmacodynamic considerations in the treatment of the elderly patient with hypertension. Expert Opin. Drug Metab. Toxicol. 2019, 15, 287–297. [Google Scholar] [CrossRef]
- Barr, R.G.; Bluemke, D.A.; Ahmed, F.S.; Carr, J.J.; Enright, P.L.; Hoffman, E.A.; Jiang, R.; Kawut, S.M.; Kronmal, R.A.; Lima, J.A.; et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N. Engl. J. Med. 2010, 362, 217–227. [Google Scholar] [CrossRef]
- Petrescu, C.; Schlink, U.; Richter, M.; Suciu, O.; Ionovici, R.; Herbarth, O. Risk assessment of the respiratory health effects due to air pollution and meteorological factors in a population from Drobeta Turnu Severin, Romania. In Proceedings of the 17th European Symposium on Computer Aided Process Engineering, Cluj, Romania, 27–30 May 2007; Volume 24, pp. 1205–1210. [Google Scholar]
- Monfredi, O.; Lakatta, E.G. Complexities in cardiovascular rhythmicity: Perspectives on circadian normality, ageing and disease. Cardiovasc. Res. 2019, 115, 1576–1595. [Google Scholar] [CrossRef] [PubMed]
- Savarese, G.; D’Amario, D. Sex Differences in Heart Failure. Adv. Exp. Med. Biol. 2018, 1065, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Barison, A.; Maffei, S.; Borrelli, C.; Morrone, D.; Cameli, M.; Palazzuoli, A.; Ambrosio, G.; Coiro, S.; et al. Sex-related differences in chronic heart failure. Int. J. Cardiol. 2018, 255, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Kim, J.K. The Role of Estrogen and Estrogen Receptors on Cardiomyocytes: An Overview. Can. J. Cardiol. 2016, 32, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Bouma, W.; Noma, M.; Kanemoto, S.; Matsubara, M.; Leshnower, B.G.; Hinmon, R.; Gorman, J.H., 3rd; Gorman, R.C. Sex-related resistance to myocardial ischemia-reperfusion injury is associated with high constitutive ARC expression. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1510–H1517. [Google Scholar] [CrossRef]
- Jia, M.; Dahlman-Wright, K.; Gustafsson, J.Å. Estrogen receptor alpha and beta in health and disease. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 557–568. [Google Scholar] [CrossRef]
- Tasevska-Dinevska, G.; Kennedy, L.M.; Cline-Iwarson, A.; Cline, C.; Erhardt, L.; Willenheimer, R. Gender differences in variables related to B-natriuretic peptide, left ventricular ejection fraction and mass, and peak oxygen consumption, in patients with heart failure. Int. J. Cardiol. 2011, 149, 364–371. [Google Scholar] [CrossRef]
- Elmariah, S.; Goldberg, L.R.; Allen, M.T.; Kao, A. Effects of gender on peak oxygen consumption and the timing of cardiac transplantation. J. Am. Coll. Cardiol. 2006, 47, 2237–2242. [Google Scholar] [CrossRef]
- vanDeursen, V.M.; Urso, R.; Laroche, C.; Damman, K.; Dahlström, U.; Tavazzi, L.; Maggioni, A.P.; Voors, A.A. Co-morbidities in patients with heart failure: An analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 2014, 16, 103–111. [Google Scholar] [CrossRef]
- Hudson, M.; Rahme, E.; Behlouli, H.; Sheppard, R.; Pilote, L. Sex differences in the effectiveness of angiotensin receptor blockers and angiotensin converting enzyme inhibitors in patients with congestive heart failure—A population study. Eur. J. Heart Fail. 2007, 9, 602–609. [Google Scholar] [CrossRef]
- Ghali, J.K.; Lindenfeld, J. Sex differences in response to chronic heart failure therapies. Expert Rev. Cardiovasc. Ther. 2008, 6, 555–565. [Google Scholar] [CrossRef]
- Adams, K.F., Jr.; Patterson, J.H.; Gattis, W.A.; O’Connor, C.M.; Lee, C.R.; Schwartz, T.A.; Gheorghiade, M. Relationship of serum digoxin concentration to mortality and morbidity in women in the digitalis investigation group trial: A retrospective analysis. J. Am. Coll. Cardiol. 2005, 46, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Valentova, M.; von Haehling, S. An overview of recent developments in the treatment of heart failure: Update from the ESC Congress 2013. Expert Opin. Investig. Drugs 2014, 23, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Arutyunov, G.P.; Kostyukevich, O.I.; Serov, R.A.; Rylova, N.V.; Bylova, N.A. Collagen accumulation and dysfunctional mucosal barrier of the small intestine in patients with chronic heart failure. Int. J. Cardiol. 2008, 125, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef]
- Schwartz, J.B. The current state of knowledge on age, sex, and their interactions on clinical pharmacology. Clin. Pharmacol. Ther. 2007, 82, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sica, D.A.; Wood, M.; Hess, M. Gender and its effect in cardiovascular pharmacotherapeutics: Recent considerations. Congest. Heart Fail. 2005, 11, 163–166. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R.; Stachnik, J.M.; Echizen, H. Clinical pharmacokinetics of drugs in patients with heart failure: An update (part 1, drugs administered intravenously). Clin. Pharmacokinet. 2013, 52, 169–185. [Google Scholar] [CrossRef]
- Valentová, M.; von Haehling, S.; Doehner, W.; Murín, J.; Anker, S.D.; Sandek, A. Liver dysfunction and its nutritional implications in heart failure. Nutrition 2013, 29, 370–378. [Google Scholar] [CrossRef]
- Ogawa, R.; Stachnik, J.M.; Echizen, H. Clinical pharmacokinetics of drugs in patients with heart failure: An update (part 2, drugs administered orally). Clin. Pharmacokinet. 2014, 53, 1083–1114. [Google Scholar] [CrossRef]
- Mangoni, A.A.; Jarmuzewska, E.A. The influence of heart failure on the pharmacokinetics of cardiovascular and non-cardiovascular drugs: A critical appraisal of the evidence. Br. J. Clin. Pharmacol. 2019, 85, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Bader, F.; Atallah, B.; Brennan, L.F.; Rimawi, R.H.; Khalil, M.E. Heart failure in the elderly: Ten peculiar management considerations. Heart Fail. Rev. 2017, 22, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.A.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W.; et al. Cardiorenal Syndrome: Classification, Pathophysiology, Diagnosis, and Treatment Strategies: A Scientific Statement From the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Mathew, R.O. Pathophysiological Mechanisms in Cardiorenal Syndrome. Adv. Chronic. Kidney Dis. 2018, 25, 400–407. [Google Scholar] [CrossRef]
- Kousa, O.; Mullane, R.; Aboeata, A. Cardiorenal Syndrome. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK542305/ (accessed on 25 January 2022).
- Damman, K.; Testani, J.M. The kidney in heart failure: An update. Eur. Heart J. 2015, 36, 1437–1444. [Google Scholar] [CrossRef]
- Ronco, C.; Chionh, C.Y.; Haapio, M.; Anavekar, N.S.; House, A.; Bellomo, R. The cardiorenal syndrome. Blood Purif. 2009, 27, 114–126. [Google Scholar] [CrossRef]
- Anthony, J.; Sliwa, K. Decompensated Heart Failure in Pregnancy. Card Fail. Rev. 2016, 2, 20–26. [Google Scholar] [CrossRef]
- Stergiopoulos, K.; Lima, F.V.; Butler, J. Heart Failure in Pregnancy: A Problem Hiding in Plain Sight. J. Am. Heart Assoc. 2019, 8, e012905. [Google Scholar] [CrossRef]
- Dorn, G.W., 2nd. The fuzzy logic of physiological cardiac hypertrophy. Hypertension 2007, 49, 962–970. [Google Scholar] [CrossRef]
- Kearney, L.; Wright, P.; Fhadil, S.; Thomas, M. Postpartum Cardiomyopathy and Considerations for Breastfeeding. Card Fail. Rev. 2018, 4, 112–118. [Google Scholar] [CrossRef]
- Tschiderer, L.; Seekircher, L.; Kunutsor, S.K.; Peters, S.A.E.; O’Keeffe, L.M.; Willeit, P. Breastfeeding Is Associated with a Reduced Maternal Cardiovascular Risk: Systematic Review and Meta-Analysis Involving Data from 8 Studies and 1 192 700 Parous Women. J. Am. Heart Assoc. 2022, 11, e022746. [Google Scholar] [CrossRef] [PubMed]
- Von Lueder, T.G.; Atar, D. Comorbidities and polypharmacy. Heart Fail. Clin. 2014, 10, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Brockhattingen, K.K.; Anru, P.L.; Masud, T.; Petrovic, M.; Ryg, J. Association between number of medications and mortality in geriatric inpatients: A Danish nationwide register-based cohort study. Eur. Geriatr. Med. 2020, 11, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Page, R.L., 2nd; O’Bryant, C.L.; Cheng, D.; Dow, T.J.; Ky, B.; Stein, C.M.; Spencer, A.P.; Trupp, R.J.; Lindenfeld, J.; American Heart Association Clinical Pharmacology and Heart Failure and Transplantation Committees of the Council on Clinical Cardiology; et al. Drugs That May Cause or Exacerbate Heart Failure: A Scientific Statement from the American Heart Association. Circulation 2016, 134, e32–e69. [Google Scholar] [CrossRef] [PubMed]
- Sunaga, T.; Yokoyama, A.; Nakamura, S.; Miyamoto, N.; Watanabe, S.; Tsujiuchi, M.; Nagumo, S.; Nogi, A.; Maezawa, H.; Mizukami, T.; et al. Association of Potentially Inappropriate Medications with All-Cause Mortality in the Elderly Acute Decompensated Heart Failure Patients: Importance of Nonsteroidal Anti-Inflammatory Drug Prescription. Cardiol. Res. 2020, 11, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Jödicke, A.M.; Burden, A.M.; Zellweger, U.; Tomka, I.T.; Neuer, T.; Roos, M.; Kullak-Ublick, G.A.; Curkovic, I.; Egbring, M. Medication as a risk factor for hospitalization due to heart failure and shock: A series of case-crossover studies in Swiss claims data. Eur. J. Clin. Pharmacol. 2020, 76, 979–989. [Google Scholar] [CrossRef] [PubMed]
- Huerta, C.; Varas-Lorenzo, C.; Castellsague, J.; García Rodríguez, L.A. Non-steroidal anti-inflammatory drugs and risk of first hospital admission for heart failure in the general population. Heart 2006, 92, 1610–1615. [Google Scholar] [CrossRef]
- Silva Almodóvar, A.; Nahata, M.C. Potentially Harmful Medication Use among Medicare Patients with Heart Failure. Am. J. Cardiovasc. Drugs 2020, 20, 603–610. [Google Scholar] [CrossRef]
- Arfè, A.; Scotti, L.; Varas-Lorenzo, C.; Zambon, A.; Kollhorst, B.; Schink, T.; Garbe, E.; Herings, R.; Straatman, H.; Schade, R.; et al. Safety of Non-steroidal Anti-inflammatory Drugs (SOS) Project Consortium. Non-steroidal anti-inflammatory drugs and risk of heart failure in four European countries: Nested case-control study. BMJ 2016, 354, i4857. [Google Scholar] [CrossRef]
- Alvarez, P.A.; Putney, D.; Ogunti, R.; Puppala, M.; Ganduglia, C.; Torre-Amione, G.; Schutt, R.; Wong, S.T.C.; Estep, J.D. Prevalence of in-hospital nonsteroidal antiinflammatory drug exposure in patients with a primary diagnosis of heart failure. Cardiovasc. Ther. 2017, 35, e12256. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, P.A.; Gao, Y.; Girotra, S.; Mentias, A.; Briasoulis, A.; Vaughan Sarrazin, M.S. Potentially harmful drug prescription in elderly patients with heart failure with reduced ejection fraction. ESC Heart Fail. 2020, 7, 1862–1871. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Li, M.; Wagner, M.B.; Chen, G.; Song, X. Doxazosin Stimulates Galectin-3 Expression and Collagen Synthesis in HL-1 Cardiomyocytes Independent of Protein Kinase C Pathway. Front. Pharmacol. 2016, 7, 495. [Google Scholar] [CrossRef] [PubMed]
- Edwards, L.P.; Brown-Bryan, T.A.; McLean, L.; Ernsberger, P. Pharmacological properties of the central antihypertensive agent, moxonidine. Cardiovasc. Ther. 2012, 30, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Valembois, L.; Audureau, E.; Takeda, A.; Jarzebowski, W.; Belmin, J.; Lafuente-Lafuente, C. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst. Rev. 2019, 9, CD005049. [Google Scholar] [CrossRef]
- Frommeyer, G.; Milberg, P.; Witte, P.; Stypmann, J.; Koopmann, M.; Lücke, M.; Osada, N.; Breithardt, G.; Fehr, M.; Eckardt, L. A new mechanism preventing proarrhythmia in chronic heart failure: Rapid phase-III repolarization explains the low proarrhythmic potential of amiodarone in contrast to sotalol in a model of pacing-induced heart failure. Eur. J. Heart Fail. 2011, 13, 1060–1069. [Google Scholar] [CrossRef]
- Finks, S.W.; Rogers, K.C.; Manguso, A.H. Assessment of sotalol prescribing in a community hospital: Opportunities for clinical pharmacist involvement. Int. J. Pharm. Pract. 2011, 19, 281–286. [Google Scholar] [CrossRef]
- Kongwatcharapong, J.; Dilokthornsakul, P.; Nathisuwan, S.; Phrommintikul, A.; Chaiyakunapruk, N. Effect of dipeptidyl peptidase-4 inhibitors on heart failure: A meta-analysis of randomized clinical trials. Int. J. Cardiol. 2016, 211, 88–95. [Google Scholar] [CrossRef]
- Savarese, G.; Schrage, B.; Cosentino, F.; Lund, L.H.; Rosano, G.M.C.; Seferovic, P.; Butler, J. Non-insulin antihyperglycaemic drugs and heart failure: An overview of current evidence from randomized controlled trials. ESC Heart Fail. 2020, 7, 3438–3451. [Google Scholar] [CrossRef]
- Patel, K.V.; Sarraju, A.; Neeland, I.J.; McGuire, D.K. Cardiovascular Effects of Dipeptidyl Peptidase-4 Inhibitors and Glucagon-Like Peptide-1 Receptor Agonists: A Review for the General Cardiologist. Curr. Cardiol. Rep. 2020, 22, 105. [Google Scholar] [CrossRef]
- Nassif, M.E.; Kosiborod, M. A Review of Cardiovascular Outcomes Trials of Glucose-Lowering Therapies and Their Effects on Heart Failure Outcomes. Am. J. Cardiol. 2019, 124 (Suppl. S1), S12–S19. [Google Scholar] [CrossRef]
- Hantson, P. Mechanisms of toxic cardiomyopathy. Clin. Toxicol. 2019, 57, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wallach, J.D.; Wang, K.; Zhang, A.D.; Cheng, D.; Grossetta Nardini, H.K.; Lin, H.; Bracken, M.B.; Desai, M.; Krumholz, H.M.; Ross, J.S. Updating insights into rosiglitazone and cardiovascular risk through shared data: Individual patient and summary level meta-analyses. BMJ 2020, 368, l7078. [Google Scholar] [CrossRef] [PubMed]
- Teaford, H.R.; Abu Saleh, O.M.; Villarraga, H.R.; Enzler, M.J.; Rivera, C.G. The Many Faces of Itraconazole Cardiac Toxicity. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Abraham, A.O.; Panda, P.K. Itraconazole Induced Congestive Heart Failure, A Case Study. Curr. Drug Saf. 2018, 13, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Paul, V.; Rawal, H. Cardiotoxicity with Itraconazole. BMJ Case Rep. 2017, 2017, bcr2017219376. [Google Scholar] [CrossRef]
- Soares, J.R.; Nunes, M.C.; Leite, A.F.; Falqueto, E.B.; Lacerda, B.E.; Ferrari, T.C. Reversible dilated cardiomyopathy associated with amphotericin B therapy. J. Clin. Pharm. Ther. 2015, 40, 333–335. [Google Scholar] [CrossRef]
- Mégarbane, B.; Leprince, P.; Deye, N.; Guerrier, G.; Résière, D.; Bloch, V.; Baud, F.J. Extracorporeal life support in a case of acute carbamazepine poisoning with life-threatening refractory myocardial failure. Intensive Care Med. 2006, 32, 1409–1413. [Google Scholar] [CrossRef]
- Faisy, C.; Guerot, E.; Diehl, J.L.; Rezgui, N.; Labrousse, J. Carbamazepine-associated severe left ventricular dysfunction. J. Toxicol. Clin. Toxicol. 2000, 38, 339–342. [Google Scholar] [CrossRef]
- Takamiya, M.; Aoki, Y.; Niitsu, H.; Saigusa, K. A case of carbamazepine overdose with focal myocarditis. Leg. Med. 2006, 8, 243–247. [Google Scholar] [CrossRef]
- Lund, M.; Poulsen, G.; Pasternak, B.; Worm Andersson, N.; Melbye, M.; Svanström, H. Use of Pregabalin and Worsening Heart Failure: A Nationwide Cohort Study. Drug Saf. 2020, 43, 1035–1044. [Google Scholar] [CrossRef]
- Awwad, Z.M.; El-Ganainy, S.O.; ElMallah, A.I.; Khedr, S.M.; Khattab, M.M.; El-Khatib, A.S. Assessment of Pregabalin-Induced Cardiotoxicity in Rats: Mechanistic Role of Angiotensin 17–7. Cardiovasc. Toxicol. 2020, 20, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.M.; Tricco, A.C.; Perrier, L.; Chen, M.; Juurlink, D.N.; Straus, S.E. Risk of heart failure and edema associated with the use of pregabalin: A systematic review. Syst. Rev. 2013, 2, 25. [Google Scholar] [CrossRef] [PubMed]
- Nezafati, M.H.; Vojdanparast, M.; Nezafati, P. Antidepressants and cardiovascular adverse events: A narrative review. ARYA Atheroscler. 2015, 11, 295–304. [Google Scholar] [PubMed]
- Biffi, A.; Rea, F.; Scotti, L.; Lucenteforte, E.; Vannacci, A.; Lombardi, N.; Chinellato, A.; Onder, G.; Vitale, C.; Cascini, S.; et al. Antidepressants and the Risk of Cardiovascular Events in Elderly Affected by Cardiovascular Disease: A Real-Life Investigation From Italy. J. Clin. Psychopharmacol 2020, 40, 112–121. [Google Scholar] [CrossRef]
- Deshmukh, A.; Ulveling, K.; Alla, V.; Abuissa, H.; Airey, K. Prolonged QTc interval and torsades de pointes induced by citalopram. Tex. Heart Inst. J. 2012, 39, 68–70. [Google Scholar]
- Assimon, M.M.; Brookhart, M.A.; Flythe, J.E. Comparative Cardiac Safety of Selective Serotonin Reuptake Inhibitors among Individuals Receiving Maintenance Hemodialysis. J. Am. Soc. Nephrol. 2019, 30, 611–623. [Google Scholar] [CrossRef]
- Tran, T.; Brophy, J.M.; Suissa, S.; Renoux, C. Risks of Cardiac Valve Regurgitation and Heart Failure Associated with Ergot- and Non-Ergot-Derived Dopamine Agonist Use in Patients with Parkinson’s Disease: A Systematic Review of Observational Studies. CNS Drugs 2015, 29, 985–998. [Google Scholar] [CrossRef]
- Montastruc, F.; Moulis, F.; Araujo, M.; Chebane, L.; Rascol, O.; Montastruc, J.L. Ergot and non-ergot dopamine agonists and heart failure in patients with Parkinson’s disease. Eur. J. Clin. Pharmacol. 2017, 73, 99–103. [Google Scholar] [CrossRef]
- Renoux, C.; Dell’Aniello, S.; Brophy, J.M.; Suissa, S. Dopamine agonist use and the risk of heart failure. Pharmacoepidemiol. Drug Saf. 2012, 21, 34–41. [Google Scholar] [CrossRef]
- Patuszynski, D.; Applegate, P.M. Suspected Clozapine-Induced Cardiomyopathy and Heart Failure with Reduced Ejection Fraction. Fed. Pract. 2017, 34, 20–22. [Google Scholar]
- Whiskey, E.; Yuen, S.; Khosla, E.; Piper, S.; O’Flynn, D.; Taylor, D. Resolution without discontinuation: Heart failure during clozapine treatment. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320924786. [Google Scholar] [CrossRef]
- Garg, A.; Bath, A.S.; Kalavakunta, J.K. Non-ischemic Cardiomyopathy: A Rare Adverse Effect of Clozapine. Cureus 2020, 12, e7901. [Google Scholar] [CrossRef] [PubMed]
- Chow, V.; Yeoh, T.; Ng, A.C.; Pasqualon, T.; Scott, E.; Plater, J.; Whitwell, B.; Hanzek, D.; Chung, T.; Thomas, L.; et al. Asymptomatic left ventricular dysfunction with long-term clozapine treatment for schizophrenia: A multicentre cross-sectional cohort study. Open Heart 2014, 1, e000030. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Gholamifar, E.; Naserzadeh, P.; Hosseini, M.J.; Pourahmad, J. Toxicity of lithium on isolated heart mitochondria and cardiomyocyte: A justification for its cardiotoxic adverse effect. J. Biochem. Mol. Toxicol. 2017, 31, e21836. [Google Scholar] [CrossRef] [PubMed]
- Mezni, A.; Aoua, H.; Khazri, O.; Limam, F.; Aouani, E. Lithium induced oxidative damage and inflammation in the rat’s heart: Protective effect of grape seed and skin extract. Biomed. Pharmacother. 2017, 95, 1103–1111. [Google Scholar] [CrossRef]
- Asim, K.; Selman, Y.; Suleyman, Y.; Ozgur, K.; Ozlem, B.; Gokhan, E. Heart Attack in the Course of Lithium Overdose. Iran. Red Crescent Med. J. 2016, 18, e21731. [Google Scholar] [CrossRef]
- Acharya, S.; Siddiqui, A.H.; Anwar, S.; Habib, S.; Anwar, S. Lithium-induced Cardiotoxicity: A Rare Clinical Entity. Cureus 2020, 12, e7286, Erratum in Cureus 2020, 12, c33. [Google Scholar] [CrossRef]
- Ataallah, B.; Al-Zakhari, R.; Sharma, A.; Tofano, M.; Haggerty, G. A Rare but Reversible Cause of Lithium-Induced Bradycardia. Cureus 2020, 12, e8600. [Google Scholar] [CrossRef]
- Wang, Y.; Yuan, J.; Qian, Z.; Zhang, X.; Chen, Y.; Hou, X.; Zou, J. β2 adrenergic receptor activation governs cardiac repolarization and arrhythmogenesis in a guinea pig model of heart failure. Sci. Rep. 2015, 5, 7681. [Google Scholar] [CrossRef]
- Say, B.; Degirmencioglu, H.; Kutman, H.; Uras, N.; Dilmen, U. Supraventricular tachycardia after nebulized salbutamol therapy in a neonate: Case report. Arch. Argent. Pediatr. 2015, 113, e98–e100, (In English and Spanish). [Google Scholar] [CrossRef]
- Yu, Y.; Wei, S.G.; Weiss, R.M.; Felder, R.B. TNF-α receptor 1 knockdown in the subfornical organ ameliorates sympathetic excitation and cardiac hemodynamics in heart failure rats. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H744–H756. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Cao, Y.; Bell, B.; Chen, X.; Weiss, R.M.; Felder, R.B.; Wei, S.G. Brain TACE (Tumor Necrosis Factor-α-Converting Enzyme) Contributes to Sympathetic Excitation in Heart Failure Rats. Hypertension 2019, 74, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.M.; Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Suroowan, S.; Mahomoodally, F. Common phyto-remedies used against cardiovascular diseases and their potential to induce adverse events in cardiovascular patients. Clin. Phytoscience 2015, 1, 1. [Google Scholar] [CrossRef][Green Version]
- Kim, E.J.Y.; Chen, Y.; Huang, J.Q.; Li, K.M.; Razmovski-Naumovski, V.; Poon, J.; Li, K.M.; Razmovski-Naumovski, V.; Poon, J.; Chan, K.; et al. Evidence-based toxicity evaluation and scheduling of Chinese herbal medicines. J. Ethnopharmacol. 2013, 146, 40–61. [Google Scholar] [CrossRef]
- Alternative Medicine Review. Aesculus hippocacastanum. 2009, 14, 278–283.
- Tachjian, A.; Maria, V.; Jahangir, A. Use of herbal products and potential interactions in patients with cardiovascular diseases. J. Am. Coll. Cardiol. 2010, 55, 515–525. [Google Scholar] [CrossRef]
- World Health Organization. Aloe. Folium Sennae, Fructus Sennae. Radix Glycyrrhizae. WHO Monograph on Selected Medicinal Plants—Volume 1. ISBN: 9241545178. Available online: http://apps.who.int/medicinedocs/en/d/Js2200e/5.html (accessed on 25 January 2022)ISBN 9241545178.
- World Health Organization. Radix Angelicae Sinensis. WHO Monograph on Selected Medicinal Plants—Volume 2. Available online: http://apps.who.int/medicinedocs/en/d/Js4927e/5.html (accessed on 25 January 2022).
- Agosti, S.; Casalino, L.; Bertero, G.; Barsotti, A.; Brunelli, C.; Morelloni, S. A dangerous fruit juice. Am. J. Emerg. Med. 2012, 30, 248.e5–248.e8. [Google Scholar] [CrossRef]
- Papandreou, D.; Phily, A. An updated mini review on grapefruit: Interactions with drugs, obesity and cardiovascular Risk factors. Food Nutr. Sci. 2014, 5, 376–381. [Google Scholar] [CrossRef][Green Version]
- Tassell, M.; Kingston, R.; Gilroy, D.; Lehane, M.; Furey, A. Hawthorn (Crataegus spp.) in the treatment of cardiovascular disease. Pharmacogn. Rev. 2010, 4, 32. [Google Scholar]
- Pittler, M.H.; Schmidt, K.; Ernst, E. Hawthorn extract for treating chronic heart failure: Meta-analysis of randomized trials. Am. J Med 2003, 114, 665–674. [Google Scholar] [CrossRef]
- Chen, W.L.; Tsai, T.H.; Yang, C.C.H.; Kuo, T.B.J. Effects of ephedra on autonomic nervous modulation in healthy young adults. J. Ethnopharmacol. 2010, 130, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Koch, E. Inhibition of platelet activating factor (PAF)-induced aggregation of human thrombocytes by ginkgolides: Considerations on possible bleeding complications after oral intake of Ginkgo biloba extracts. Phytomed 2005, 12, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Bone, K.M. Potential interaction of Ginkgo biloba leaf with antiplatelet or anticoagulant drugs: What is the evidence? Mol. Nutr. Food Res. 2008, 52, 764–771. [Google Scholar] [CrossRef]
- Pengelly, A. Harpagophytum procumbens. Altern. Med. Rev. 2008, 13, 248–252. [Google Scholar]
- Calitz, C.; Steenekamp, J.H.; Steyn, J.D.; Gouws, C.; Viljoen, J.M.; Hamman, J.H. Impact of traditional African medicine on drug metabolism and transport. Expert Opin. Drug Metab. Toxicol. 2014, 10, 991–1003. [Google Scholar] [CrossRef]
- Johne, A.; Brockmoller, J.; Bauer, S.; Maurer, A.; Langheinrich, M.; Roots, I. Pharmacokinetic interactionof digoxin with an herbal extract from St John’s wort (Hypericum perforatum). Clin. Pharmacol. Ther. 1999, 66, 338–345. [Google Scholar] [CrossRef]
- Yue, Q.Y.; Bergquist, C.; Gerden, B. Safety of St John’s wort (Hypericum perforatum). Lancet 2002, 355, 576–577. [Google Scholar] [CrossRef]
- Henderson, L.; Yue, Q.Y.; Bergquist, C.; Gerden, B.; Arlett, P. St John’s wort (Hypericum perforatum): Drug interactions and clinical outcomes. Br. J. Clin. Pharmacol. 2002, 54, 349–356. [Google Scholar] [CrossRef]
- World Health Organization. Oleum OenotheraeBiennis. WHO Monograph on Selected Medicinal Plants—Volume 2. Available online: http://apps.who.int/medicinedocs/en/d/Js4927e/22.html (accessed on 25 January 2022).
- Fecker, R.; Buda, V.; Alexa, E.; Avram, S.; Pavel, I.Z.; Muntean, D.; Cocan, I.; Watz, C.; Minda, D.; Dehelean, C.A.; et al. Phytochemical and biological screening of Oenothera biennis L. Hydroalcoholic extract. Biomolecules 2020, 10, 818. [Google Scholar] [CrossRef]
- Frishman, W.H.; Beravol, P.; Carosella, C. Alternative and complementary medicine for preventing and treating cardiovascular disease. Dis. Mon. 2009, 55, 121–192. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. RhizomaZingiberis. WHO Monograph on Selected Medicinal Plants–Volume 1. Available online: http://apps.who.int/medicinedocs/en/d/Js2200e/30.html (accessed on 25 January 2022).
- Georgiev, K.D.; Hvarchanova, N.; Georgieva, M.; Kanazirev, B. The role of the clinical pharmacist in the prevention of potential drug interactions in geriatric heart failure patients. Int. J. Clin. Pharm. 2019, 41, 1555–1561. [Google Scholar] [CrossRef] [PubMed]
- Bhagat, A.A.; Greene, S.J.; Vaduganathan, M.; Fonarow, G.C.; Butler, J. Initiation, Continuation, Switching, and Withdrawal of Heart Failure Medical Therapies During Hospitalization. JACC Heart Fail. 2019, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.L.; Marrs, J.C. A Review of the Role of the Pharmacist in Heart Failure Transition of Care. Adv. Ther. 2018, 35, 311–323. [Google Scholar] [CrossRef]
- Investigators of the MAGIC-PHARM Study; Khazaka, M.; Laverdière, J.; Li, C.C.; Correal, F.; Mallet, L.; Poitras, M.; Nguyen, P.V. Medication appropriateness on an acute geriatric care unit: The impact of the removal of a clinical pharmacist. Age Ageing 2020, 50, afaa175. [Google Scholar] [CrossRef]
- Association Nationale Des Enseignants de Pharmacie Clinique; Limat, S.; Dupuis, A.; Fagnoni, P.; Deamore, B.; Fernandez, C.; Aulagner, G.; Cazin, J.L. Pharmacie Clinique et Therapeutique; Elsevier: Amsterdam, The Netherlands, 2018; ISBN 9782294750779. [Google Scholar]
- Preston, C.L. Stockley’s Drug Interactions, 12th ed.; Pharmaceutical Press: London, UK, 2019; ISBN 978-0-85-711347-4. [Google Scholar]
- Sica, D.A. Angiotensin-converting enzyme inhibitors side effects—Physiologic and non-physiologic considerations. J. Clin. Hypertens 2004, 6, 410–416. [Google Scholar] [CrossRef]
- Wiggins, B.S.; Saseen, J.J.; Page, R.L., 2nd; Reed, B.N.; Sneed, K.; Kostis, J.B.; Lanfear, D.; Virani, S.; Morris, P.B.; American Heart Association Clinical Pharmacology Committee of the Council on Clinical Cardiology; et al. Recommendations for Management of Clinically Significant Drug-Drug Interactions With Statins and Select Agents Used in Patients With Cardiovascular Disease: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e468–e495. [Google Scholar] [CrossRef]
- Hsiao, H.L.; Langenickel, T.H.; Petruck, J.; Kode, K.; Ayalasomayajula, S.; Schuehly, U.; Greeley, M.; Pal, P.; Zhou, W.; Prescott, M.F.; et al. Evaluation of Pharmacokinetic and Pharmacodynamic Drug-Drug Interaction of Sacubitril/Valsartan (LCZ696) and Sildenafil in Patients with Mild-to-Moderate Hypertension. Clin. Pharmacol. Ther. 2018, 103, 468–476. [Google Scholar] [CrossRef]
- Referentiel National des Interactions Medicamenteuses—ANSM. Thesaurus des Interactions Medicamenteuses 2019. Available online: https://ansm.sante.fr/var/ansm_site/storage/original/application/0002510e4ab3a9c13793a1fdc0d4c955.pdf (accessed on 25 January 2022).
- Singh, G.; Correa, R. Methimazole. StatPearls [Internet]. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK545223/ (accessed on 25 January 2022).
- Steffel, J.; Verhamme, P.; Potpara, T.S.; Albaladejo, P.; Antz, M.; Desteghe, L.; Haeusler, K.G.; Oldgren, J.; Reinecke, H.; Roldan-Schilling, V.; et al. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur. Heart J. 2018, 39, 1330–1393. [Google Scholar] [CrossRef]
- Frost, C.E.; Byon, W.; Song, Y.; Wang, J.; Schuster, A.E.; Boyd, R.A.; Zhang, D.; Yu, Z.; Dias, C.; Shenker, A.; et al. Effect of ketoconazole and diltiazem on the pharmacokinetics of apixaban, an oral direct factor Xa inhibitor. Br. J. Clin. Pharmacol. 2015, 79, 838–846. [Google Scholar] [CrossRef]
- Bundhun, P.K.; Teeluck, A.R.; Bhurtu, A.; Huang, W.Q. Is the concomitant use of clopidogrel and Proton Pump Inhibitors still associated with increased adverse cardiovascular outcomes following coronary angioplasty? A systematic review and meta-analysis of recently published studies (2012–2016). BMC Cardiovasc. Disord. 2017, 17, 3. [Google Scholar] [CrossRef] [PubMed]
- Kariyanna, P.T.; Haseeb, S.; Chowdhury, Y.S.; Jayarangaiah, A.; Maryniak, A.; Mo, G.; Hegde, S.; Marmur, J.D.; McFarlane, I.M. Ticagrelor and Statin Interaction Induces Rhabdomyolysis and Acute Renal Failure: Case reports and Scoping Review. Am. J. Med. Case Rep. 2019, 7, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Oscanoa, T.J.; Lizaraso, F.; Carvajal, A. Hospital admissions due to adverse drug reactions in the elderly. A meta-analysis. Eur. J. Clin. Pharmacol. 2017, 73, 759–770. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.; Banerjee, P. Iatrogenic Decompensated Heart Failure. Curr. Heart Fail. Rep. 2020, 17, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Rich, M.W.; Shah, A.S.; Vinson, J.M.; Freedland, K.E.; Kuru, T.; Sperry, J.C. Iatrogenic congestive heart failure in older adults: Clinical course and prognosis. J. Am. Geriatr. Soc. 1996, 44, 638–643. [Google Scholar] [CrossRef]
- Bots, S.H.; Groepenhoff, F.; Eikendal, A.L.M.; Tannenbaum, C.; Rochon, P.A.; Regitz-Zagrosek, V.; Miller, V.M.; Day, D.; Asselbergs, F.W.; den Ruijter, H.M. Adverse Drug Reactions to Guideline-Recommended Heart Failure Drugs in Women: A Systematic Review of the Literature. JACC Heart Fail. 2019, 7, 258–266. [Google Scholar] [CrossRef]
- Tamargo, J.; Rosano, G.; Walther, T.; Duarte, J.; Niessner, A.; Kaski, J.C.; Ceconi, C.; Drexel, H.; Kjeldsen, K.; Savarese, G.; et al. Gender differences in the effects of cardiovascular drugs. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 163–182. [Google Scholar] [CrossRef]
- Soldin, O.P.; Chung, S.; Mattison, D.R. Sex differences in drug disposition. J. Biomed. Biotechnol. 2011, 2011, 187103. [Google Scholar] [CrossRef]
- Soldin, O.P.; Mattison, D.R. Sex differences in pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 2009, 48, 143–158. [Google Scholar] [CrossRef]
- Drici, M.D.; Clement, N. Is gender a risk factor for adverse drug reactions? The example of drug-induced long QT syndrome. Drug Saf. 2001, 24, 575–585. [Google Scholar] [CrossRef]
- Yap, Y.G.; Camm, A.J. Drug induced QT prolongation and torsades de pointes. Heart 2003, 89, 1363–1372. [Google Scholar] [CrossRef] [PubMed]
- Pratt, C.M.; Camm, A.J.; Cooper, W.; Friedman, P.L.; MacNeil, D.J.; Moulton, K.M.; Pitt, B.; Schwartz, P.J.; Veltri, E.P.; Waldo, A.L.; et al. Mortality in the Survival WithORal D-Sotalol (SWORD) trial: Why did patients die? Am. J. Cardiol. 1998, 81, 869–876. [Google Scholar] [CrossRef]
- Jochmann, N.; Stangl, K.; Garbe, E.; Baumann, G.; Stangl, V. Female-specific aspects in the pharmacotherapy of chronic cardiovascular diseases. Eur. Heart J. 2005, 26, 1585–1595. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Angiolillo, D.J. Impact of race and gender on antithrombotic therapy. Thromb. Haemost. 2012, 104, 471–484. [Google Scholar] [CrossRef]
- Gilstrap, L.G.; Fonarow, G.C.; Desai, A.S.; Fonarow, G.C.; Butler, J. Initiation, Continuation, or Withdrawal of Angiotensin-Converting Enzyme Inhibitors/Angiotensin Receptor Blockers and Outcomes in Patients Hospitalized with Heart Failure with Reduced Ejection Fraction. J. Am. Heart Assoc. 2017, 6, e004675. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.T. Beta-blockade withdrawal. Lancet 1975, 2, 819. [Google Scholar] [CrossRef]
- Miller, R.R.; Olson, H.G.; Amsterdam, E.A.; Mason, D.T. Propranolol-withdrawal rebound phenomenon. Exacerbation of coronary events after abrupt cessation of antianginal therapy. N. Engl. J. Med. 1975, 293, 416–418. [Google Scholar] [CrossRef]
- Clark, H.; Krum, H.; Hopper, I. Worsening renal function during renin-angiotensin-aldosterone system inhibitor initiation and long-term outcomes in patients with left ventricular systolic dysfunction. Eur. J. Heart Fail. 2014, 16, 41–48. [Google Scholar] [CrossRef]
- Clark, A.L.; Kalra, P.R.; Petrie, M.C.; Mark, P.B.; Tomlinson, L.A.; Tomson, C.R. Change in renal function associated with drug treatment in heart failure: National guidance. Heart 2019, 105, 904–910. [Google Scholar] [CrossRef]
- Jondeau, G.; Neuder, Y.; Eicher, J.C.; Jourdain, P.; Fauveau, E.; Galinier, M.; Jegou, A.; Bauer, F.; Trochu, J.N.; Bouzamondo, A.; et al. B-CONVINCED: Beta-blocker CONtinuationVs. INterruption in patients with Congestive heart failure hospitalizED for a decompensation episode. Eur. Heart J. 2009, 30, 2186–2192. [Google Scholar] [CrossRef]
- Fonarow, G.C.; Abraham, W.T.; Albert, N.M.; Stough, W.G.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Nunez, E.; Yancy, C.W.; Young, J.B. A smoker’s paradox in patients hospitalized for heart failure: Findings from OPTIMIZE-HF. Eur. Heart J. 2008, 29, 1983–1991. [Google Scholar] [CrossRef] [PubMed]
- Halliday, B.P.; Wassall, R.; Lota, A.S.; Khalique, Z.; Gregson, J.; Newsome, S.; Jackson, R.; Rahneva, T.; Wage, R.; Smith, G.; et al. Withdrawal of pharmacological treatment for heart failure in patients with recovered dilated cardiomyopathy (TRED-HF): An open-label, pilot, randomized trial. Lancet 2019, 393, 61–73. [Google Scholar] [CrossRef]
| ARNI | SGLT2 Inhibitors | |
|---|---|---|
| Indications | ▪ HFrEF (≤40%) ▪ NYHA class II-IV ▪ Alternative of ACEI/ARB | ▪ HFrEF (≤40%) ± diabetes mellitus ▪ NYHA class II-IV |
| Contra-indications | - hypersensitivity to the active substances - history of angioedema - severe hepatic impairment - ≤36 h of the last ACEI dose | - hypersensitivity to the active substance - type I diabetes - dialysis - eGFR < 30 mL/min/1.73 m2 (dapagliflozin) - eGFR < 20 mL/min/1.73 m2 (empagliflozin) |
| Cautions | ◊ severe renal impairment (starting dose: 24/26 mg × 2/day) ◊ moderate hepatic impairment (starting dose: 24/26 mg × 2/day) ◊ SBP < 100 mmHg ◊ volume depletion ◊ renal artery stenosis ◊ pregnancy/lactation | ◊ high risk of genital infections (especially mycotic) and urinary infections ◊ hypovolemia ◊ ketoacidosis ◊ acute renal impairment ◊ necrotizing fasciitis of the perineum (Fournier gangrene) ◊ bladder cancer ◊ pregnancy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buda, V.; Prelipcean, A.; Cozma, D.; Man, D.E.; Negres, S.; Scurtu, A.; Suciu, M.; Andor, M.; Danciu, C.; Crisan, S.; et al. An Up-to-Date Article Regarding Particularities of Drug Treatment in Patients with Chronic Heart Failure. J. Clin. Med. 2022, 11, 2020. https://doi.org/10.3390/jcm11072020
Buda V, Prelipcean A, Cozma D, Man DE, Negres S, Scurtu A, Suciu M, Andor M, Danciu C, Crisan S, et al. An Up-to-Date Article Regarding Particularities of Drug Treatment in Patients with Chronic Heart Failure. Journal of Clinical Medicine. 2022; 11(7):2020. https://doi.org/10.3390/jcm11072020
Chicago/Turabian StyleBuda, Valentina, Andreea Prelipcean, Dragos Cozma, Dana Emilia Man, Simona Negres, Alexandra Scurtu, Maria Suciu, Minodora Andor, Corina Danciu, Simina Crisan, and et al. 2022. "An Up-to-Date Article Regarding Particularities of Drug Treatment in Patients with Chronic Heart Failure" Journal of Clinical Medicine 11, no. 7: 2020. https://doi.org/10.3390/jcm11072020
APA StyleBuda, V., Prelipcean, A., Cozma, D., Man, D. E., Negres, S., Scurtu, A., Suciu, M., Andor, M., Danciu, C., Crisan, S., Dehelean, C. A., Petrescu, L., & Rachieru, C. (2022). An Up-to-Date Article Regarding Particularities of Drug Treatment in Patients with Chronic Heart Failure. Journal of Clinical Medicine, 11(7), 2020. https://doi.org/10.3390/jcm11072020










