Speckled Tracking of Pleura—A Novel Tool for Lung Ultrasound; Distinguishing COVID-19 from Acute Heart Failure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects and Study Design
2.2. Collection of Ultrasound Clips
2.3. Classifying Patient Groups
2.4. Analysis of B Mode
2.5. Statistical Analysis
3. Results
4. Discussion
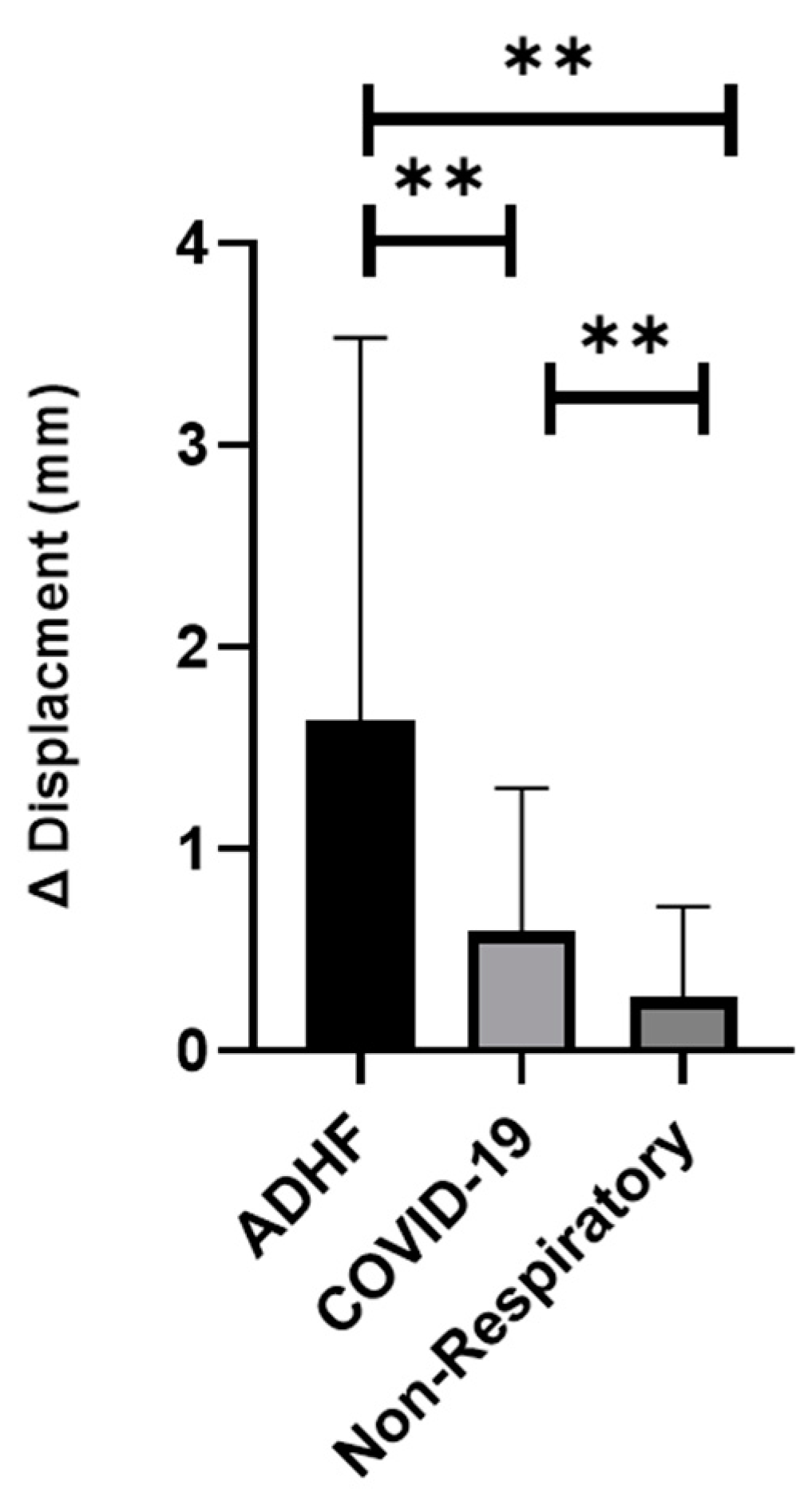
- The displacement of the pleura, when quantitated by a speckled tracking tool, varies significantly between ADHF patients, COVID-19 patients, and non-respiratory patients. ADHF pleura showed larger movements than in COVID-19, while the non-respiratory patients had a relatively small displacement (Figure 3, p < 0.01).
- The velocity of the pleural movement is significantly larger for the ADHF patients than for COVID-19 pneumonia patients; while the velocity for the non-respiratory patients was very small (Figure 4, p < 0.01).
- The agreement between the physicians about the diagnosis of the LUS was poor and an objective tool is required.
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Jozwiak, M.; Teboul, J.L.; Monnet, X. Extravascular Lung Water in Critical Care: Recent Advances and Clinical Applications. Ann. Intensive Care 2015, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Baggish, A.L.; Lloyd-Jones, D.M.; Blatt, J.; Richards, A.M.; Lainchbury, J.; O’Donoghue, M.; Sakhuja, R.; Chen, A.A.; Januzzi, J.L. A Clinical and Biochemical Score for Mortality Prediction in Patients with Acute Dyspnoea: Derivation, Validation and Incorporation into a Bedside Programme. Heart 2008, 94, 1032–1037. [Google Scholar] [CrossRef] [PubMed]
- McManus, N.M.; Offman, R.; Oetman, J.D. Emergency Department Management of COVID-19: An Evidence-Based Approach. West. J. Emerg. Med. 2020, 21, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.; Axler, O. Intensive Use of General Ultrasound in the Intensive Care Unit—Prospective Study of 150 Consecutive Patients. Intensive Care Med. 1993, 19, 353–355. [Google Scholar] [CrossRef]
- Lichtenstein, D.A.; Mezière, G.A. Relevance of Lung Ultrasound in the Diagnosis of Acute Respiratory Failure the BLUE Protocol. Chest 2008, 134, 117–125. [Google Scholar] [CrossRef]
- Conangla, L.; Domingo, M.A.R.; Lupón, J.; Wilke, A.; Juncà, G.; Tejedor, X.; Volpicelli, G.; Evangelista, L.; Pera, G.; Toran, P.E.R.E.; et al. Lung Ultrasound for Heart Failure Diagnosis in Primary Care. J. Card. Fail. 2020, 26, 824–831. [Google Scholar] [CrossRef]
- Karimi, E. Comparing Sensitivity of Ultrasonography and Plain Chest Radiography in Detection of Pneumonia; a Diagnostic Value Study. Arch. Acad. Emerg. Med. 2019, 7, 1–5. [Google Scholar] [CrossRef]
- Copetti, R.; Soldati, G.; Copetti, P. Chest Sonography: A Useful Tool to Differentiate Acute Cardiogenic Pulmonary Edema from Acute Respiratory Distress Syndrome. Cardiovasc. Ultrasound 2008, 6, 16. [Google Scholar] [CrossRef]
- Bekgoz, B.; Kilicaslan, I.; Bildik, F.; Keles, A.; Demircan, A.; Hakoglu, O.; Coskun, G.; Demir, H.A. BLUE Protocol Ultrasonography in Emergency Department Patients Presenting with Acute Dyspnea. Am. J. Emerg. Med. 2019, 37, 2020–2027. [Google Scholar] [CrossRef]
- Vetrugno, L.; Baciarello, M.; Bignami, E.; Bonetti, A.; Saturno, F.; Orso, D.; Girometti, R.; Cereser, L.; Bove, T. The “Pandemic” Increase in Lung Ultrasound Use in Response to COVID-19: Can We Complement Computed Tomography Findings? A Narrative Review. Ultrasound J. 2020, 12, 39. [Google Scholar] [CrossRef]
- Brenner, D.S.; Liu, G.Y.; Omron, R.; Tang, O.; Garibaldi, B.T.; Fong, T.C. Diagnostic Accuracy of Lung Ultrasound for SARS-CoV-2: A Retrospective Cohort Study. Ultrasound J. 2021, 13, 12. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, A.O.; Costa, R.M.; Uzun, R.; Fraga, A.M.A.; Ribeiro, J.D.; Marson, F.A.L. Applicability of Lung Ultrasound in COVID-19 Diagnosis and Evaluation of the Disease Progression: A Systematic Review. Pulmonology 2021, 27, 529–562. [Google Scholar] [CrossRef] [PubMed]
- Matthay, M.A.; Zemans, R.L. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 147–163. [Google Scholar] [CrossRef]
- Suffoletto, M.S.; Dohi, K.; Cannesson, M.; Saba, S.; Gorcsan, J. Novel Speckle-Tracking Radial Strain from Routine Black-and-White Echocardiographic Images to Quantify Dyssynchrony and Predict Response to Cardiac Resynchronization Therapy. Circulation 2006, 113, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Duclos, G.; Bobbia, X.; Markarian, T.; Muller, L.; Cheyssac, C.; Castillon, S.; Resseguier, N.; Boussuges, A.; Volpicelli, G.; Leone, M.; et al. Speckle Tracking Quantification of Lung Sliding for the Diagnosis of Pneumothorax: A Multicentric Observational Study. Intensive Care Med. 2019, 45, 1212–1218. [Google Scholar] [CrossRef]
- Fissore, E.; Zieleskiewicz, L.; Markarian, T.; Muller, L.; Duclos, G.; Bourgoin, M.; Michelet, P.; Leone, M.; Claret, P.G.; Bobbia, X. Pneumothorax Diagnosis with Lung Sliding Quantification by Speckle Tracking: A Prospective Multicentric Observational Study. Am. J. Emerg. Med. 2021, 49, 14–17. [Google Scholar] [CrossRef]
- Colombi, D.; Petrini, M.; Maffi, G.; Villani, G.D.; Bodini, F.C.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Comparison of Admission Chest Computed Tomography and Lung Ultrasound Performance for Diagnosis of COVID-19 Pneumonia in Populations with Different Disease Prevalence. Eur. J. Radiol. 2020, 133, 109344. [Google Scholar] [CrossRef]
- Duggan, N.M.; Shokoohi, H.; Liteplo, A.S.; Huang, C.; Goldsmith, A.J. Best Practice Recommendations for Point-of-Care Lung Ultrasound in Patients with Suspected COVID-19. J. Emerg. Med. 2020, 59, 515–520. [Google Scholar] [CrossRef]
- Green, S.M.; Martinez-Rumayor, A.; Gregory, S.A.; Baggish, A.L.; O’Donoghue, M.L.; Green, J.A.; Lewandrowski, K.B.; Januzzi, J.L. Clinical Uncertainty, Diagnostic Accuracy, and Outcomes in Emergency Department Patients Presenting with Dyspnea. Arch. Intern. Med. 2008, 168, 741–748. [Google Scholar] [CrossRef]
- Jung, Y.J.; Yoon, J.L.; Kim, H.S.; Lee, A.Y.; Kim, M.Y.; Cho, J.J. Atypical Clinical Presentation of Geriatric Syndrome in Elderly Patients with Pneumonia or Coronary Artery Disease. Ann. Geriatr. Med. Res. 2017, 21, 158–163. [Google Scholar] [CrossRef]
- Lichtenstein, D.A. BLUE-Protocol and FALLS-Protocol: Two Applications of Lung Ultrasound in the Critically Ill. Chest 2015, 147, 1659–1670. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, B.; Higgins, K.E. The Geriatric Assessment. Am. Fam. Physician 2011, 83, 48–56. [Google Scholar] [PubMed]



| ADHF | COVID-19 | Non-Respiratory | |
|---|---|---|---|
| N | 25 | 21 | 19 |
| LUS loops | 66 | 60 | 82 |
| M/F | 6/19 | 10/11 | 13/6 |
| age | 80 ± 8 | 63 ± 13 | 51 ± 13 |
| Comorbidities | |||
| Congestive Heart Failure | 13 | 2 | - |
| Rheumatic Heart Failure | 1 | - | |
| Asthma | 1 | 2 | - |
| Chronic obstructive pulmonary disese | 4 | 3 | - |
| Major complaints | |||
| Dyspnea | 19 | 12 | 4 |
| Weakness | 6 | 8 | - |
| Head Injury | 1 | 1 | - |
| Chest Pain | 6 | 6 | 3 |
| Leg Edema | 3 | 11 | 2 |
| Fall | 1 | 3 | 1 |
| Palpitations | 1 | 1 | - |
| Cough | 1 | 1 | - |
| Hypotension | 1 | 1 | - |
| Electrocardiographic Changes | 1 | 1 | 1 |
| Orthopnea | 1 | 1 | - |
| Back Pain | 1 | 1 | 1 |
| Abdominal Pain | - | 1 | 5 |
| flank pain | - | - | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tzadok, B.; Blumberg, Y.; Shubert, M.; Halabi, M.; Tal-Or, E.; Bachner-Hinenzon, N.; Carasso, S. Speckled Tracking of Pleura—A Novel Tool for Lung Ultrasound; Distinguishing COVID-19 from Acute Heart Failure. J. Clin. Med. 2022, 11, 4846. https://doi.org/10.3390/jcm11164846
Tzadok B, Blumberg Y, Shubert M, Halabi M, Tal-Or E, Bachner-Hinenzon N, Carasso S. Speckled Tracking of Pleura—A Novel Tool for Lung Ultrasound; Distinguishing COVID-19 from Acute Heart Failure. Journal of Clinical Medicine. 2022; 11(16):4846. https://doi.org/10.3390/jcm11164846
Chicago/Turabian StyleTzadok, Batsheva, Yair Blumberg, Moti Shubert, Majdi Halabi, Eran Tal-Or, Noa Bachner-Hinenzon, and Shemy Carasso. 2022. "Speckled Tracking of Pleura—A Novel Tool for Lung Ultrasound; Distinguishing COVID-19 from Acute Heart Failure" Journal of Clinical Medicine 11, no. 16: 4846. https://doi.org/10.3390/jcm11164846
APA StyleTzadok, B., Blumberg, Y., Shubert, M., Halabi, M., Tal-Or, E., Bachner-Hinenzon, N., & Carasso, S. (2022). Speckled Tracking of Pleura—A Novel Tool for Lung Ultrasound; Distinguishing COVID-19 from Acute Heart Failure. Journal of Clinical Medicine, 11(16), 4846. https://doi.org/10.3390/jcm11164846







