Severe Pulmonary Bleeding after Assist Device Implantation: Incidence, Risk Factors and Prognostic Impact
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Inclusion and Exclusion Criteria
2.3. Study Aim
2.4. Statistics
2.5. Ethics
2.6. Surgical Procedure
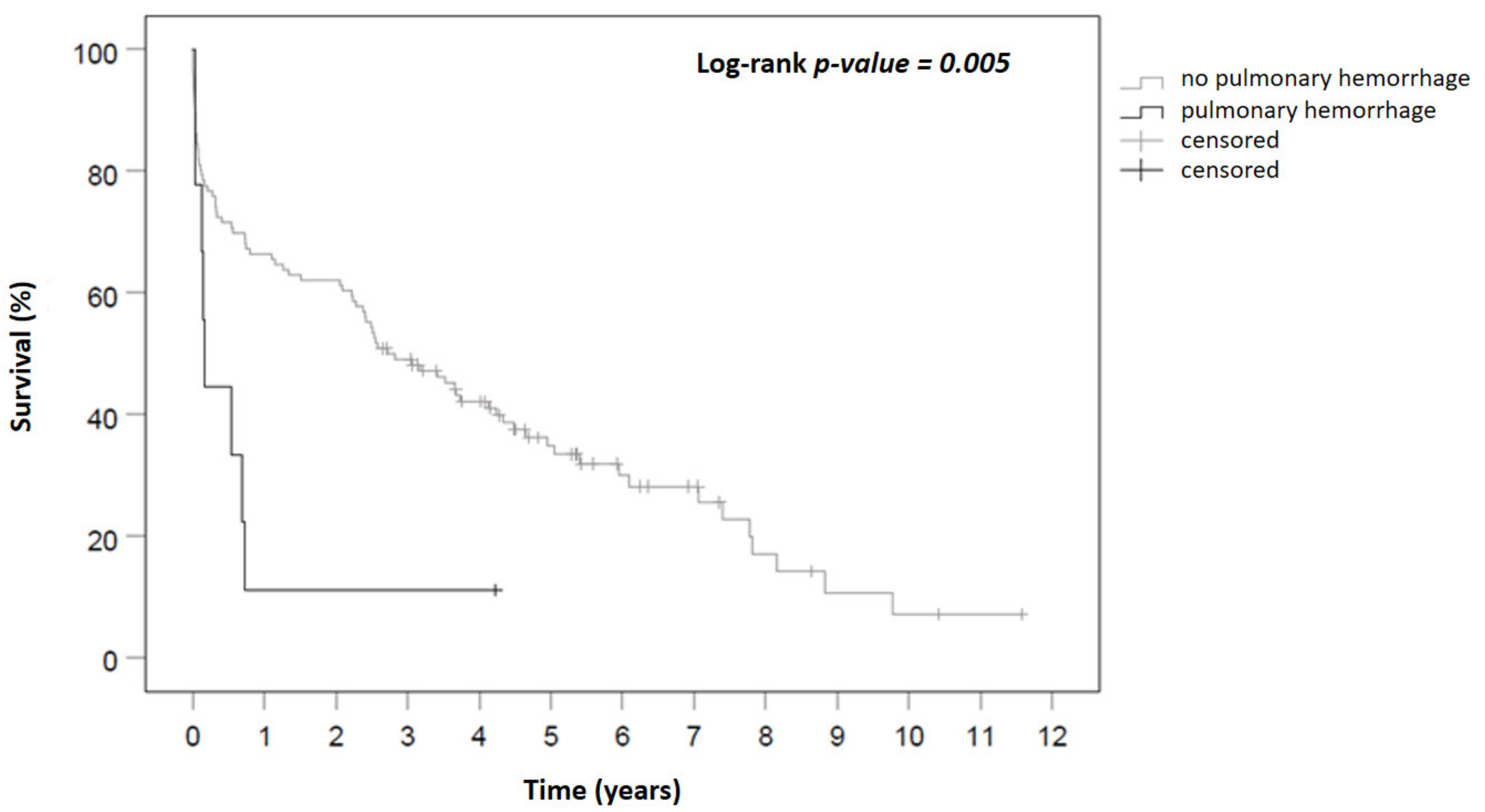
3. Results
3.1. Comparison of Patients with SPB vs. Patients without SPB
3.2. Management of SPB and Anti-Coagulation after SPB
4. Discussion
5. Conclusions
6. Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jefferson, H.L.; Kent, W.D.T.; MacQueen, K.T.; Miller, R.J.H.; Holloway, D.D.; Hassanabad, A.F. Left ventricular assist devices: A comprehensive review of major clinical trials, devices, and future directions. J. Card. Surg. 2021, 36, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Mihalj, M.; Heinisch, P.P.; Schober, P.; Dobner, S.; Fuerholz, M.; Martinelli, M.; Hugi-Mayr, B.; De By, T.M.M.H.; Mohacsi, P.; Schefold, J.C.; et al. Third generation continuous flow left ventricular assist devices: A comparative outcome analysis by device type. Eur. Heart J. 2021, 42, ehab724-0946. [Google Scholar] [CrossRef]
- Clement, A.; Anghel, L.; Sascău, R.; Stătescu, C. Left Ventricular Assist Device-Related Complications. J. Cardiovasc. Emerg. 2020, 6, 50–58. [Google Scholar] [CrossRef]
- Muslem, R.; Caliskan, K.; Leebeek, F. Acquired coagulopathy in patients with left ventricular assist devices. J. Thromb. Haemost. 2018, 16, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Tantry, U.S.; Bliden, K.P.; Gurbel, P.A. Bleeding and thrombosis associated with ventricular assist device therapy. J. Heart Lung Transplant. 2017, 36, 1164–1173. [Google Scholar] [CrossRef]
- Birks, E.J. Stopping LVAD Bleeding: A Piece of the Puzzle. Circ. Res. 2017, 121, 902–904. [Google Scholar] [CrossRef]
- Huenges, K.; Panholzer, B.; Cremer, J.; Haneya, A. Left Ventricular Assist Device Implantation with Concomitant Aortic Valve and Ascending Aortic Replacement. Case Rep. Med. 2018, 2018, 9057351. [Google Scholar] [CrossRef]
- Aubron, C.; Depuydt, J.; Belon, F.; Bailey, M.; Schmidt, M.; Sheldrake, J.; Murphy, D.; Scheinkestel, C.; Cooper, D.J.; Capellier, G.; et al. Predictive factors of bleeding events in adults undergoing extracorporeal membrane oxygenation. Ann. Intensiv. Care 2016, 6, 97. [Google Scholar] [CrossRef]
- Welp, H.; Sindermann, J.R.; Deschka, H.; Martens, S.; Scherer, M. Pulmonary Bleeding During Right Ventricular Support After Left Ventricular Assist Device Implantation. J. Cardiothorac. Vasc. Anesth. 2016, 30, 627–631. [Google Scholar] [CrossRef]
- Oude Lansink-Hartgring, A.; de Vries, A.J.; Droogh, J.M.; van den Bergh, W.M. Hemorrhagic complications during extracorporeal membrane oxygenation–The role of anticoagulation and platelets. J. Crit. Care 2019, 54, 239–243. [Google Scholar] [CrossRef]
- Kilic, A.; Acker, M.A.; Atluri, P. Dealing with surgical left ventricular assist device complications. J. Thorac. Dis. 2015, 7, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Kataria, R.; Jorde, U.P. Gastrointestinal Bleeding During Continuous-Flow Left Ventricular Assist Device Support: State of the Field. Cardiol. Rev. 2019, 27, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Nascimbene, A.; Neelamegham, S.; Frazier, O.H.; Moake, J.L.; Dong, J.-F. Acquired von Willebrand syndrome associated with left ventricular assist device. Blood 2016, 127, 3133–3141. [Google Scholar] [CrossRef] [PubMed]
- Ittrich, H.; Bockhorn, M.; Klose, H.; Simon, M. The Diagnosis and Treatment of Hemoptysis. Dtsch. Arztebl Int. 2017, 114, 371–381. [Google Scholar] [CrossRef]
- Agarwal, B.; Gatt, A.; Riddell, A.; Wright, G.; Chowdary, P.; Jalan, R.; Burroughs, A.K.; Davenport, A. Hemostasis in patients with acute kidney injury secondary to acute liver failure. Kidney Int. 2013, 84, 158–163. [Google Scholar] [CrossRef]
- Bartoli, C.R.; Zhang, D.M.; Hennessy-Strahs, S.; Kang, J.; Restle, D.J.; Bermudez, C.; Atluri, P.; Acker, M.A. Clinical and In Vitro Evidence That Left Ventricular Assist Device–Induced von Willebrand Factor Degradation Alters Angiogenesis. Circ. Heart Fail. 2018, 11, e004638. [Google Scholar] [CrossRef]
- Xu, Y.; Stout, L.C. Complete step section microscopic study of a Swan-Ganz catheter-related pulmonary artery rupture: A fre-quently lethal complication that to our knowledge has not had a comprehensive microscopic examination: Case report and literature review. Cardiovasc. Pathol. 2017, 31, 17–19. [Google Scholar] [CrossRef]
- Cantu, J.; Wang, D.; Safdar, Z. Clinical implications of haemoptysis in patients with pulmonary arterial hypertension. Int. J. Clin. Pr. 2012, 66, 5–12. [Google Scholar] [CrossRef][Green Version]
- Chew, S.T.H.; Hwang, N.C. Acute Kidney Injury After Cardiac Surgery: A Narrative Review of the Literature. J. Cardiothorac. Vasc. Anesth. 2019, 33, 1122–1138. [Google Scholar] [CrossRef]
- Shaz, B.H.; Hillyer, C.D. Is there transfusion-related acute renal injury? Anesthesiology 2010, 113, 1012–1013. [Google Scholar] [CrossRef]
- American Association for Respiratory Care. AARC Clinical Practice Guidelines. Endotracheal suctioning of mechanically ventilated patients with artificial airways 2010. Respir. Care. 2010, 55, 758–764. [Google Scholar]
- Deppe, S.A.; Kelly, J.W.; Thoi, L.L.; Chudy, J.H.; Longfield, R.N.; Ducey, J.P.; Truwit, C.L.; Antopol, M.R. Incidence of colonization, nosocomial pneumonia, and mortality in critically ill patients using a Trach Care closed-suction system versus an open-suction system: Prospective, randomized study. Crit. Care Med. 1990, 18, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Maggiore, S.M.; Lellouche, F.; Pignataro, C.; Girou, E.; Maître, B.; Richard, J.-C.M.; Lemaire, F.; Brun-Buisson, C.; Brochard, L. Decreasing the Adverse Effects of Endotracheal Suctioning During Mechanical Ventilation by Changing Practice. Respir. Care 2013, 58, 1588–1597. [Google Scholar] [CrossRef] [PubMed]
- Gaffey, A.C.; Chen, C.W.; Chung, J.J.; Han, J.; Bermudez, C.A.; Wald, J.; Atluri, P. Is there a difference in bleeding after left ventricular assist device implant: Centrifugal versus axial? J. Cardiothorac. Surg. 2018, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Kawabori, M.; Kurihara, C.; Critsinelis, A.C.; Sugiura, T.; Kaku, Y.; Civitello, A.B.; Rosengart, T.K.; Morgan, J.A. Gastrointestinal Bleeding After HeartMate II or HVAD Implantation: Incidence, Location, Etiology, and Effect on Survival. ASAIO J. 2020, 66, 283–290. [Google Scholar] [CrossRef] [PubMed]
| Total (n = 125) | No SPB (n = 116) | SPB (n = 9) | p-Value | |
|---|---|---|---|---|
| Age, years | 61 (52; 67) | 61 (52; 66) | 65 (50; 73) | 0.194 |
| Female gender | 20 (16.0%) | 17 (14.7%) | 3 (33.3%) | 0.156 |
| Logistic EuroSCORE (%) | 44.1 (32.0; 60.0) | 42.5 (32.0; 59.9) | 49.6 (39.5; 75.4) | 0.221 |
| Additive EuroSCORE (%) | 14 (12; 16) | 14 (12; 16) | 15 (12.5; 16) | 0.456 |
| Body mass index, kg/m2, (%) | 25.4 (22.6; 29.5) | 25.3 (22.6; 29.9) | 25.4 (22.6; 27.5) | 0.771 |
| INTERMACS level | 3.0 (2.0; 4.0) | 3.0 (2.0; 4.0) | 3.0 (2.0; 3.0) | 0.060 |
| Acute cardiac decompensation | 77 (65.3%) | 73 (66.4%) | 4 (50%) | 0.446 |
| Prior cardiac decompensation | 109 (94.8%) | 102 (96.2%) | 7 (77.8%) | 0.070 |
| Arterial hypertension | 68 (55.3%) | 62 (54.4%) | 6 (66.7%) | 0.730 |
| Pulmonary hypertension | 65 (57.0%) | 62 (58.5%) | 3 (37.5%) | 0.286 |
| Heart rhythm | ||||
| Sinus rhythm | 66 (57.9%) | 63 (60.0%) | 3 (33.3%) | 0.163 |
| Atrial fibrillation | 29 (25.4%) | 25 (23.8%) | 4 (44.4%) | 0.229 |
| Other | 19 (16.8%) | 17 (16.3%) | 2 (22.2%) | 0.646 |
| Diabetes mellitus | 37 (29.6%) | 35 (30.2%) | 2 (22.2%) | 1.000 |
| Hyperlipoproteinemia | 59 (50.4%) | 56 (51.9%) | 3 (33.3%) | 0.322 |
| Chronic renal insufficiency | 76 (61.3%) | 69 (60.0%) | 7 (77.8%) | 0.480 |
| Decompensated renal insufficiency | 22 (17.9%) | 20 (17.5%) | 2 (22.2%) | 0.662 |
| Chronic dialysis | 7 (5.6%) | 7 (6.1%) | 0 (0%) | 1.000 |
| COPD | 14 (11.3%) | 14 (12.2%) | 0 (0%) | 0.596 |
| Currently smoking | 18 (15.1%) | 17 (15.5%) | 1 (11.1%) | 1.000 |
| Previous smoking | 38 (32.8%) | 37 (34.6%) | 1 (11.1%) | 0.268 |
| Coronary heart disease (CHD) | 64 (54.2%) | 61 (56.0%) | 3 (33.3%) | 0.298 |
| No CHD | 54 (45.8%) | 48 (44.0%) | 6 (66.7%) | ----- |
| One-vessel disease | 15 (12.7%) | 15 (13.8%) | 0 (0%) | ----- |
| Two-vessel disease | 12 (10.2%) | 12 (11.0%) | 0 (0%) | ----- |
| Three-vessel disease | 37 (31.4%) | 34 (31.2%) | 3 (33.3%) | ----- |
| Prior myocardial infarction | 49 (39.2%) | 49 (42.2%) | 0 (0%) | 0.012 |
| Previous PCI (+/-DES) | 49 (39.2%) | 46 (39.7%) | 3 (33.3%) | 1.000 |
| Previous thoracic surgery | 44 (35.8%) | 40 (35.1%) | 4 (44.4%) | 0.720 |
| Peripheral vascular disease | 14 (11.4%) | 14 (12.3%) | 0 (0%) | 0.596 |
| Clinical presentation | ||||
| Acute myocardial infarction (<14 d) | 5 (4.2%) | 5 (4.6%) | 0 (0%) | 1.000 |
| Cardiogenic shock (<14 d) | 14 (11.9%) | 12 (11.0%) | 2 (22.2%) | 0.289 |
| CPR (<24 h) | 7 (5.9%) | 6 (5.5%) | 1 (11.1%) | 0.435 |
| Transfer from intensive care unit | 59 (50.0%) | 53 (48.6%) | 6 (66.7%) | 0.490 |
| Intubated prior surgery | 25 (21.2%) | 23 (21.1%) | 2 (22.2%) | 1.000 |
| Cardiomyopathy | 0.046 | |||
| ICM | 58 (48.3%) | 56 (50.0%) | 2 (25.0%) | ----- |
| DCM | 56 (46.7%) | 52 (46.4%) | 4 (50.0%) | ----- |
| HCM | 1 (0.8%) | 1 (0.9%) | 0 (0.0%) | ----- |
| Others | 5 (4.2%) | 3 (2.7%) | 2 (25.0%) | ----- |
| Acute myocarditis | 15 (12.1%) | 14 (12.2%) | 1 (11.1%) | 1.000 |
| Coagulation disorder | 7 (5.7%) | 7 (6.1%) | 0 (0%) | 1.000 |
| Apoplex preoperative | 13 (10.4%) | 13 (11.2%) | 0 (0%) | 0.596 |
| Neurologic disease | 1 (0.8%) | 1 (0.9%) | 0 (0%) | 1.000 |
| LVAD type | 125 (100%) | 116 (110%) | 9 (100%) | ----- |
| HVAD | 102 (81.6%) | 93 (80.2%) | 9 (100%) | ----- |
| HM2 | 12 (9.8%) | 12 (10.3%) | 0 (0%) | ----- |
| HM3 | 11 (8.9%) | 11 (9.5%) | 0 (0%) | ----- |
| RVAD (temporary) | 6 (4.9%) | 6 (5.3%) | 0 (0%) | 1.000 |
| BiVAD | 1 (0.8%) | 1 (0.9%) | 0 (0%) | 1.000 |
| Total (n = 125) | No SPB (n = 116) | SPB (n = 9) | p-Value | |
|---|---|---|---|---|
| Urgency | 0.506 | |||
| Elective | 89 (82.4%) | 83 (83.0%) | 6 (75.0%) | ----- |
| Urgent | 15 (13.9%) | 13 (13.0%) | 2 (25.0%) | ----- |
| Emergency | 4 (3.7%) | 4 (4.0%) | 0 (0.0%) | ----- |
| Length of surgery, min | 249 (200; 316) | 249 (200; 311) | 290 (188; 476) | 0.435 |
| Cardiopulmonary bypass time, min | 118 (98; 158) h | 118 (99; 145) | 149 (93; 246) | 0.492 |
| Cross-clamp time, min | 71 (60; 86) | 71 (60; 85) | 91 (56; 137) | 0.287 |
| Circulatory arrest, min | 0 (0; 0) | 0 (0; 0) | 0 (0; 0) | ----- |
| Number of PRBC | 2 (1; 5) | 2 (1; 5) | 2 (1; 9) | 0.657 |
| Number of fresh frozen plasma | 2 (0; 6) | 2 (0; 6) | 1 (0; 7) | 0.975 |
| Number of platelets | 2 (1; 2) | 2 (1; 2) | 2 (1; 2) | 0.618 |
| Fibrinogen | 3 (0; 4) | 3 (0; 4) | 4 (2.5; 7.5) | 0.181 |
| PCC | 87 (72.5%) | 80 (72.1%) | 7 (77.8%) | 1.000 |
| PCC (IE) | 2400 (2000; 4000) | 2500 (2000; 4150) | 2400 (1800; 2400) | 0.065 |
| Coagulation factor XIII | 24 (20.0%) | 21 (18.9%) | 3 (33.3%) | 0.381 |
| Coagulation factor XIII (IE) | 1875 (1250; 2500) | 2500 (1250; 2500) | 1250 (1250; 1250) | 0.172 |
| Novoseven | 4 (3.3%) | 4 (3.6%) | 0 (0.0%) | 1.000 |
| Novoseven (mg) | 6.5 (5.3; 15.3) | 6.5 (5.3; 15.3) | 0 (0; 0) | ----- |
| Surgical procedure | ||||
| CABG | 4 (3.3%) | 4 (3.5%) | 0 (0%) | 1.000 |
| Aortic valve replacement | 11 (8.9%) | 8 (7.0%) | 3 (33.3%) | 0.034 |
| Tricuspid valve replacement / repair | 3 (2.4%) | 2 (1.8%) | 1 (11.1%) | 0.205 |
| PFO-closure | 7 (5.7%) | 6 (5.3%) | 1 (11.1%) | 0.421 |
| Other | ||||
| Ventavis | 3 (2.5%) | 3 (2.7%) | 0 (0%) | 1.000 |
| NO | 52 (43.3%) | 47 (42.3%) | 5 (55.6%) | 0.499 |
| Perfan | 40 (33.6%) | 37 (33.6%) | 3 (33.3%) | 1.000 |
| Adrenalin | 111 (92.5%) | 102 (91.9%) | 9 (100%) | 1.000 |
| Milrinone | 113 (94.2%) | 104 (93.7%) | 9 (100%) | 1.000 |
| ECLS | 25 (20.8%) | 23 (20.5%) | 2 (25.0%) | 0.671 |
| Total (n = 125) | No SPB (n = 116) | SPB (n = 9) | p-Value | |
|---|---|---|---|---|
| Drainage loss, <24 h postoperative (mL) | 850 (600; 1213) | 850 (600; 1175) | 930 (675; 1800) | 0.307 |
| Drainage loss, total (mL) | 4100 (2100; 9220) | 3800 (2063; 8575) | 9950 (6325; 16770) | 0.012 |
| Number of packed red blood cells, <24 h | 2 (0; 3) | 2 (0; 3) | 3 (1.5; 5) | 0.053 |
| Number of fresh frozen plasma, <24 h | 4 (0; 7) | 4 (0; 6) | 7 (2.5; 15) | 0.051 |
| Number of platelets, <24 h | 0 (0; 1) | 0 (0; 1) | 1(0; 2) | 0.041 |
| Number of packed red blood cells, total | 8 (4; 20) | 7 (3; 17) | 26 (23; 46) | <0.001 |
| Number of fresh frozen plasma, total | 6 (3.3; 14.8) | 6 (3; 13) | 18 (12; 32) | 0.002 |
| Number of platelets, total | 1 (0; 5) | 1 (0; 3) | 8 (2.; 5; 12.5) | 0.001 |
| Noradrenalin at admission ICU (µg/min) | 0.23 (0.07; 0.62) | 0.21 (0.07; 0.61) | 0.42 (0.23; 0.85) | 0.123 |
| Noradrenalin 1. POD (µg/min) | 3.9 (0; 20) | 2.00 (0.00; 20.00) | 22.0 (5.0; 48.0) | 0.099 |
| Adrenalin at admission ICU (µg/kg/min) | 0.05 (0.02; 0.09) | 0.04 (0.02; 0.09) | 0.08 (0.02; 0.10) | 0.365 |
| Adrenalin 1. POD (µg/min) | 0.0 (0.0; 2.0) | 0.00 (0.00; 2.00) | 1.00 (0.00; 3.50) | 0.468 |
| Milrinone at admission ICU (µg/kg/min) | 0.31 (0.21; 0.37) | 0.31 (0.21; 0.37) | 0.37 (0.29; 0.39) | 0.160 |
| Milrinone 1. POD (µg/min) | 27.0 (13.2; 27.0) | 27.0 (13.2; 27.0) | 27.0 (23.4: 27.0) | 0.204 |
| Fluid intake <24 h mL) | 5530 (3498; 7608) | 5360 (3440; 7525) | 7830 (5138; 9775) | 0.030 |
| AKI KDIGO any stage | 44 (41.5%) | 36 (36.7%) | 8 (100%) | 0.001 |
| RRT | 31 (30.7%) | 23 (24.7%) | 8 (100%) | <0.001 |
| Reintubation | 21 (18.1%) | 19 (17.8%) | 2 (22.2%) | 0.665 |
| Tracheotomy | 44 (37.9%) | 36 (33.6%) | 8 (88.9%) | 0.002 |
| Re-admission to the ICU | 11 (9.6%) | 10 (9.5%) | 1 (11.1%) | 1.000 |
| Postoperative delirium | 20 (17.1%) | 16 (14.8%) | 4 (44.4%) | 0.045 |
| TIA/Stroke (CT-proofed) | 12 (10.3%) | 12 (11.2%) | 0 (0%) | 0.595 |
| CPR | 3 (2.6%) | 3 (2.8%) | 0 (0%) | 1.000 |
| Pneumonia | 33 (28.2%) | 29 (26.9%) | 4 (44.4%) | 0.268 |
| Sepsis | 28 (24.3%) | 24 (22.6%) | 4 (44.4%) | 0.218 |
| Rethoracotomy | 33 (28.2%) | 29 (26.9%) | 4 (44.4%) | 0.268 |
| Sternal wound infection/VAC revision | 2 (1.7%) | 2 (1.9%) | 0 (0%) | 1.000 |
| Driveline infection | 2 (1.7%) | 2 (1.9%) | 0 (0%) | 1.000 |
| ENT bleeding | 8 (6.8%) | 5 (4.6%) | 3 (33.3%) | 0.015 |
| GI bleeding | 19 (16.2%) | 17 (15.7%) | 2 (22.2%) | 0.638 |
| Cerebral bleeding | 6 (5.1%) | 6 (5.6%) | 0 (0%) | 1.000 |
| ICU time (days) | 14.5 (6.3; 35.8) | 13 (6; 33) | 58 (29.5; 71) | 0.002 |
| Ventilation time, h | 207 (26.5; 946.0) | 171 (22; 783) | 1206 (810; 1330) | 0.001 |
| 30 d mortality, % | 24 (20.0%) | 22 (19.8%) | 2 (22.2%) | 1.000 |
| Hospital mortality, % | 37 (31.9%) | 31 (29.0%) | 6 (66.7%) | 0.029 |
| Total (n = 125) | No SPB (n = 116) | SPB (n = 9) | p-Value | |
|---|---|---|---|---|
| Lactate levels prior surgery | 1.0 (0.8; 1.3) | 1.0 (0.8; 11.4) | 0.9 (0.7; 1.3) | 0.462 |
| Lactate levels admission ICU | 2.9 (1.8; 4.9) | 2.9 (1.9; 4.7) | 2.9 (1.5; 7.0) | 0.995 |
| Lactate levels 1. POD | 1.7 (1.2; 2.5) | 1.7 (1.2; 2.5) | 2.0 (1.2; 4.5) | 0.550 |
| Creatinine prior surgery | 116.4 (90.0; 171.3) | 113.0 (89.6; 158.3) | 141.0 (124.0; 190.5) | 0.163 |
| Creatinine admission ICU | 117.6 (92.1: 167.7) | 116.0 (91.6; 164.7) | 150.0 (117.5; 181.2) | 0.221 |
| Creatinine 1. POD | 122.0 (95.0; 168.0) | 119.5 (94.7; 168.4) | 149.0 (106.0; 176.0) | 0.411 |
| CK prior surgery | 46.5 (30.8; 114.0) | 49.0 (30.5; 118.0) | 43.0 (31.0; 145.0) | 0.733 |
| CK admission ICU | 338 (262; 525) | 339.0 (258.5; 531.0) | 316.0 (292.5; 464.0) | 0.971 |
| CK 1. POD (µmol/L) | 463 (272; 819) | 464.5 (268.5; 822.3) | 421.0 (250.5; 812.5) | 0.929 |
| GOT prior surgery | 28.9 (19.1; 40.0) | 29.1 (18.4; 40.0) | 26.9 (21.1; 64.8) | 0.733 |
| GOT admission ICU _ | 59.0 (49.7; 93.1) | 60.9 (49.0; 93.6) | 57.9 (51.9; 98.9) | 0.983 |
| GOT 1. POD | 107.0 (67.0; 157.4) | 106.9 (66.8; 157.0) | 119.0 (96.2; 180.0) | 0.439 |
| GPT prior surgery | 24.0 (14.7; 48.9) | 24.4 (15.0; 49.0) | 18.1 (12.8; 48.0) | 0.292 |
| GPT admission ICU | 24.5 (17.7; 41.5) | 24.4 (17.4; 40.5) | 24.9 (19.8; 62.7) | 0.538 |
| GPT1. POD | 28.0 (19.4; 45.1) | 27.1 (19.0; 42.0) | 38.2 (22.2; 74.5) | 0.332 |
| Bilirubin 1 POD | 28.8 (16.3; 51.6) | 28.8 (15.9; 51.6) | 32.4 (21.1; 52.2) | 0.359 |
| INR prior surgery | 1.30 (1.16; 1.47) | 1.30 (1.16; 1.43) | 1.41 (1.17; 1.64) | 0.349 |
| INR admission ICU | 1.18 (1.09; 1.31) | 1.18 (1.09; 1.29) | 1.23 (1.05; 1.57) | 0.568 |
| INR 1. POD | 1.18 (1.10; 1.31) | 1.18 (1.10; 1.32) | 1.20 (1.13; 1.29) | 0.933 |
| INR_3. POD | 1.27 (1.18; 1.40) | 1.26 (1.18; 1.37) | 1.55 (1.20; 2.06) | 0.055 |
| CRP prior surgery | 14.4 (4.2; 47.4) | 13.5 (4.2; 46.6) | 14.9 (3.3; 58.5) | 0.922 |
| CRP admission ICU | 76.4 (50.6; 112.2) | 75.8 (50.4; 113.7) | 93.4 (74.5; 108.0) | 0.413 |
| CRP 3. POD | 176.5 (111.3; 238.5) | 179 (111; 242) | 139.0 (110.2; 200.5) | 0.383 |
| WBC prior surgery | 7.23 (5.75; 9.88) | 7.22 (5.77; 9.89) | 7.34 (4.80; 10.09) | 0.742 |
| WBC admission ICU | 14.00 (9.83; 19.03) | 14.32 (9.98; 19.05) | 13.66 (8.14; 19.64) | 0.655 |
| WBC 1. POD | 11.45 (7.92; 14.67) | 11.63 (7.86; 14.68) | 9.67 (7.76; 15.11) | 0.659 |
| WBC 3. POD | 10.57 (7.79; 14.16) | 10.35 (7.78; 14.18) | 11.15 (7.96; 14.13) | 0.588 |
| Platelet count prior surgery | 174.5 (120.5; 219.8) | 179.0 (122.0; 228.5) | 140.0 (78.5; 213.0) | 0.311 |
| Platelet count admission ICU | 153.0 (119.5; 189.5) | 154.5 (119.3; 192.5) | 139.0 (117.5; 161.5) | 0.332 |
| Platelet count 1. POD | 130.0 (98.0; 160.8) | 130.0 (99.5; 167.0) | 119.0 (55.5; 149.0) | 0.459 |
| Platelet count 3.POD | 96.0 (74.5; 119.3) | 96.0 (74.0; 119.5) | 95.0 (69.5; 120.5) | 0.904 |
| Hemoglobin prior surgery | 10.3 (9.4; 11.6) | 10.3 (9.3; 11.7) | 9.6 (9.3; 10.3) | 0.201 |
| Hemoglobine admission ICU | 10.4 (9.3; 11.4) | 10.4 (9.4; 11.4) | 9.0 (8.7; 10.4) | 0.032 |
| Hemoglobin 1. POD | 9.6 (9.1; 10.5) | 9.6 (9.0; 10.4) | 10.0 (8.2; 11.5) | 0.577 |
| LDH 1. POD | 412.0 (348.5; 528.0) | 412.0 (339.8; 528.0) | 442.0 (362.8; 572.3) | 0.665 |
| LDH3. POD | 362.0 (300.0; 464.0) | 362.5 (295.8; 452.0) | 348.0 (329.0; 585.0) | 0.392 |
| SPB onset after Implantation (d) | 11 (2; 15) |
|---|---|
| Duration of SPB (d) | 11 (7; 33) |
| Days of mechanical ventilation until onset of SPB (d) | 10 (6; 15) |
| Number of packed red blood cells during SPB | 19 (12; 27) |
| Number of fresh frozen plasma during SPB | 13 (10; 20) |
| Number of platelets during SPB | 6 (4; 12) |
| Fibrinogen during SPB | 4 (2; 8) |
| PCC during SPB (IE) | 2400 (1800; 4200) |
| Faktor XIII during SPB (IE) | 6000 (5000; 11,250) |
| von Willebrand factor during SPB (IE) | 19,000 (4000; 31,000) |
| Days of von Willebrand factor therapy (d) | 8 (4; 27) |
| Novoseven® during SPB (mg) | 0 (0; 0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panholzer, B.; Pilarczyk, K.; Huenges, K.; Aldinger, C.; Friedrich, C.; Nowak-Göttl, U.; Cremer, J.; Haneya, A. Severe Pulmonary Bleeding after Assist Device Implantation: Incidence, Risk Factors and Prognostic Impact. J. Clin. Med. 2022, 11, 1908. https://doi.org/10.3390/jcm11071908
Panholzer B, Pilarczyk K, Huenges K, Aldinger C, Friedrich C, Nowak-Göttl U, Cremer J, Haneya A. Severe Pulmonary Bleeding after Assist Device Implantation: Incidence, Risk Factors and Prognostic Impact. Journal of Clinical Medicine. 2022; 11(7):1908. https://doi.org/10.3390/jcm11071908
Chicago/Turabian StylePanholzer, Bernd, Kevin Pilarczyk, Katharina Huenges, Charlotte Aldinger, Christine Friedrich, Ulrike Nowak-Göttl, Jochen Cremer, and Assad Haneya. 2022. "Severe Pulmonary Bleeding after Assist Device Implantation: Incidence, Risk Factors and Prognostic Impact" Journal of Clinical Medicine 11, no. 7: 1908. https://doi.org/10.3390/jcm11071908
APA StylePanholzer, B., Pilarczyk, K., Huenges, K., Aldinger, C., Friedrich, C., Nowak-Göttl, U., Cremer, J., & Haneya, A. (2022). Severe Pulmonary Bleeding after Assist Device Implantation: Incidence, Risk Factors and Prognostic Impact. Journal of Clinical Medicine, 11(7), 1908. https://doi.org/10.3390/jcm11071908






