Effects of COPD on Left Ventricular and Left Atrial Deformation in Patients with Acute Myocardial Infarction: Strain Analysis Using Speckle-Tracking Echocardiography
Abstract
:1. Introduction
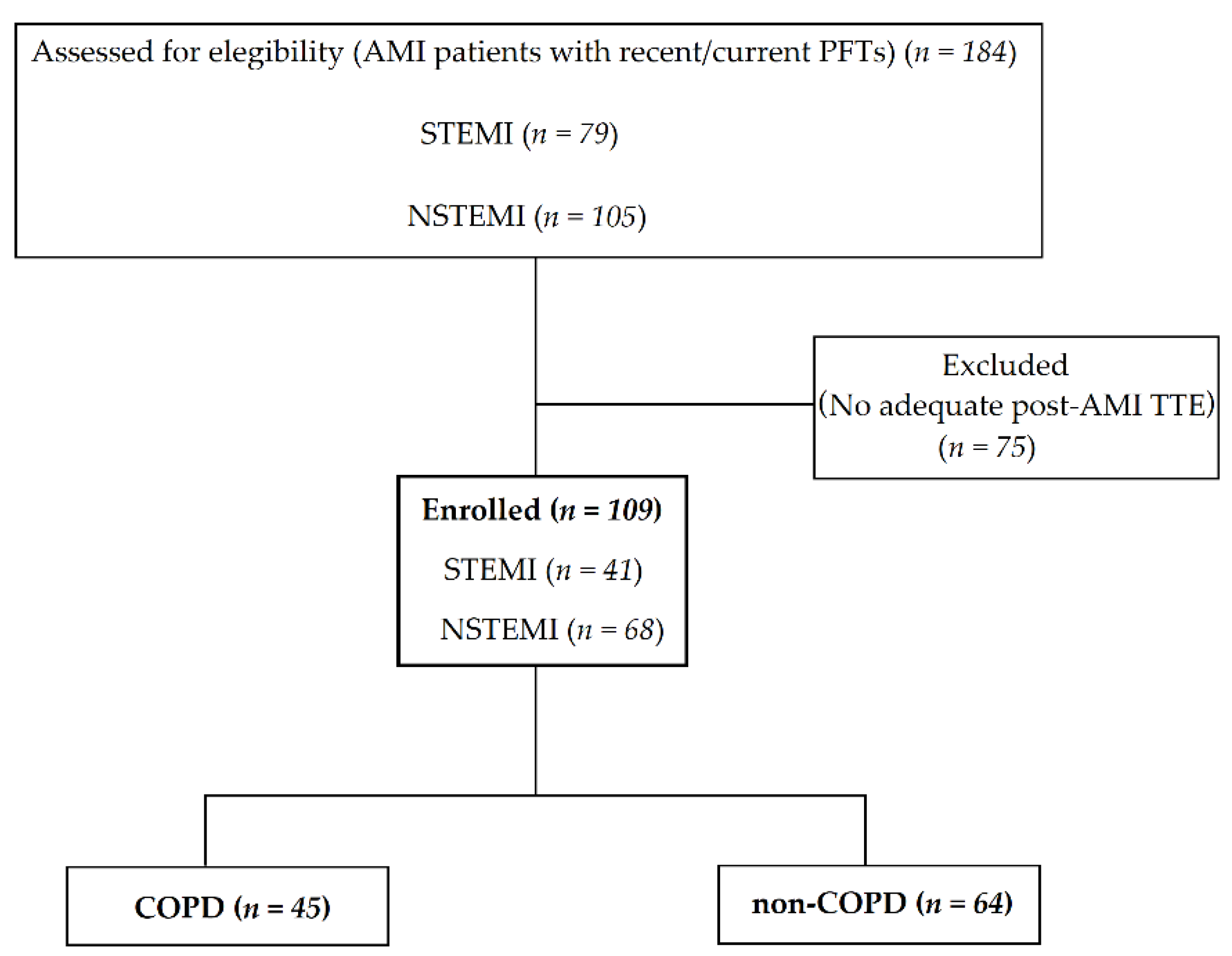
2. Materials and Methods
- -
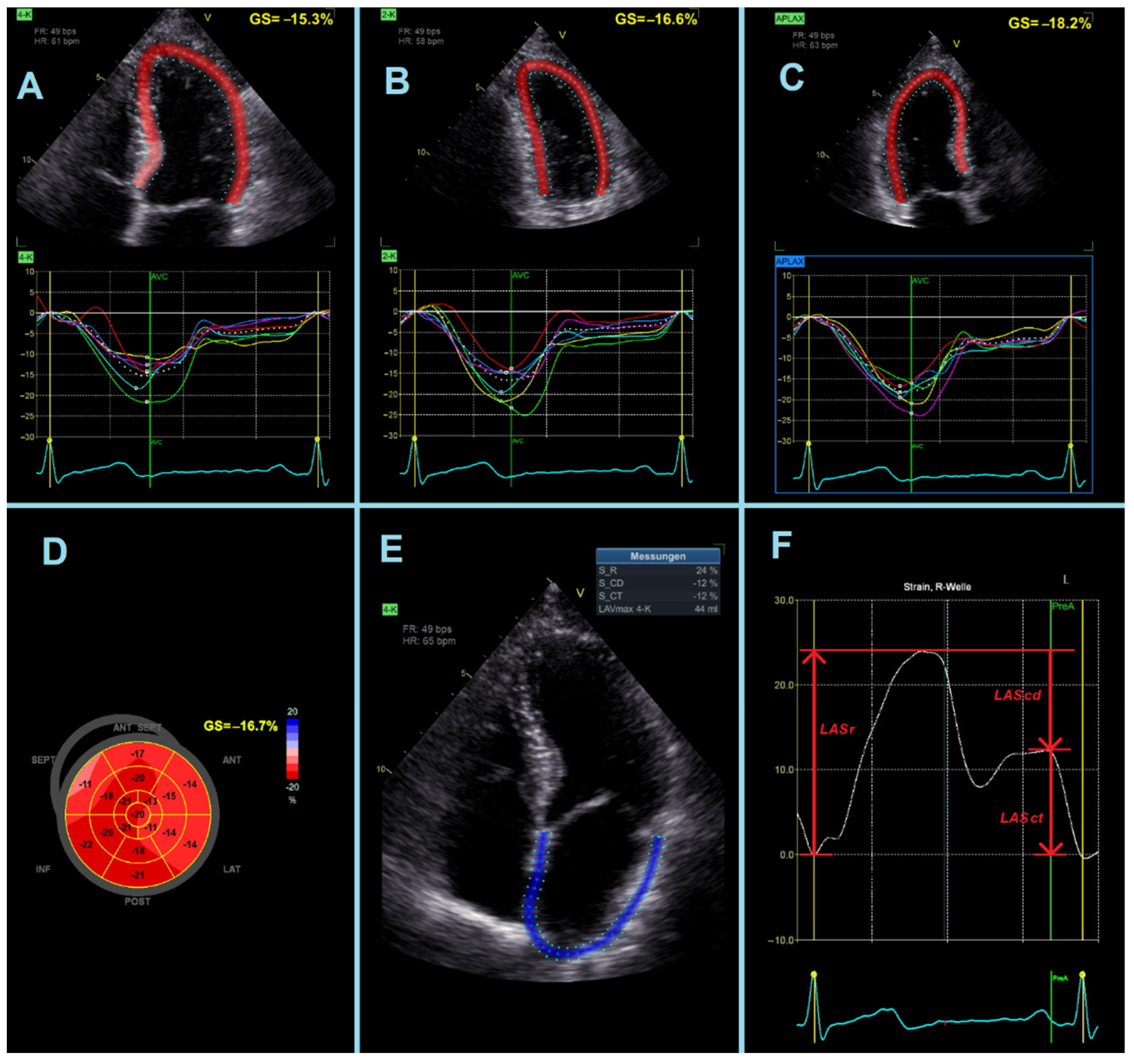
- LASr = strain during reservoir phase, measured as the strain value from the ventricular end-diastole to the mitral valve opening at ventricular end-systole (positive value).
- -
- LAScd = strain during conduit phase, measured as the strain value from the mitral valve opening to the onset of atrial contraction (negative value). In patients with atrial fibrillation, LAScd has the same value as LASr, but with a negative sign.
- -
- LASct = strain during contraction phase, measured only in patients in sinus rhythm as the strain value from the onset of atrial contraction to ventricular end-diastole (negative value).
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sin, D.D.; Anthonisen, N.R.; Soriano, J.B.; Agusti, A.G. Mortality in COPD: Role of comorbidities. Eur. Respir. J. 2006, 28, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Daher, A.; Dreher, M. The bidirectional relationship between chronic obstructive pulmonary disease and coronary artery disease. Herz 2020, 45, 110–117. [Google Scholar] [CrossRef] [PubMed]
- López-Campos, J.L.; Tan, W.; Soriano, J.B. Global burden of COPD. Respirology 2016, 21, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Campo, G.; Guastaroba, P.; Marzocchi, A.; Santarelli, A.; Varani, E.; Vignali, L.; Sangiorgio, P.; Tondi, S.; Serenelli, C.; De Palma, R.; et al. Impact of COPD on long-term outcome after ST-segment elevation myocardial infarction receiving primary percutaneous coronary intervention. Chest 2013, 144, 750–757. [Google Scholar] [CrossRef] [Green Version]
- Jatene, T.; Biering-Sørensen, T.; Nochioka, K.; Mangione, F.M.; Hansen, K.W.; Sørensen, R.; Jensen, J.S.; Jørgensen, P.G.; Jeger, R.; Kaiser, C.; et al. Frequency of Cardiac Death and Stent Thrombosis in Patients with Chronic Obstructive Pulmonary Disease Undergoing Percutaneous Coronary Intervention (from the BASKET-PROVE I and II Trials). Am. J. Cardiol. 2017, 119, 14–19. [Google Scholar] [CrossRef] [PubMed]
- Andell, P.; Koul, S.; Martinsson, A.; Sundström, J.; Jernberg, T.; Smith, J.G.; James, S.; Lindahl, B.; Erlinge, D. Impact of chronic obstructive pulmonary disease on morbidity and mortality after myocardial infarction. Open Heart 2014, 1, e000002. [Google Scholar] [CrossRef]
- Enriquez, J.R.; de Lemos, J.A.; Parikh, S.V.; Peng, S.A.; Spertus, J.A.; Holper, E.M.; Roe, M.T.; Rohatgi, A.; Das, S.R. Association of chronic lung disease with treatments and outcomes patients with acute myocardial infarction. Am. Heart J. 2013, 165, 43–49. [Google Scholar] [CrossRef]
- Hawkins, N.M.; Huang, Z.; Pieper, K.S.; Solomon, S.D.; Kober, L.; Velazquez, E.J.; Swedberg, K.; Pfeffer, M.A.; McMurray, J.J.; Maggioni, A.P. Chronic obstructive pulmonary disease is an independent predictor of death but not atherosclerotic events in patients with myocardial infarction: Analysis of the Valsartan in Acute Myocardial Infarction Trial (VALIANT). Eur. J. Heart Fail. 2009, 11, 292–298. [Google Scholar] [CrossRef] [Green Version]
- Enriquez, J.R.; Parikh, S.V.; Selzer, F.; Jacobs, A.K.; Marroquin, O.; Mulukutla, S.; Srinivas, V.; Holper, E.M. Increased adverse events after percutaneous coronary intervention in patients with COPD: Insights from the National Heart, Lung, and Blood Institute dynamic registry. Chest 2011, 140, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Steg, P.G.; James, S.K.; Atar, D.; Badano, L.P.; Blömstrom-Lundqvist, C.; Borger, M.A.; Di Mario, C.; Dickstein, K.; Ducrocq, G.; Fernandez-Aviles, F.; et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur. Heart J. 2012, 33, 2569–2619. [Google Scholar] [CrossRef]
- Su, T.H.; Chang, S.H.; Chen, P.C.; Chan, Y.L. Temporal Trends in Treatment and Outcomes of Acute Myocardial Infarction in Patients with Chronic Obstructive Pulmonary Disease: A Nationwide Population-Based Observational Study. J. Am. Heart Assoc. 2017, 6, e004525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedemans, L.; Abou, R.; Hoogslag, G.E.; Marsan, N.A.; Taube, C.; Delgado, V.; Bax, J.J. Comparison of Left Ventricular Function and Myocardial Infarct Size Determined by 2-Dimensional Speckle Tracking Echocardiography in Patients with and Without Chronic Obstructive Pulmonary Disease After ST-Segment Elevation Myocardial Infarction. Am. J. Cardiol. 2017, 120, 734–739. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potter, E.; Marwick, T.H. Assessment of Left Ventricular Function by Echocardiography: The Case for Routinely Adding Global Longitudinal Strain to Ejection Fraction. JACC. Cardiovasc. Imaging 2018, 11, 260–274. [Google Scholar] [CrossRef]
- Goedemans, L.; Abou, R.; Hoogslag, G.E.; Marsan, N.A.; Delgado, V.; Bax, J.J. Left ventricular global longitudinal strain and long-term prognosis in patients with chronic obstructive pulmonary disease after acute myocardial infarction. European heart journal. Cardiovasc. Imaging 2019, 20, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Zielinski, J.; Price, D. Screening for and early detection of chronic obstructive pulmonary disease. Lancet 2009, 374, 721–732. [Google Scholar] [CrossRef]
- Walker, P.P.; Mitchell, P.; Diamantea, F.; Warburton, C.J.; Davies, L. Effect of primary-care spirometry on the diagnosis and management of COPD. Eur. Respir. J. 2006, 28, 945–952. [Google Scholar] [CrossRef] [Green Version]
- Januszek, R.; Siudak, Z.; Dziewierz, A.; Rakowski, T.; Dudek, D.; Bartuś, S. Chronic obstructive pulmonary disease affects the angiographic presentation and outcomes of patients with coronary artery disease treated with percutaneous coronary interventions. Pol. Arch. Intern. Med. 2018, 128, 24–34. [Google Scholar] [CrossRef] [Green Version]
- Antoni, M.L.; ten Brinke, E.A.; Atary, J.Z.; Marsan, N.A.; Holman, E.R.; Schalij, M.J.; Bax, J.J.; Delgado, V. Left atrial strain is related to adverse events in patients after acute myocardial infarction treated with primary percutaneous coronary intervention. Heart 2011, 97, 1332–1337. [Google Scholar] [CrossRef]
- Wanger, J.; Clausen, J.L.; Coates, A.; Pedersen, O.F.; Brusasco, V.; Burgos, F.; Casaburi, R.; Crapo, R.; Enright, P.; van der Grinten, C.P.; et al. Standardisation of the measurement of lung volumes. Eur. Respir. J. 2005, 26, 511–522. [Google Scholar] [CrossRef]
- Miller, M.R.; Hankinson, J.; Brusasco, V.; Burgos, F.; Casaburi, R.; Coates, A.; Crapo, R.; Enright, P.; van der Grinten, C.P.; Gustafsson, P.; et al. Standardisation of spirometry. Eur. Respir. J. 2005, 26, 319–338. [Google Scholar] [CrossRef] [Green Version]
- Matthys, H.; Sorichter, S. Lungenfunktionsuntersuchungen. In Klinische Pneumologie, 3rd ed.; Matthys, H., Seeger, W., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; Volume 2, pp. 56–78. [Google Scholar]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Pulmonary Disease: 2021 Report; GOLD: Fontana, WI, USA, 2021. [Google Scholar]
- Voigt, J.U.; Pedrizzetti, G.; Lysyansky, P.; Marwick, T.H.; Houle, H.; Baumann, R.; Pedri, S.; Ito, Y.; Abe, Y.; Metz, S.; et al. Definitions for a common standard for 2D speckle tracking echocardiography: Consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. J. Am. Soc. Echocardiogr. 2015, 28, 183–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badano, L.P.; Kolias, T.J.; Muraru, D.; Abraham, T.P.; Aurigemma, G.; Edvardsen, T.; D’Hooge, J.; Donal, E.; Fraser, A.G.; Marwick, T.; et al. Standardization of left atrial, right ventricular, and right atrial deformation imaging using two-dimensional speckle tracking echocardiography: A consensus document of the EACVI/ASE/Industry Task Force to standardize deformation imaging. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Goedemans, L.; Bax, J.J.; Delgado, V. COPD and acute myocardial infarction. Eur. Respir. Rev. 2020, 29, 190139. [Google Scholar] [CrossRef]
- Konecny, T.; Somers, K.R.; Park, J.Y.; John, A.; Orban, M.; Doshi, R.; Scanlon, P.D.; Asirvatham, S.J.; Rihal, C.S.; Brady, P.A. Chronic obstructive pulmonary disease as a risk factor for ventricular arrhythmias independent of left ventricular function. Heart Rhythm 2018, 15, 832–838. [Google Scholar] [CrossRef] [PubMed]
- Rothnie, K.J.; Yan, R.; Smeeth, L.; Quint, J.K. Risk of myocardial infarction (MI) and death following MI in people with chronic obstructive pulmonary disease (COPD): A systematic review and meta-analysis. BMJ Open 2015, 5, e007824. [Google Scholar] [CrossRef] [PubMed]
- Dreher, M.; Daher, A.; Keszei, A.; Marx, N.; Müller, T.; Cornelissen, C.; Brandenburg, V. Whole-Body Plethysmography and Blood Gas Analysis in Patients with Acute Myocardial Infarction Undergoing Percutaneous Coronary Intervention. Respiration 2019, 97, 24–33. [Google Scholar] [CrossRef]
- Mayr, A.; Mair, J.; Klug, G.; Schocke, M.; Pedarnig, K.; Trieb, T.; Pachinger, O.; Jaschke, W.; Metzler, B. Cardiac troponin T and creatine kinase predict mid-term infarct size and left ventricular function after acute myocardial infarction: A cardiac MR study. J. Magn. Reson. Imaging 2011, 33, 847–854. [Google Scholar] [CrossRef]
- Ko, F.W.; Yan, B.P.; Lam, Y.Y.; Chu, J.H.; Chan, K.P.; Hui, D.S. Undiagnosed airflow limitation is common in patients with coronary artery disease and associated with cardiac stress. Respirology 2016, 21, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Ashitani, J.; Mukae, H.; Arimura, Y.; Matsukura, S. Elevated plasma procoagulant and fibrinolytic markers in patients with chronic obstructive pulmonary disease. Intern. Med. 2002, 41, 181–185. [Google Scholar] [CrossRef] [Green Version]
- MacNee, W. Systemic inflammatory biomarkers and co-morbidities of chronic obstructive pulmonary disease. Ann. Med. 2013, 45, 291–300. [Google Scholar] [CrossRef]
- Sabit, R.; Bolton, C.E.; Fraser, A.G.; Edwards, J.M.; Edwards, P.H.; Ionescu, A.A.; Cockcroft, J.R.; Shale, D.J. Sub-clinical left and right ventricular dysfunction in patients with COPD. Respir. Med. 2010, 104, 1171–1178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoos, M.M.; Dalsgaard, M.; Kjærgaard, J.; Moesby, D.; Jensen, S.G.; Steffensen, I.; Iversen, K.K. Echocardiographic predictors of exercise capacity and mortality in chronic obstructive pulmonary disease. BMC Cardiovasc. Disord. 2013, 13, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neilan, T.G.; Bakker, J.P.; Sharma, B.; Owens, R.L.; Farhad, H.; Shah, R.V.; Abbasi, S.A.; Kohli, P.; Wilson, J.; DeMaria, A.; et al. T1 measurements for detection of expansion of the myocardial extracellular volume in chronic obstructive pulmonary disease. Can. J. Cardiol. 2014, 30, 1668–1675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salisbury, A.C.; Reid, K.J.; Spertus, J.A. Impact of chronic obstructive pulmonary disease on post-myocardial infarction outcomes. Am. J. Cardiol. 2007, 99, 636–641. [Google Scholar] [CrossRef]
- Farsalinos, K.E.; Daraban, A.M.; Ünlü, S.; Thomas, J.D.; Badano, L.P.; Voigt, J.U. Head-to-Head Comparison of Global Longitudinal Strain Measurements among Nine Different Vendors: The EACVI/ASE Inter-Vendor Comparison Study. J. Am. Soc. Echocardiogr. 2015, 28, 1171–1181.e2. [Google Scholar] [CrossRef]
| All Patients (n = 109) | COPD (n = 45) | Non-COPD (n = 64) | p-Value | |
|---|---|---|---|---|
| Age, years | 65 ± 12 | 68 ± 11 | 63 ± 12 | 0.05 |
| Male | 85 (78%) | 34 (76%) | 51 (80%) | 0.61 |
| Female | 24 (22%) | 11 (24%) | 13 (20%) | 0.61 |
| Body-Mass-Index, kg/m2 | 27 ± 5 | 27 ± 5 | 27 ± 4 | 0.67 |
| NSTEMI | 68 (62%) | 31 (69%) | 37 (58%) | 0.24 |
| STEMI | 41 (38%) | 14 (31%) | 27 (42%) | 0.24 |
| Laboratory tests | ||||
| - Serum creatinine, mg/dL | 0.99 ± 0.34 | 1.05 ± 0.45 | 0.95 ± 0.23 | 0.56 |
| - GFR, mL/min/1.73m2 | 78 ± 21 | 73 ± 23 | 82 ± 19 | 0.06 |
| - NT-proBNP, pg/mL | 1273 (425; 2344) | 1379 (422; 2323) | 1238 (449; 2316) | 0.74 |
| - CRP, mg/dL | 9 (3; 37) | 5 (2; 29) | 13 (5; 42) | 0.14 |
| - CKmax, U/L | 535 (226; 1385) | 395 (133; 1197) | 607 (259; 1478) | 0.32 |
| - cTnTmax, pg/mL | 767 (169; 2390) | 365 (102; 1632) | 1125 (210; 2784) | 0.17 |
| Smoking history | ||||
| - Current smoker | 49 (45%) | 23 (51%) | 26 (41%) | 0.07 |
| - Ex-smoker | 43 (39%) | 22 (49%) | 21 (33%) | 0.07 |
| - Never smoked | 17 (27%) | 0 (0%) | 17 (27%) | 0.07 |
| - Period of smoking, years | 33 ± 14 | 35 ± 13 | 31 ± 15 | 0.45 |
| - Smoking, Pack-year | 37 ± 29 | 45 ± 29 | 33 ± 28 | 0.03 |
| Co-morbidities | ||||
| - Hypertension | 81 (74%) | 34 (76%) | 47 (73%) | 0.80 |
| - Diabetes | 28 (26%) | 14 (31%) | 14 (22%) | 0.28 |
| - Bronchial asthma | 5 (5%) | 3 (7%) | 2 (3%) | 0.38 |
| - Atrial fibrillation | 27 (25%) | 10 (22%) | 8 (13%) | 0.18 |
| Medications at admission | ||||
| - ACE inhibitor/AT1 antagonist | 69 (63%) | 29 (64%) | 40 (63%) | 0.99 |
| - Aspirin | 64 (59%) | 30 (67%) | 34 (53%) | 0.22 |
| - Beta blocker | 58 (53%) | 21 (47%) | 37 (58%) | 0.34 |
| - Statin | 58 (53%) | 28 (62%) | 30 (47%) | 0.17 |
| - P2Y12 inhibitor | 19 (17%) | 10 (22%) | 9 (14%) | 0.40 |
| - CCB | 17 (16%) | 10 (22%) | 7 (11%) | 0.18 |
| - Loop diuretic | 14 (13%) | 9 (20%) | 5 (8%) | 0.11 |
| - Thiazide | 13 (12%) | 8 (18%) | 5 (8%) | 0.20 |
| - Antimineralocorticoid | 6 (6%) | 3 (7%) | 3 (5%) | 0.98 |
| - LABA | 18 (17%) | 16 (36%) | 2 (3%) | <0.001 |
| - LAMA | 17 (16%) | 17 (38%) | 0 (0%) | <0.001 |
| - ICS | 5 (5%) | 5 (11%) | 0 (0%) | 0.02 |
| - OCS | 0 (0%) | 0 (0%) | 0 (0%) | |
| Pulmonary function tests | ||||
| - FEV1/FVC, % | 71 ± 13 | 58 ± 9 | 80 ± 5 | <0.001 |
| - FEV1, % of predicted | 80 ± 24 | 63 ± 18 | 92 ± 21 | <0.001 |
| - TLC, % of predicted | 104 ± 20 | 113 ± 22 | 97 ± 16 | <0.001 |
| - RV, % of predicted | 141 ± 47 | 169 ± 52 | 121 ± 30 | <0.001 |
| - RV/TLC, % of predicted | 125 ± 23 | 138 ± 22 | 116 ± 19 | <0.001 |
| - DLCO/VA, % of predicted | 68 ± 19 | 58 ± 22 | 71 ± 17 | 0.06 |
| ABGs | ||||
| - SpO2, % | 94 ± 3 | 92 ± 3 | 94 ± 3 | 0.02 |
| - PaO2, mmHg | 65 ± 9 | 63 ± 9 | 66 ± 10 | 0.20 |
| - PaCO2, mmHg | 36 ± 5 | 37 ± 5 | 35 ± 5 | 0.05 |
| - PH | 7.4 ± 0.03 | 7.5 ± 0.02 | 7.4 ± 0.03 | 0.10 |
| - Bicarbonate, mmHg | 23 ± 2 | 24 ± 2 | 23 ± 2 | 0.81 |
| COPD (n = 45) | Non-COPD (n = 64) | p-Value | |
|---|---|---|---|
| LVEF, % | 48 ± 9 | 53 ± 7 | 0.01 |
| LVEDD, mm | 48 ± 8 | 49 ± 5 | 0.40 |
| LVESD, mm | 36 ± 10 | 33 ± 6 | 0.13 |
| LAVol, mL | 61 ± 29 | 49 ± 17 | 0.03 |
| LAVI, mL/m2 | 32 ± 14 | 25 ± 9 | 0.01 |
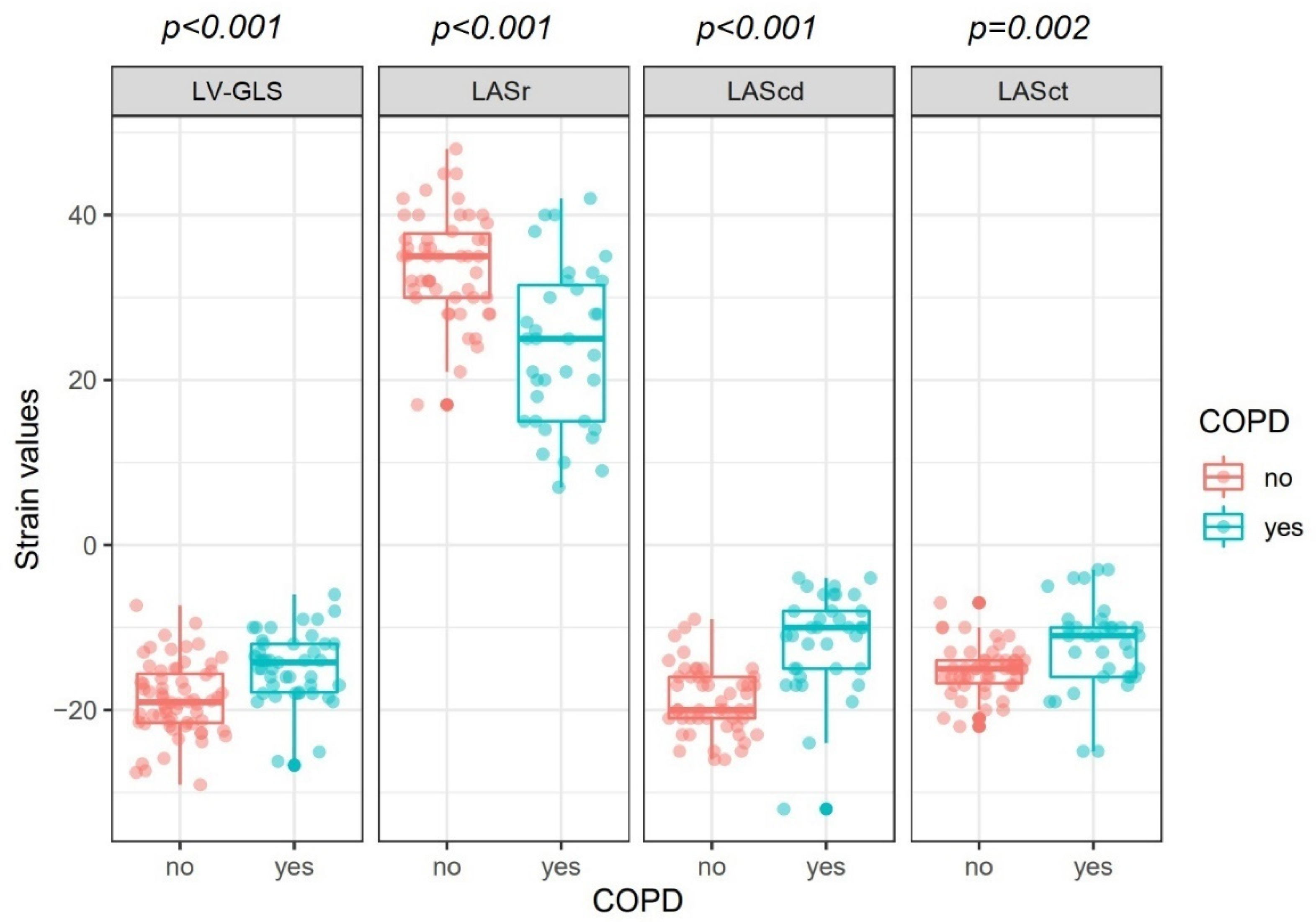
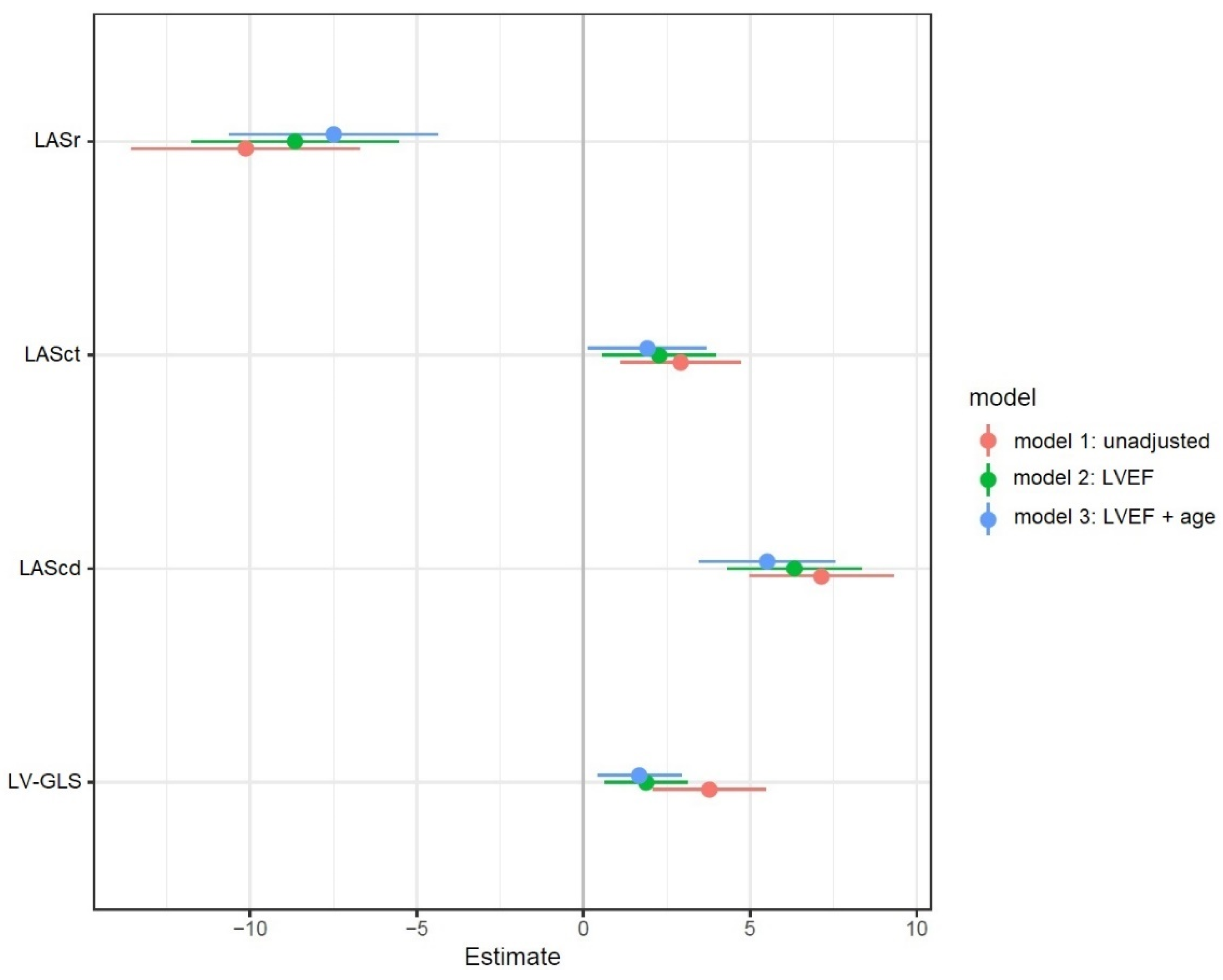
| LV-GLS, % | −15 ± 4 | −18 ± 4 | <0.001 |
| LASr, % | 24 ± 10 | 34 ± 6 | <0.001 |
| LAScd, % | −12 ± 6 | −19 ± 4 | <0.001 |
| LASct, % | −12 ± 5 | −15 ± 3 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grebe, J.; Müller, T.; Altiok, E.; Becker, M.; Keszei, A.P.; Marx, N.; Dreher, M.; Daher, A. Effects of COPD on Left Ventricular and Left Atrial Deformation in Patients with Acute Myocardial Infarction: Strain Analysis Using Speckle-Tracking Echocardiography. J. Clin. Med. 2022, 11, 1917. https://doi.org/10.3390/jcm11071917
Grebe J, Müller T, Altiok E, Becker M, Keszei AP, Marx N, Dreher M, Daher A. Effects of COPD on Left Ventricular and Left Atrial Deformation in Patients with Acute Myocardial Infarction: Strain Analysis Using Speckle-Tracking Echocardiography. Journal of Clinical Medicine. 2022; 11(7):1917. https://doi.org/10.3390/jcm11071917
Chicago/Turabian StyleGrebe, Julian, Tobias Müller, Ertunc Altiok, Michael Becker, András P. Keszei, Nikolaus Marx, Michael Dreher, and Ayham Daher. 2022. "Effects of COPD on Left Ventricular and Left Atrial Deformation in Patients with Acute Myocardial Infarction: Strain Analysis Using Speckle-Tracking Echocardiography" Journal of Clinical Medicine 11, no. 7: 1917. https://doi.org/10.3390/jcm11071917
APA StyleGrebe, J., Müller, T., Altiok, E., Becker, M., Keszei, A. P., Marx, N., Dreher, M., & Daher, A. (2022). Effects of COPD on Left Ventricular and Left Atrial Deformation in Patients with Acute Myocardial Infarction: Strain Analysis Using Speckle-Tracking Echocardiography. Journal of Clinical Medicine, 11(7), 1917. https://doi.org/10.3390/jcm11071917







