Surgery for Liver Metastasis of Non-Colorectal and Non-Neuroendocrine Tumors
Abstract
1. Introduction
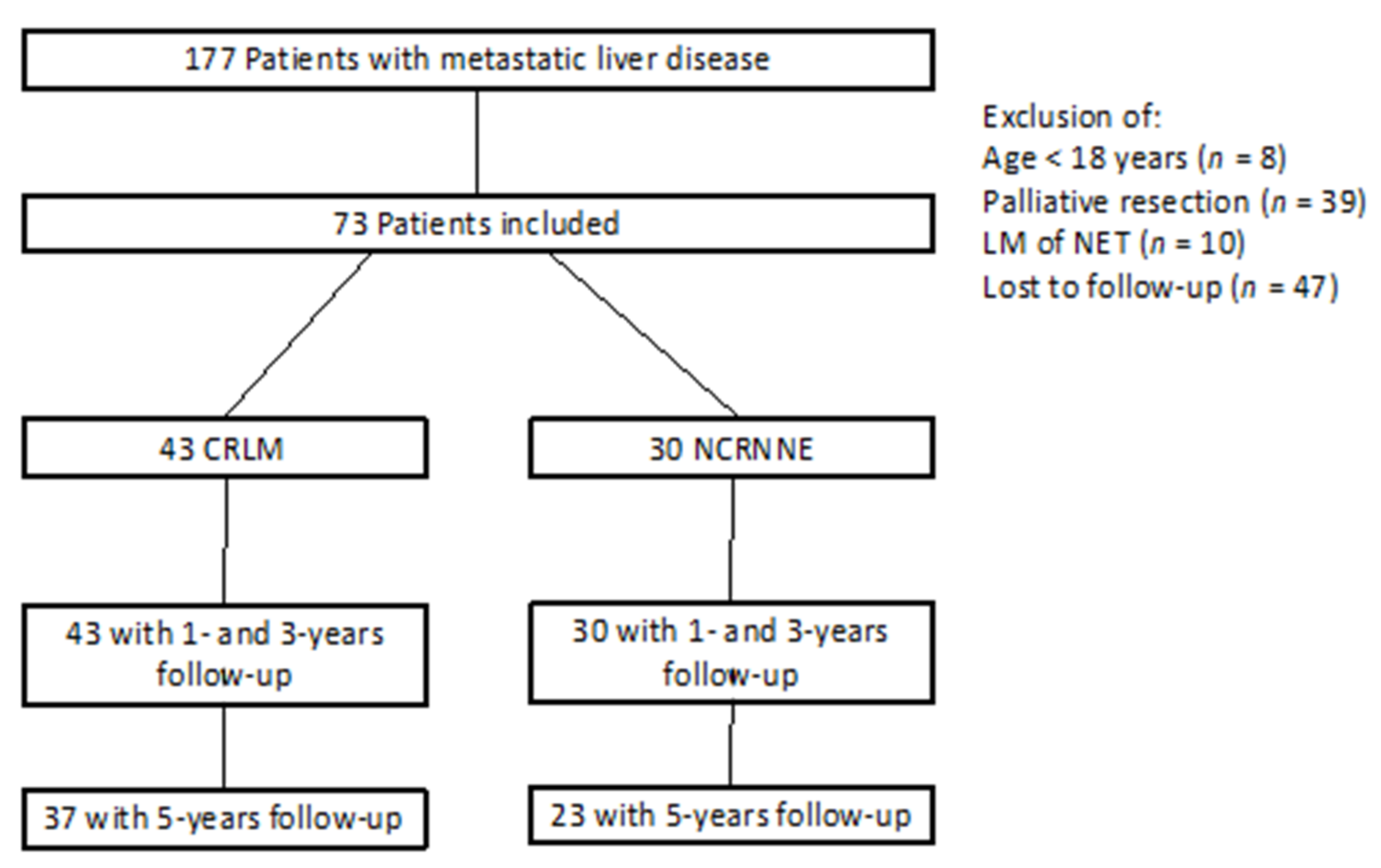
2. Materials and Methods
Statistics
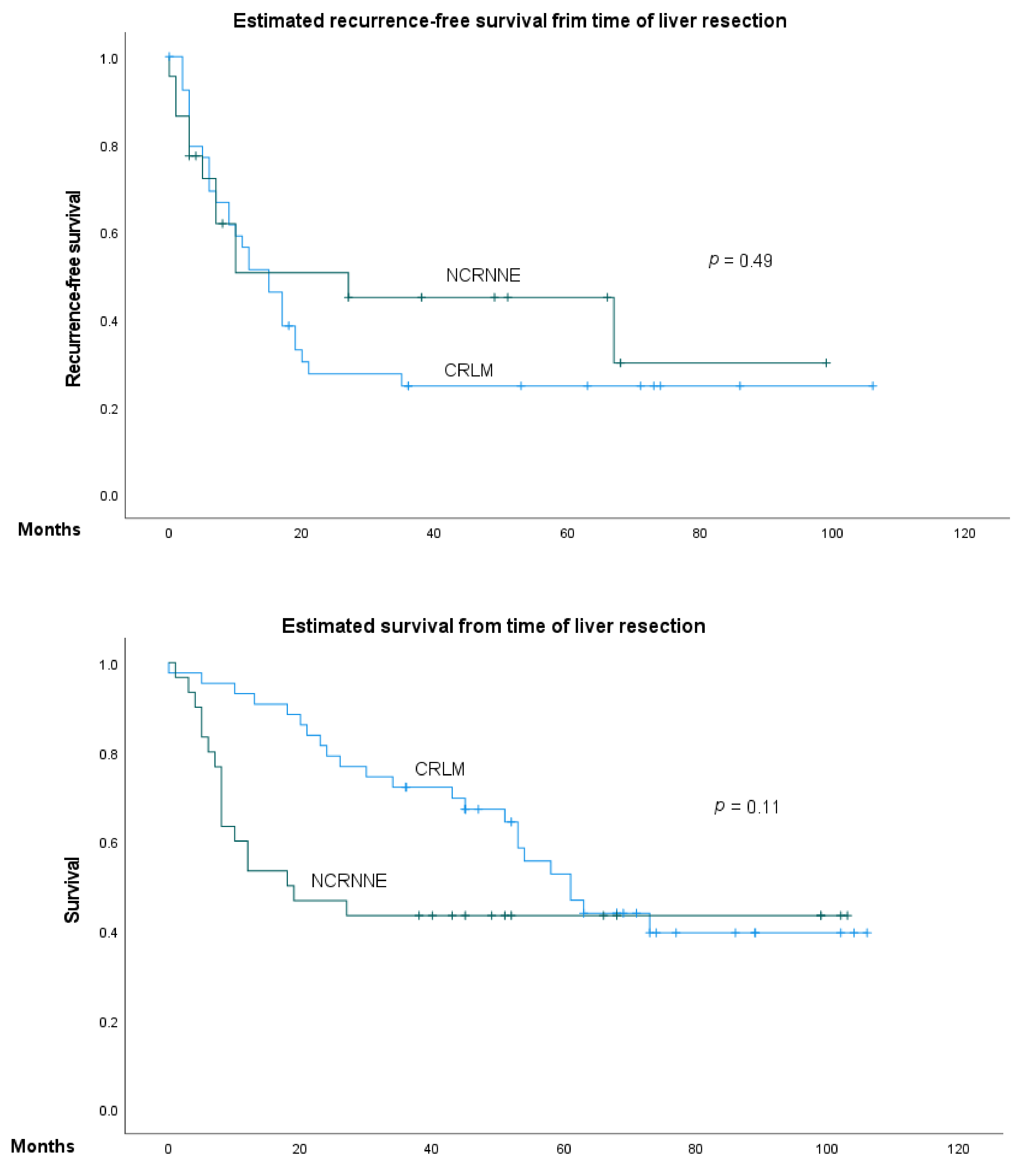
3. Results
3.1. Characteristic Data
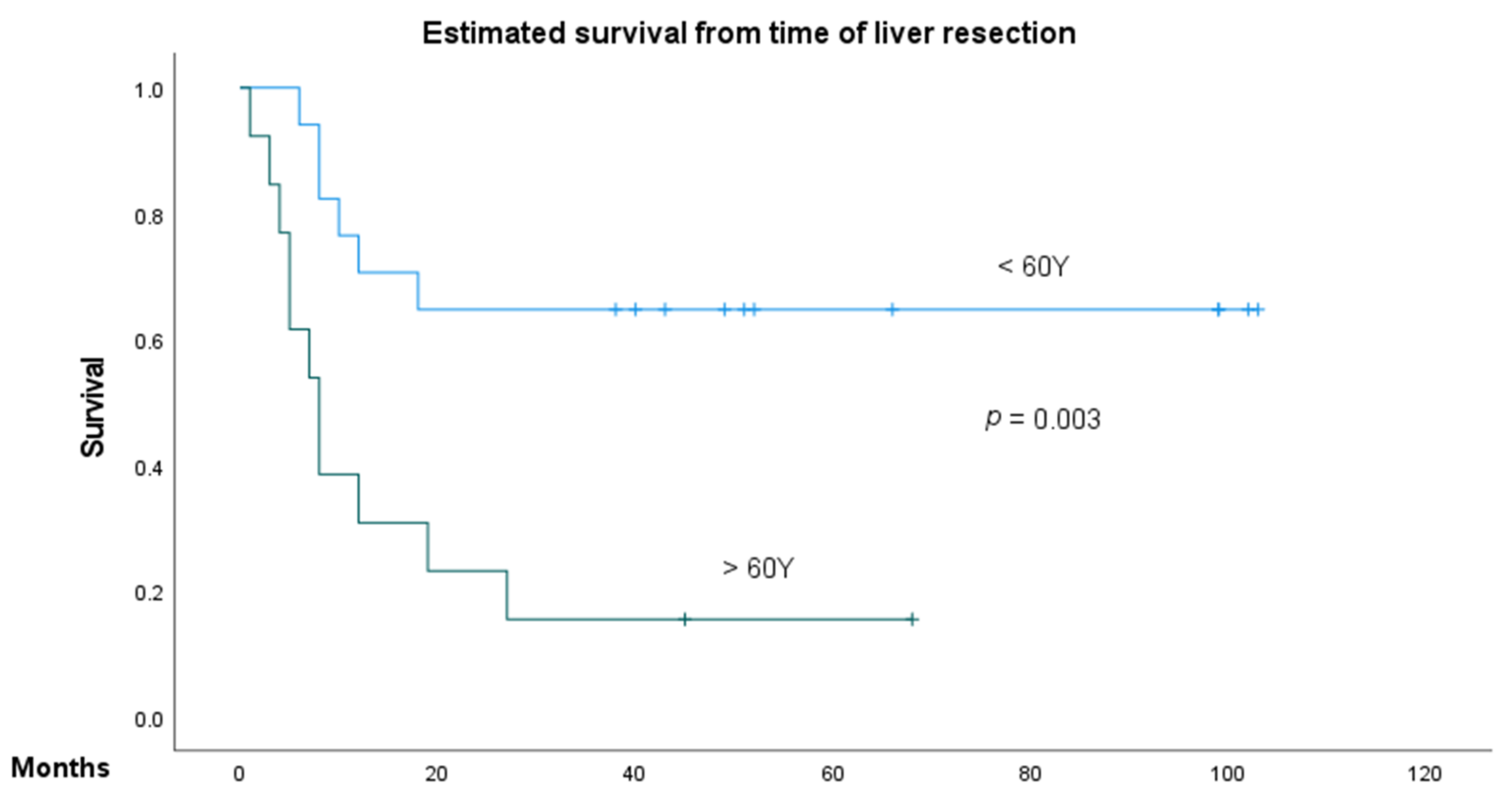
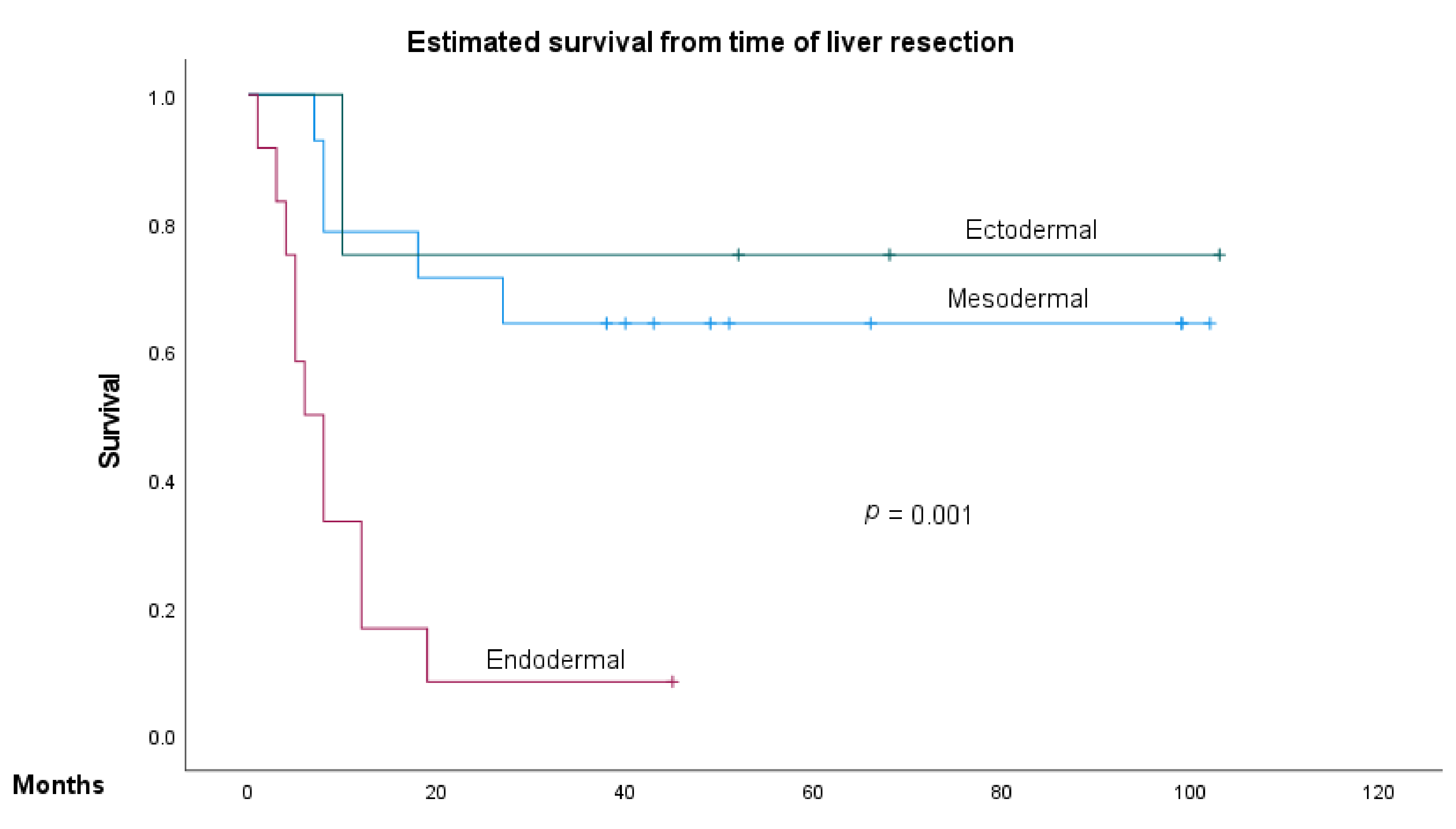
3.2. Predictive Factors of Overall Survival and Recurrence-Free Survival in NCRNNELM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abbas, S.; Lam, V.; Hollands, M. Ten-year survival after liver resection for colorectal metastases: Systematic review and meta-analysis. ISRN Oncol. 2011, 2011, 763245. [Google Scholar] [CrossRef][Green Version]
- Creasy, J.M.; Sadot, E.; Koerkamp, B.G.; Chou, J.F.; Gonen, M.; Kemeny, N.E.; Balachandran, V.P.; Kingham, T.P.; DeMatteo, R.P.; Allen, P.J.; et al. Actual 10-year survival after hepatic resection of colorectal liver metastases: What factors preclude cure? Surgery 2018, 163, 1238–1244. [Google Scholar] [CrossRef]
- Engstrand, J.; Nilsson, H.; Stromberg, C.; Jonas, E.; Freedman, J. Colorectal cancer liver metastases—A population-based study on incidence, management and survival. BMC Cancer 2018, 18, 78. [Google Scholar] [CrossRef]
- Hackl, C.; Neumann, P.; Gerken, M.; Loss, M.; Klinkhammer-Schalke, M.; Schlitt, H.J. Treatment of colorectal liver metastases in Germany: A ten-year population-based analysis of 5772 cases of primary colorectal adenocarcinoma. BMC Cancer 2014, 14, 810. [Google Scholar] [CrossRef]
- Kanas, G.P.; Taylor, A.; Primrose, J.N.; Langeberg, W.J.; Kelsh, M.A.; Mowat, F.S.; Alexander, D.D.; Choti, M.A.; Poston, G. Survival after liver resection in metastatic colorectal cancer: Review and meta-analysis of prognostic factors. Clin Epidemiol. 2012, 4, 283–301. [Google Scholar]
- Rees, M.; Tekkis, P.P.; Welsh, F.K.; O’Rourke, T.; John, T.G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: A multifactorial model of 929 patients. Ann. Surg. 2008, 247, 125–135. [Google Scholar] [CrossRef]
- Dorr, N.M.; Bartels, M.; Morgul, M.H. Current treatment of colorectal liver metastasis as a chronic disease. Anticancer Res. 2020, 40, 1–7. [Google Scholar] [CrossRef]
- Petrowsky, H.; Fritsch, R.; Guckenberger, M.; De Oliveira, M.L.; Dutkowski, P.; Clavien, P.A. Modern therapeutic approaches for the treatment of malignant liver tumours. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 755–772. [Google Scholar] [CrossRef]
- Frilling, A.; Clift, A.K. Therapeutic strategies for neuroendocrine liver metastases. Cancer 2015, 121, 1172–1186. [Google Scholar] [CrossRef]
- Mayo, S.C.; de Jong, M.C.; Pulitano, C.; Clary, B.M.; Reddy, S.K.; Gamblin, T.C.; Celinksi, S.A.; Kooby, D.A.; Staley, C.A.; Stokes, J.B.; et al. Surgical management of hepatic neuroendocrine tumor metastasis: Results from an international multi-institutional analysis. Ann. Surg. Oncol. 2010, 17, 3129–3136. [Google Scholar] [CrossRef]
- Fairweather, M.; Swanson, R.; Wang, J.; Brais, L.K.; Dutton, T.; Kulke, M.H.; Clancy, T.E. Management of neuroendocrine tumor liver metastases: Long-term outcomes and prognostic factors from a large prospective database. Ann. Surg Oncol. 2017, 24, 2319–2325. [Google Scholar] [CrossRef]
- Yedibela, S.; Gohl, J.; Graz, V.; Pfaffenberger, M.K.; Merkel, S.; Hohenberger, W.; Meyer, T. Changes in indication and results after resection of hepatic metastases from noncolorectal primary tumors: A single-institutional review. Ann. Surg Oncol. 2005, 12, 778–785. [Google Scholar] [CrossRef]
- Kassahun, W.T. Controversies in defining prognostic relevant selection criteria that determine long-term effectiveness of liver resection for noncolorectal nonneuroendocrine liver metastasis. Int. J. Surg. 2015, 24 Pt A, 85–90. [Google Scholar] [CrossRef]
- Takemura, N.; Saiura, A. Role of surgical resection for non-colorectal non-neuroendocrine liver metastases. World J. Hepatol. 2017, 9, 242–251. [Google Scholar] [CrossRef]
- De Ridder, J.; De Wilt, J.H.; Simmer, F.; Overbeek, L.; Lemmens, V.; Nagtegaal, I. Incidence and origin of histologically confirmed liver metastases: An explorative case-study of 23,154 patients. Oncotarget 2016, 7, 55368–55376. [Google Scholar] [CrossRef]
- Chiapponi, C.; Berlth, F.; Plum, P.S.; Betzler, C.; Stippel, D.L.; Popp, F.; Bruns, C.J. Oligometastatic Disease in Upper Gastrointestinal Cancer—How to Proceed? Visc. Med. 2017, 33, 31–34. [Google Scholar] [CrossRef]
- Leporrier, J.; Maurel, J.; Chiche, L.; Bara, S.; Segol, P.; Launoy, G. A population-based study of the incidence, management and prognosis of hepatic metastases from colorectal cancer. Br. J. Surg. 2006, 93, 465–474. [Google Scholar] [CrossRef]
- Sasaki, K.; Margonis, G.A.; Andreatos, N.; Zhang, X.F.; Buettner, S.; Wang, J.; Deshwar, A.; He, J.; Wolfgang, C.L.; Weiss, M.; et al. The prognostic utility of the “Tumor Burden Score” based on preoperative radiographic features of colorectal liver metastases. J. Surg. Oncol. 2017, 116, 515–523. [Google Scholar] [CrossRef]
- Adam, R.; Chiche, L.; Aloia, T.; Elias, D.; Salmon, R.; Rivoire, M.; Jaeck, D.; Saric, J.; Le Treut, Y.P.; Belghiti, J.; et al. Hepatic resection for noncolorectal nonendocrine liver metastases: Analysis of 1452 patients and development of a prognostic model. Ann. Surg. 2006, 244, 524–535. [Google Scholar]
- Bohlok, A.; Lucidi, V.; Bouazza, F.; Daher, A.; Germanova, D.; Van Laethem, J.L.; Hendlisz, A.; Donckier, V. The lack of selection criteria for surgery in patients with non-colorectal non-neuroendocrine liver metastases. World J. Surg Oncol. 2020, 18, 106. [Google Scholar] [CrossRef]
- Holzner, P.A.; Makowiec, F.; Klock, A.; Glatz, T.; Fichtner-Feigl, S.; Lang, S.A.; Neeff, H.P. Outcome after hepatic resection for isolated non-colorectal, non-neuroendocrine liver metastases in 100 patients—The role of the embryologic origin of the primary tumor. BMC Surg. 2018, 18, 89. [Google Scholar] [CrossRef]
- Parisi, A.; Trastulli, S.; Ricci, F.; Regina, R.; Cirocchi, R.; Grassi, V.; Gemini, A.; Pironi, D.; D’Andrea, V.; Santoro, A.; et al. Analysis of long-term results after liver surgery for metastases from colorectal and non-colorectal tumors: A retrospective cohort study. Int. J. Surg. 2016, 30, 25–30. [Google Scholar] [CrossRef] [PubMed]
- O’Rourke, T.R.; Tekkis, P.; Yeung, S.; Fawcett, J.; Lynch, S.; Strong, R.; Wall, D.; John, T.G.; Welsh, F.; Rees, M. Long-term results of liver resection for non-colorectal, non-neuroendocrine metastases. Ann. Surg Oncol. 2008, 15, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Groeschl, R.T.; Nachmany, I.; Steel, J.L.; Reddy, S.K.; Glazer, E.S.; de Jong, M.C.; Pawlik, T.M.; Geller, D.A.; Tsung, A.; Marsh, J.W.; et al. Hepatectomy for noncolorectal non-neuroendocrine metastatic cancer: A multi-institutional analysis. J. Am. Coll. Surg. 2012, 214, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Uggeri, F.; Ronchi, P.A.; Goffredo, P.; Garancini, M.; Degrate, L.; Nespoli, L.; Gianotti, L.; Romano, F. Metastatic liver disease from non-colorectal, non-neuroendocrine, non-sarcoma cancers: A systematic review. World J. Surg. Oncol. 2015, 13, 191. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sano, K.; Yamamoto, M.; Mimura, T.; Endo, I.; Nakamori, S.; Konishi, M.; Miyazaki, M.; Wakai, T.; Nagino, M.; Kubota, K.; et al. Outcomes of 1639 hepatectomies for non-colorectal non-neuroendocrine liver metastases: A multicenter analysis. J. Hepatobiliary Pancreat Sci. 2018, 25, 465–475. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Hibi, T.; Yoneda, G.; Iwao, Y.; Sawada, Y.; Hoshino, H.; Uemura, S.; Ban, D.; Kudo, A.; Takemura, T.; et al. Predictive model for survival after liver resection for noncolorectal liver metastases in the modern era: A Japanese multicenter analysis. J. Hepatobiliary Pancreat Sci. 2019, 26, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Schiergens, T.S.; Luning, J.; Renz, B.W.; Thomas, M.; Pratschke, S.; Feng, H.; Lee, S.M.L.; Engel, J.; Rentsch, M.; Guba, M.; et al. Liver resection for non-colorectal non-neuroendocrine metastases: Where do we stand today compared to colorectal cancer? J. Gastrointest. Surg. 2016, 20, 1163–1172. [Google Scholar] [CrossRef]
- Aghayan, D.L.; Kalinowski, P.; Kazaryan, A.M.; Fretland, A.A.; Sahakyan, M.A.; Rosok, B.I.; Pelanis, E.; Bjørnbeth, B.A.; Edwin, B. Laparoscopic liver resection for non-colorectal non-neuroendocrine metastases: Perioperative and oncologic outcomes. World J. Surg. Oncol. 2019, 17, 156. [Google Scholar] [CrossRef]
- Knitter, S.; Andreou, A.; Kradolfer, D.; Beierle, A.S.; Pesthy, S.; Eichelberg, A.-C.; Kästner, A.; Feldbrügge, L.; Krenzien, F.; Schulz, M.; et al. Minimal-invasive versus open hepatectomy for colorectal liver metastases: Bicentric analysis of postoperative outcomes and long-term survival using propensity score matching analysis. J. Clin. Med. 2020, 9, 4027. [Google Scholar] [CrossRef]
- Feldbrügge, L.; Ortiz Galindo, S.A.; Frisch, O.; Benzing, C.; Krenzien, F.; Riddermann, A.; Kästner, A.; Nevermann, N.F.; Malinka, T.; Schöning, W.; et al. Safety and feasibility of robotic liver resection after previous abdominal surgeries. Surg. Endosc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ren, Y.; Hu, Y.; Cui, N.; Wang, X.; Cui, Y. Neoadjuvant chemotherapy versus neoadjuvant chemoradiotherapy for cancer of the esophagus or the gastroesophageal junction: A meta-analysis based on clinical trials. PLoS ONE 2018, 13, e0202185. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, K.; Pucher, P.H.; Armstrong, T.; Bateman, A.; Hamady, Z. Systemic neoadjuvant chemotherapy in modern pancreatic cancer treatment: A systematic review and meta-analysis. Ann. R. Coll. Surg. Engl. 2019, 101, 453–462. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Feng, J.; Hu, Z.; Wu, J.; Zhang, M.; Shen, Y.; Zheng, S. Feasibility of pancreaticoduodenectomy with synchronous liver metastasectomy for oligometastatic pancreatic ductal adenocarcinoma—A case-control study. Ann. Med. Surg. 2020, 62, 490–494. [Google Scholar] [CrossRef] [PubMed]
- Turgeman, I.; Ben-Aharon, I. Evolving treatment paradigms in esophageal cancer. Ann. Transl. Med. 2021, 9, 903. [Google Scholar] [CrossRef] [PubMed]
- Hau, H.M.; Thalmann, F.; Lübbert, C.; Morgul, M.H.; Schmelzle, M.; Atanasov, G.; Benzing, C.; Lange, U.; Ascherl, R.; Ganzer, R.; et al. The value of hepatic resection in metastasic renal cancer in the Era of Tyrosinkinase Inhibitor Therapy. BMC Surg. 2016, 16, 49. [Google Scholar] [CrossRef]
- Lok, H.; Fung, A.; Chong, C.; Lee, K.; Wong, J.; Cheung, S.; Lai, P.; Ng, K. Comparison of long-term survival outcome after curative hepatectomy between selected patients with non-colorectal and colorectal liver metastasis: A propensity score matching analysis. Asian J. Surg. 2021, 44, 459–464. [Google Scholar] [CrossRef]
- Patkar, S.; Niyogi, D.; Parray, A.; Goel, M. Is resection for noncolorectal, nonneuroendocrine liver metastases justified? J. Surg. Oncol. 2021, 123, 957–962. [Google Scholar] [CrossRef]
- Elfrink, A.; Kok, N.; Swijnenburg, R.; den Dulk, M.; van den Boezem, P.; Hartgrink, H.; te Riele, W.W.; Patijn, G.; Leclercq, W.; Lips, D.; et al. Nationwide oncological networks for resection of colorectal liver metastases in the Netherlands: Differences and postoperative outcomes. Eur. J. Surg. Oncol. 2021, 48, 435–448. [Google Scholar] [CrossRef]
| Parameter | CRLM n = 43 | NCRNNE n = 30 | p (<0.05) |
|---|---|---|---|
| All patients | 43 (100.0) | 30 (100.0) | |
| Age (median and range) | 64.5 (35–90) | 54.3 (20–80) | 0.03 a |
| Sex (n, %) | |||
| Male | 26 (60.5) | 15 (50.0) | n.s. |
| Female | 17 (39.5) | 15 (50.0) | n.s. |
| BMI (kg/m2, mean ± SD) | 27.2 | 26.0 | n.s. |
| ASA score (n, %) | |||
| ≤2 | 28 (65.1) * | 22 (73.3) | n.s. |
| >2 | 12 (27.9) | 8 (26.7) | |
| Synchronicity (n, %) | |||
| synchronous | 19 (44.2) | 12 (40.0) | n.s. |
| metachronous | 24 (55.8) | 18 (60.0) | n.s. |
| Extrahepatic metastasis (n, %) | |||
| Yes | 2 (4.7) | 9 (30.0) | 0.003 b |
| No | 41 (95.3) | 21 (70.0) | |
| Number of metastases (n, %) | |||
| Solitary | 22 (51.1) * | 24 (80.0) | |
| Oligo | 12 (27.9) | 5 (16.7) | 0.02 b |
| Multiple | 6 (13.9) | 1 (3.3) | |
| Size of biggest lesion (n, %) | |||
| ≤5 cm | 36 (83.7) * | 25 (83.3) | n.s. |
| >5 cm | 4 (9.3) | 5 (16.7) | |
| TBS (mean ± SD) | 4.5 ± 4.6 | 3.9 ± 3.4 | n.s. |
| Location (n, %) | |||
| Right lobe | 25 (58.1) | 17 (56.7) | |
| Left lobe | 7 (16.3) | 11 (36.7) | 0.03 |
| Bilobar | 11 (25.6) | 2 (6.7) | |
| Preoperative chemotherapy (n, %) | |||
| Yes | 28 (65.1) | 17 (56.7) | n.s. |
| No | 15 (34.9) | 13 (43.3) | |
| Postoperative chemotherapy (n, %) | |||
| Yes | 21 (48.8) * | 12 (40.0) * | n.s. |
| No | 20 (46.5) | 16 (53.3) | |
| Liver resection (n, %) | |||
| minor | 36 (83.7) | 29 (96.7) | n.s. |
| major | 7 (16.3) | 1 (3.3) | |
| Surgery time (min, mean ± SD) | 214 | 237.8 | n.s. |
| ICU (day, mean ± SD) | 5.6 | 4.1 | n.s. |
| Blood Transfusion (n, %) | |||
| Yes | 7 (16.2) * | 2 (6.66) * | n.s. |
| No | 34 (79.0) | 26 (86.6) | |
| R-status (n, %) | |||
| R0 | 40 (93.0) | 27 (90.0) | n.s. |
| R1 | 3 (7.0) | 3 (10.0) | |
| Reoperation (n, %) | |||
| Yes | 7 (16.3) | 0 (0) | 0.02 b |
| No | 36 (83.7) | 30 (100.0) | |
| Complications (n, %) | |||
| none | 18 (41.8) * | 14 (46.7) | |
| CD ≤ 3a | 16 (37.2) | 14 (46.7) | n.s. |
| CD > 3a | 8 (18.6) | 2 (6.7) | |
| ICU readmission (n, %) | |||
| yes | 2 (5.0) | 1 (3.3) | n.s. |
| no | 41 (95.0) | 29 (96.7) |
| Variables | Recurrence-Free Survival | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariante | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| ASA, ≤2 vs. >2 | 1.22 | 0.32–4.66 | 0.76 | |||
| Sex, male vs. female | 0.56 | 0.16–1.89 | 0.35 | |||
| Age, ≤60 years vs. >60 years | 1.36 | 0.41–4.45 | 0.60 | |||
| Primary embryology, mesoderm vs. ectoderm vs. endoderm | 3.41 | 1.34–8.67 | 0.01 | 2.96 | 1.10–7.94 | 0.03 |
| Extra-hepatic disease manifestation, yes vs. No | 0.76 | 0.16–3.55 | 0.73 | |||
| Synchronicity, synchronous vs. metachronous | 1.44 | 0.43–4.81 | 0.55 | |||
| Timing of metastases, ≤24 months vs. >24 months | 0.99 | 0.29–3.35 | 0.99 | |||
| Number of metastases, solitary vs. Multiple | 12.59 | 2.09–75.74 | 0.006 | 5.71 | 0.92–35.47 | 0.06 |
| Location of metastases, right vs. Left vs. Bilobar | 0.94 | 0.31–2.85 | 0.92 | |||
| Size of biggest lesion, ≤5 cm vs. >5 cm | 0.26 | 0.31–2.21 | 0.21 | |||
| Neoadjuvant chemotherapy, yes vs. no | 0.56 | 0.16–1.92 | 0.36 | |||
| Adjuvant chemotherapy, yes vs. no | 0.75 | 0.19–2.83 | 0.67 | |||
| Clavien-Dindo, 0 vs. ≤3a vs. >3a | 1.67 | 0.62–4.44 | 0.30 | |||
| Variables | Overall Survival | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR | 95% CI | p | HR | 95% CI | p | |
| ASA, <2 vs. >2 | 1.76 | 0.65–4.77 | 0.26 | |||
| Sex, male vs. female | 0.74 | 0.28–1.92 | 0.53 | |||
| Age, ≤60 years vs. >60 years | 4.03 | 1.48–10.99 | 0.006 | 1.90 | 0.51–7.01 | 0.33 |
| Primary embryology, mesoderm vs. ectoderm vs. endoderm | 2.46 | 1.37–4.41 | 0.003 | 1.93 | 0.91–4.08 | 0.08 |
| Extra-hepatic disease manifestation, yes vs. no | 0.88 | 0.30–2.50 | 0.81 | |||
| Synchronicity, synchronous vs. metachronous | 0.80 | 0.30–2.12 | 0.66 | |||
| Timing of metastases, ≤24 months vs. >24 months | 1.86 | 0.65–5.30 | 0.24 | |||
| Number of metastases, solitary vs. multiple | 1.42 | 0.46–4.39 | 0.53 | |||
| Location of metastases, right vs. Left vs. bilobar | 0.96 | 0.43–2.14 | 0.93 | |||
| Size of biggest lesion, <5 cm vs. >5 cm | 1.02 | 0.29–3.56 | 0.97 | |||
| Neoadjuvant chemotherapy, yes vs. No | 0.54 | 0.20–1.40 | 0.20 | |||
| Adjuvant chemotherapy, yes vs. No | 0.65 | 0.23–1.79 | 0.40 | |||
| R status, R0 vs. R1 | 1.66 | 0.37–7.28 | 0.50 | |||
| Blood transfusion, yes vs. No | 4.63 | 0.99–21.52 | 0.05 | |||
| Clavien-Dindo, 0 vs. ≤3a vs. >3a | 1.83 | 0.89–3.78 | 0.09 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katou, S.; Schmid, F.; Silveira, C.; Schäfer, L.; Naim, T.; Becker, F.; Radunz, S.; Juratli, M.A.; Seifert, L.L.; Heinzow, H.; et al. Surgery for Liver Metastasis of Non-Colorectal and Non-Neuroendocrine Tumors. J. Clin. Med. 2022, 11, 1906. https://doi.org/10.3390/jcm11071906
Katou S, Schmid F, Silveira C, Schäfer L, Naim T, Becker F, Radunz S, Juratli MA, Seifert LL, Heinzow H, et al. Surgery for Liver Metastasis of Non-Colorectal and Non-Neuroendocrine Tumors. Journal of Clinical Medicine. 2022; 11(7):1906. https://doi.org/10.3390/jcm11071906
Chicago/Turabian StyleKatou, Shadi, Franziska Schmid, Carolina Silveira, Lina Schäfer, Tizian Naim, Felix Becker, Sonia Radunz, Mazen A. Juratli, Leon Louis Seifert, Hauke Heinzow, and et al. 2022. "Surgery for Liver Metastasis of Non-Colorectal and Non-Neuroendocrine Tumors" Journal of Clinical Medicine 11, no. 7: 1906. https://doi.org/10.3390/jcm11071906
APA StyleKatou, S., Schmid, F., Silveira, C., Schäfer, L., Naim, T., Becker, F., Radunz, S., Juratli, M. A., Seifert, L. L., Heinzow, H., Struecker, B., Pascher, A., & Morgul, M. H. (2022). Surgery for Liver Metastasis of Non-Colorectal and Non-Neuroendocrine Tumors. Journal of Clinical Medicine, 11(7), 1906. https://doi.org/10.3390/jcm11071906






