The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients
Abstract
:1. Introduction
2. Glucagon-like Peptide-1 Receptor Agonists (GLP-1RA)
2.1. Specific Medications
2.1.1. Liraglutide
2.1.2. Semaglutide
2.1.3. Dulaglutide
2.1.4. Albiglutide
2.2. Cardio-Protection Mechanism
2.3. Chronic Kidney Disease (CKD)
2.4. Adverse Outcomes/Side Effects
3. Sodium-Glucose Cotransporter-2 Inhibitors (SGLT2i)
3.1. Specific Medication
3.1.1. Empagliflozin
3.1.2. Canagliflozin
3.1.3. Dapagliflozin
3.2. Cardio-Protection Mechanisms
3.3. Heart Failure
3.3.1. Heart Failure with Reduced Ejection Fraction (HFrEF)
Dapagliflozin
Empagliflozin
Sotagliflozin
3.3.2. Heart Failure with Preserved Ejection Fraction (HFpEF)
3.3.3. Acute Heart Failure
3.4. Chronic Kidney Disease (CKD)
3.5. Adverse Outcome/Side Effects
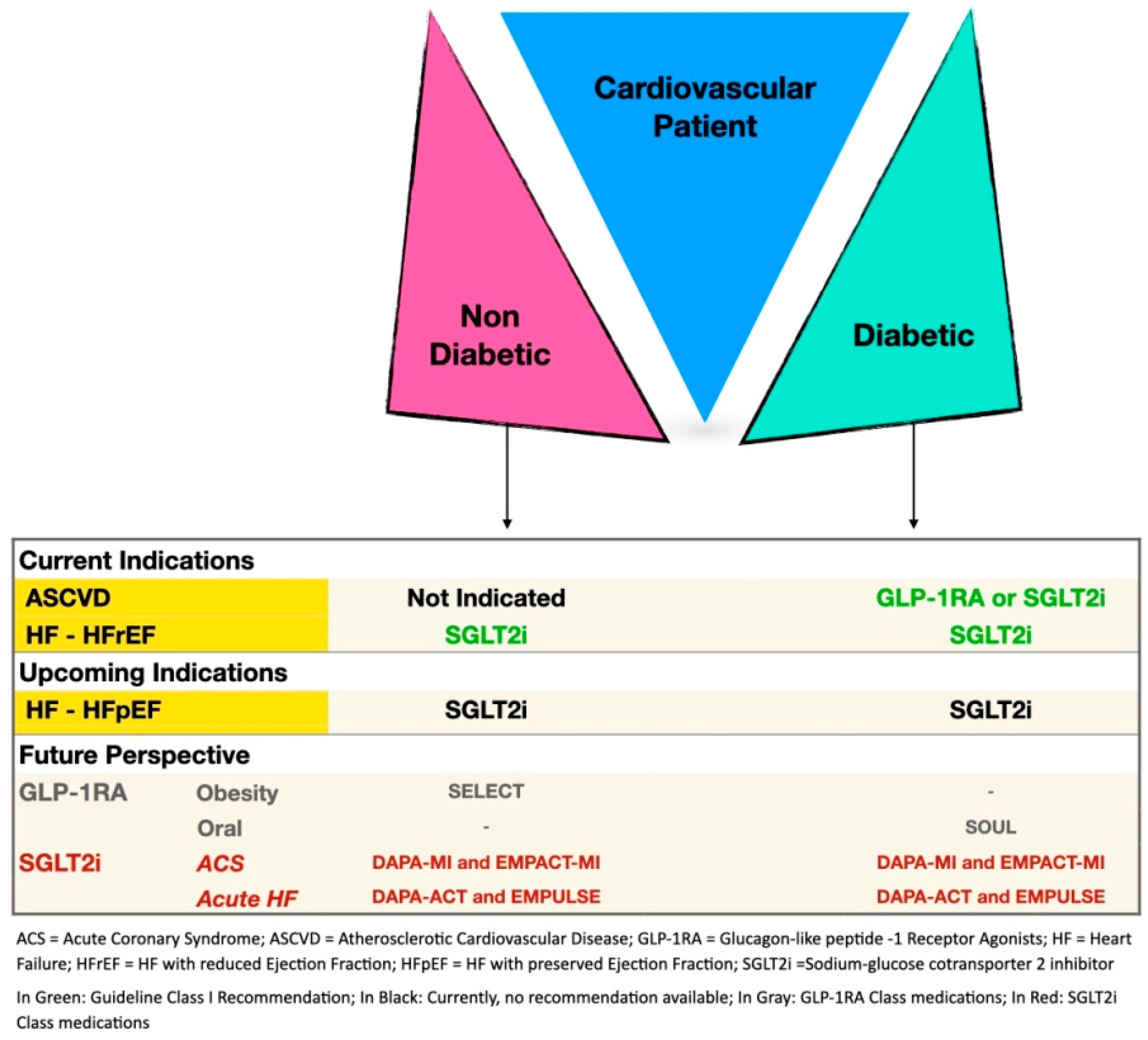
4. Current Guidelines
5. Non-Pharmacological Interventions
6. Conclusions and Future Perspective
Funding
Conflicts of Interest
Abbreviations
| ASCVD | Atherosclerotic cardiovascular disease |
| CVD | Cardiovascular disease |
| CVOTs | Cardiovascular outcome trials |
| DM | Diabetes mellitus |
| DPP-4 | Dipeptidyl peptidase-4 |
| GLP-1 | Glucagon-like peptide-1 |
| GLP-1RA | Glucagon-like peptide-1 receptor agonists |
| HFrEF | Heart Failure with reduced ejection fraction |
| HFpEF | Heart Failure with Preserved ejection fraction |
| MACE | Major adverse cardiovascular events |
| MI | Myocardial infarction |
| SGLT2i | Sodium-glucose cotransporter 2 inhibitor |
References
- Zimmet, P.Z.; Magliano, D.J.; Herman, W.H.; Shaw, J.E. Diabetes: A 21st Century Challenge. Lanect Diabetes Endocrinol. 2014, 2, 56–64. [Google Scholar] [CrossRef]
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global Estimates of Diabetes Prevalence for 2017 and Projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, J.M.; Divers, J.; Isom, S.; Saydah, S.; Imperatore, G.; Pihoker, C.; Marcovina, S.M.; Mayer-Davis, E.J.; Hamman, R.F.; Dolan, L.; et al. Trends in Prevalence of Type 1 and Type 2 Diabetes in Children and Adolescents in the US, 2001–2017. JAMA 2021, 326, 717–727. [Google Scholar] [CrossRef] [PubMed]
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2019, 41, 12–85. [Google Scholar] [CrossRef]
- Holman, R.R.; Paul, S.K.; Bethel, M.A.; Matthews, D.R.; Neil, H.A.W. 10-Year Follow-Up of Intensive Glucose Control in Type 2 Diabetes. N. Engl. J. Med. 2008, 359, 1577–1589. [Google Scholar] [CrossRef] [Green Version]
- Diabetes Control and Complications Trial Research Group. The Effect of Intensive Treatment of Diabetes on the Development and Progression of Long-Term Complications in Insulin-Dependent Diabetes Mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef]
- Gregg, E.W.; Cheng, Y.J.; Srinivasan, M.; Lin, J.; Geiss, L.S.; Albright, A.L.; Imperatore, G. Trends in Cause-Specific Mortality among Adults with and without Diagnosed Diabetes in the USA: An Epidemiological Analysis of Linked National Survey and Vital Statistics Data. Lancet 2018, 391, 2430–2440. [Google Scholar] [CrossRef]
- Collaboration, E.R.F.; Sarwar, N.; Gao, P.; Seshasai, S.R.K.; Gobin, R.; Kaptoge, S.; Angelantonio, E.D.; Ingelsson, E.; Lawlor, D.A.; Selvin, E.; et al. Diabetes Mellitus, Fasting Blood Glucose Concentration, and Risk of Vascular Disease: A Collaborative Meta-Analysis of 102 Prospective Studies. Lancet 2010, 375, 2215–2222. [Google Scholar] [CrossRef] [Green Version]
- Rawshani, A.; Rawshani, A.; Franzén, S.; Eliasson, B.; Svensson, A.-M.; Miftaraj, M.; McGuire, D.K.; Sattar, N.; Rosengren, A.; Gudbjörnsdottir, S. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N. Engl. J. Med. 2017, 376, 1407–1418. [Google Scholar] [CrossRef] [Green Version]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical PracticeDeveloped by the Task Force for Cardiovascular Disease Prevention in Clinical Practice with Representatives of the European Society of Cardiology and 12 Medical Societies with the Special Contribution of the European Association of Preventive Cardiology (EAPC). Eur. Heart J. 2021, 42, ehab484. [Google Scholar] [CrossRef]
- Newman, J.D.; Vani, A.K.; Aleman, J.O.; Weintraub, H.S.; Berger, J.S.; Schwartzbard, A.Z. The Changing Landscape of Diabetes Therapy for Cardiovascular Risk Reduction JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2018, 72, 1856–1869. [Google Scholar] [CrossRef] [PubMed]
- Pálsson, R.; Patel, U.D. Cardiovascular Complications of Diabetic Kidney Disease. Adv. Chronic. Kidney Dis. 2014, 21, 273–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nauck, M.A.; Vardarli, I.; Deacon, C.F.; Holst, J.J.; Meier, J.J. Secretion of Glucagon-like Peptide-1 (GLP-1) in Type 2 Diabetes: What Is up, What Is Down? Diabetologia 2011, 54, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Nauck, M.A. The Rollercoaster History of Using Physiological and Pharmacological Properties of Incretin Hormones to Develop Diabetes Medications with a Convincing Benefit-Risk Relationship. Metab. Clin. Exp. 2020, 103, 154031. [Google Scholar] [CrossRef]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.E.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [Green Version]
- Crowley, M.J.; McGuire, D.K.; Alexopoulos, A.-S.; Jensen, T.J.; Rasmussen, S.; Saevereid, H.A.; Verma, S.; Buse, J.B. Effects of Liraglutide on Cardiovascular Outcomes in Type 2 Diabetes Patients with and without Baseline Metformin Use: Post Hoc Analyses of the LEADER Trial. Diabetes Care 2020, 43, e108–e110. [Google Scholar] [CrossRef]
- Verma, S.; Bain, S.C.; Buse, J.B.; Idorn, T.; Rasmussen, S.; Ørsted, D.D.; Nauck, M.A. Occurence of First and Recurrent Major Adverse Cardiovascular Events with Liraglutide Treatment Among Patients with Type 2 Diabetes and High Risk of Cardiovascular Events. JAMA Cardiol. 2019, 4, 1214–1220. [Google Scholar] [CrossRef]
- Svanström, H.; Ueda, P.; Melbye, M.; Eliasson, B.; Svensson, A.-M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B. Use of Liraglutide and Risk of Major Cardiovascular Events: A Register-Based Cohort Study in Denmark and Sweden. Lancet Diabetes Endocrinol. 2019, 7, 106–114. [Google Scholar] [CrossRef]
- Marso, S.P.; Bain, S.C.; Consoli, A.; Eliaschewitz, F.G.; Jódar, E.; Leiter, L.A.; Lingvay, I.; Rosenstock, J.; Seufert, J.; Warren, M.L.; et al. Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 1834–1844. [Google Scholar] [CrossRef] [Green Version]
- Husain, M.; Birkenfeld, A.L.; Donsmark, M.; Dungan, K.; Eliaschewitz, F.G.; Franco, D.R.; Jeppesen, O.K.; Lingvay, I.; Mosenzon, O.; Pedersen, S.D.; et al. Oral Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 841–851. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerstein, H.C.; Colhoun, H.M.; Dagenais, G.R.; Diaz, R.; Lakshmanan, M.; Pais, P.; Probstfield, J.; Riesmeyer, J.S.; Riddle, M.C.; Rydén, L.; et al. Dulaglutide and Cardiovascular Outcomes in Type 2 Diabetes (REWIND): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2019, 394, 121–130. [Google Scholar] [CrossRef]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and Therapeutic Implications of Current Drugs for Type 2 Diabetes Mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernandez, A.F.; Green, J.B.; Janmohamed, S.; D’Agostino, R.B.; Granger, C.B.; Jones, N.P.; Leiter, L.A.; Rosenberg, A.E.; Sigmon, K.N.; Somerville, M.C.; et al. Albiglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes and Cardiovascular Disease (Harmony Outcomes): A Double-Blind, Randomised Placebo-Controlled Trial. Lancet 2018, 392, 1519–1529. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, X.; Lu, P.; Zhang, J.; Xu, Y.; He, W.; Li, M.; Zhang, S.; Jia, J.; Shao, S.; et al. Incretin-Based Agents in Type 2 Diabetic Patients at Cardiovascular Risk: Compare the Effect of GLP-1 Agonists and DPP-4 Inhibitors on Cardiovascular and Pancreatic Outcomes. Cardiovasc. Diabetol. 2017, 16, 31. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.S.-H.; Lee, J.-K.; Chen, W.-J. Major Adverse Cardiovascular and Limb Events in Patients with Diabetes Treated with GLP-1 Receptor Agonists vs DPP-4 Inhibitors. Diabetologia 2021, 64, 1949–1962. [Google Scholar] [CrossRef]
- Longato, E.; Camillo, B.D.; Sparacino, G.; Tramontan, L.; Avogaro, A.; Fadini, G.P. Better Cardiovascular Outcomes of Type 2 Diabetic Patients Treated with GLP-1 Receptor Agonists versus DPP-4 Inhibitors in Clinical Practice. Cardiovasc. Diabetol. 2020, 19, 74. [Google Scholar] [CrossRef]
- Anyanwagu, U.; Mamza, J.; Donnelly, R.; Idris, I. Effect of Adding GLP-1RA on Mortality, Cardiovascular Events, and Metabolic Outcomes among Insulin-Treated Patients with Type 2 Diabetes: A Large Retrospective UK Cohort Study. Am. Heart J. 2018, 196, 18–27. [Google Scholar] [CrossRef]
- Kristensen, S.L.; Rørth, R.; Jhund, P.S.; Docherty, K.F.; Sattar, N.; Preiss, D.; Køber, L.; Petrie, M.C.; McMurray, J.J.V. Cardiovascular, Mortality, and Kidney Outcomes with GLP-1 Receptor Agonists in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Cardiovascular Outcome Trials. Lancet Diabetes Endocrinol. 2019, 7, 776–785. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Barnes, E.H.; Keech, A.; et al. Efficacy and Safety of More Intensive Lowering of LDL Cholesterol: A Meta-Analysis of Data from 170,000 Participants in 26 Randomised Trials. Lancet 2010, 376, 1670–1681. [Google Scholar] [CrossRef] [Green Version]
- Rakipovski, G.; Rolin, B.; Nøhr, J.; Klewe, I.; Frederiksen, K.S.; Augustin, R.; Hecksher-Sørensen, J.; Ingvorsen, C.; Polex-Wolf, J.; Knudsen, L.B. The GLP-1 Analogs Liraglutide and Semaglutide Reduce Atherosclerosis in ApoE−/− and LDLr−/− Mice by a Mechanism That Includes Inflammatory Pathways. JACC Basic. Trans. Sci. 2018, 3, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Anholm, C.; Kumarathurai, P.; Pedersen, L.R.; Samkani, A.; Walzem, R.L.; Nielsen, O.W.; Kristiansen, O.P.; Fenger, M.; Madsbad, S.; Sajadieh, A.; et al. Liraglutide in Combination with Metformin May Improve the Atherogenic Lipid Profile and Decrease C-Reactive Protein Level in Statin Treated Obese Patients with Coronary Artery Disease and Newly Diagnosed Type 2 Diabetes: A Randomized Trial. Atherosclerosis 2019, 288, 60–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikolic, D.; Giglio, R.V.; Rizvi, A.A.; Patti, A.M.; Montalto, G.; Maranta, F.; Cianflone, D.; Stoian, A.P.; Rizzo, M. Liraglutide Reduces Carotid Intima-Media Thickness by Reducing Small Dense Low-Density Lipoproteins in a Real-World Setting of Patients with Type 2 Diabetes: A Novel Anti-Atherogenic Effect. Diabetes Ther. 2021, 12, 261–274. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Z.; Ilyas, I.; Little, P.J.; Kamato, D.; Sahebka, A.; Chen, Z.; Luo, S.; Zheng, X.; Weng, J.; et al. GLP-1 Receptor Agonists (GLP-1RAs): Cardiovascular Actions and Therapeutic Potential. Int. J. Biol. Sci. 2021, 17, 2050–2068. [Google Scholar] [CrossRef] [PubMed]
- Shaman, A.M.; Bain, S.C.; Bakris, G.L.; Buse, J.B.; Idorn, T.; Mahaffey, K.W.; Mann, J.F.E.; Nauck, M.A.; Rasmussen, S.; Rossing, P.; et al. Effect of the Glucagon-like Peptide-1 Receptor Agonists Semaglutide and Liraglutide on Kidney Outcomes in Patients with Type 2 Diabetes: A Pooled Analysis of SUSTAIN 6 and LEADER Trials. Circulation 2021, 145, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Aziz, M.A.E.; Cahyadi, O.; Meier, J.J.; Schmidt, W.E.; Nauck, M.A. Incretin-Based Glucose-Lowering Medications and the Risk of Acute Pancreatitis and Malignancies: A Meta-Analysis Based on Cardiovascular Outcomes Trials. Diabetes Obes. Metab. 2020, 22, 699–704. [Google Scholar] [CrossRef]
- Cao, C.; Yang, S.; Zhou, Z. GLP-1 Receptor Agonists and Pancreatic Safety Concerns in Type 2 Diabetic Patients: Data from Cardiovascular Outcome Trials. Endocrine 2020, 68, 518–525. [Google Scholar] [CrossRef]
- Knudsen, L.B.; Madsen, L.W.; Andersen, S.; Almholt, K.; Boer, A.S.; Drucker, D.J.; Gotfredsen, C.; Egerod, F.L.; Hegelund, A.C.; Jacobsen, H.; et al. Glucagon-like Peptide-1 Receptor Agonists Activate Rodent Thyroid C-Cells Causing Calcitonin Release and C-Cell Proliferation. Endocrinology 2010, 151, 1473–1486. [Google Scholar] [CrossRef] [Green Version]
- Aroda, V.R.; Bain, S.C.; Cariou, B.; Piletič, M.; Rose, L.; Axelsen, M.; Rowe, E.; DeVries, J.H. Efficacy and Safety of Once-Weekly Semaglutide versus Once-Daily Insulin Glargine as Add-on to Metformin (with or without Sulfonylureas) in Insulin-Naive Patients with Type 2 Diabetes (SUSTAIN 4): A Randomised, Open-Label, Parallel-Group, Multicentre, Multinational, Phase 3a Trial. Lancet Diabetes Endocrinol. 2017, 5, 355–366. [Google Scholar] [CrossRef]
- Rieg, T.; Vallon, V. Development of SGLT1 and SGLT2 Inhibitors. Diabetologia 2018, 61, 2079–2086. [Google Scholar] [CrossRef] [Green Version]
- Pasternak, B.; Ueda, P.; Eliasson, B.; Svensson, A.M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Wintzell, V.; Melbye, M.; et al. Use of Sodium Glucose Cotransporter 2 Inhibitors and Risk of Major Cardiovascular Events and Heart Failure: Scandinavian Register Based Cohort Study. BMJ 2019, 366, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Bowe, B.; Gibson, A.K.; McGill, J.B.; Maddukuri, G.; Al-Aly, Z. Comparative Effectiveness of Sodium-Glucose Cotransporter 2 Inhibitors vs Sulfonylureas in Patients with Type 2 Diabetes. JAMA Int. Med. 2021, 181, 1043–1053. [Google Scholar] [CrossRef] [PubMed]
- Kosiborod, M.; Lam, C.S.P.; Kohsaka, S.; Kim, D.J.; Karasik, A.; Shaw, J.; Tangri, N.; Goh, S.-Y.; Thuresson, M.; Chen, H.; et al. Cardiovascular Events Associated with SGLT-2 Inhibitors versus Other Glucose-Lowering Drugs The CVD-REAL 2 Study. J. Am. Coll. Cardiol. 2018, 71, 2628–2639. [Google Scholar] [CrossRef] [PubMed]
- Real, J.; Vlacho, B.; Ortega, E.; Vallés, J.A.; Mata-Cases, M.; Castelblanco, E.; Wittbrodt, E.T.; Fenici, P.; Kosiborod, M.; Mauricio, D.; et al. Cardiovascular and Mortality Benefits of Sodium–Glucose Co-Transporter-2 Inhibitors in Patients with Type 2 Diabetes Mellitus: CVD-Real Catalonia. Cardiovasc. Diabetol. 2021, 20, 139. [Google Scholar] [CrossRef]
- Jeon, J.Y.; Ha, K.H.; Kim, D.J. Cardiovascular Safety of Sodium Glucose Cotransporter 2 Inhibitors as Add-on to Metformin Monotherapy in Patients with Type 2 Diabetes Mellitus. Korean Diabetes J. 2020, 45, 505–514. [Google Scholar] [CrossRef]
- Kosiborod, M.; Cavender, M.A.; Fu, A.Z.; Wilding, J.P.; Khunti, K.; Holl, R.W.; Norhammar, A.; Birkeland, K.I.; Jørgensen, M.E.; Thuresson, M.; et al. Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors versus Other Glucose-Lowering Drugs. Circulation 2017, 136, 249–259. [Google Scholar] [CrossRef]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [Green Version]
- McGuire, D.K.; Zinman, B.; Inzucchi, S.E.; Wanner, C.; Fitchett, D.; Anker, S.D.; Pocock, S.; Kaspers, S.; George, J.T.; von Eynatten, M.; et al. Effects of Empagliflozin on First and Recurrent Clinical Events in Patients with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: A Secondary Analysis of the EMPA-REG OUTCOME Trial. Lancet Diabetes Endocrinol. 2020, 8, 949–959. [Google Scholar] [CrossRef]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R.; et al. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. 2019 ESC Guidelines on Diabetes, Pre-Diabetes, and Cardiovascular Diseases Developed in Collaboration with the EASD. Eur. Heart J. 2019, 40, 3215–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Pocock, S.J.; Carson, P.; Januzzi, J.; Verma, S.; Tsutsui, H.; Brueckmann, M.; et al. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N. Engl. J. Med. 2020, 383, 1413–1424. [Google Scholar] [CrossRef]
- Baker, W.L.; Smyth, L.R.; Riche, D.M.; Bourret, E.M.; Chamberlin, K.W.; White, W.B. Effects of Sodium-Glucose Co-Transporter 2 Inhibitors on Blood Pressure: A Systematic Review and Meta-Analysis. J. Am. Soc. Hypertens. 2014, 8, 262–275. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Perkins, B.A.; Fitchett, D.H.; Husain, M.; Cherney, D.Z.I. Sodium Glucose Cotransporter 2 Inhibitors in the Treatment of Diabetes Mellitus. Circulation 2016, 134, 752–772. [Google Scholar] [CrossRef]
- Verma, S.; Rawat, S.; Ho, K.L.; Wagg, C.S.; Zhang, L.; Teoh, H.; Dyck, J.E.; Uddin, G.M.; Oudit, G.Y.; Mayoux, E.; et al. Empagliflozin Increases Cardiac Energy Production in Diabetes: Novel Translational Insights Into the Heart Failure Benefits of SGLT2 Inhibitors. JACC Basic Trans. Sci. 2018, 3, 575–587. [Google Scholar] [CrossRef] [PubMed]
- Goonasekera, S.A.; Hammer, K.; Auger-Messier, M.; Bodi, I.; Chen, X.; Zhang, H.; Reiken, S.; Elrod, J.W.; Correll, R.N.; York, A.J.; et al. Decreased Cardiac L-Type Ca2+ Channel Activity Induces Hypertrophy and Heart Failure in Mice. J. Clin. Investig. 2012, 122, 280–290. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Ford, R.J.; Smith, B.K.; Gowans, G.J.; Mancini, S.J.; Pitt, R.D.; Day, E.A.; Salt, I.P.; Steinberg, G.R.; Hardie, D.G. The Na+/Glucose Cotransporter Inhibitor Canagliflozin Activates AMPK by Inhibiting Mitochondrial Function and Increasing Cellular AMP Levels. Diabetes 2016, 65, 2784–2794. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Wang, S.; Zhu, P.; Hu, S.; Chen, Y.; Ren, J. Empagliflozin Rescues Diabetic Myocardial Microvascular Injury via AMPK-Mediated Inhibition of Mitochondrial Fission. Redox Biol. 2018, 15, 335–346. [Google Scholar] [CrossRef]
- Packer, M. Activation and Inhibition of Sodium-Hydrogen Exchanger Is a Mechanism That Links the Pathophysiology and Treatment of Diabetes Mellitus with That of Heart Failure. Circulation 2017, 136, 1548–1559. [Google Scholar] [CrossRef]
- Zannad, F.; Ferreira, J.P.; Pocock, S.J.; Anker, S.D.; Butler, J.; Filippatos, G.; Brueckmann, M.; Ofstad, A.P.; Pfarr, E.; Jamal, W.; et al. SGLT2 Inhibitors in Patients with Heart Failure with Reduced Ejection Fraction: A Meta-Analysis of the EMPEROR-Reduced and DAPA-HF Trials. Lancet 2020, 396, 819–829. [Google Scholar] [CrossRef]
- Packer, M.; Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Pocock, S.J.; Carson, P.; Anand, I.; Doehner, W.; Haass, M.; et al. Effect of Empagliflozin on the Clinical Stability of Patients with Heart Failure and a Reduced Ejection Fraction: The EMPEROR-Reduced Trial. Circulation 2021, 143, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Jhund, P.S.; Docherty, K.F.; Murphy, S.A.; Verma, S.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Langkilde, A.M.; Martinez, F.A.; et al. Time to Clinical Benefit of Dapagliflozin and Significance of Prior Heart Failure Hospitalization in Patients with Heart Failure with Reduced Ejection Fraction. JAMA Cardiol. 2021, 6, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.N.; Murray, E.; Butler, J.; Cooper, L.B.; Cox, Z.L.; Fiuzat, M.; Green, J.B.; Lindenfeld, J.; McGuire, D.K.; Nassif, M.E.; et al. In-Hospital Initiation of Sodium-Glucose Cotransporter-2 Inhibitors for Heart Failure with Reduced Ejection Fraction. J. Am. Coll. Cardiol. 2021, 78, 2004–2012. [Google Scholar] [CrossRef]
- Powell, D.R.; Zambrowicz, B.; Morrow, L.; Beysen, C.; Hompesch, M.; Turner, S.; Hellerstein, M.; Banks, P.; Strumph, P.; Lapuerta, P. Sotagliflozin Decreases Postprandial Glucose and Insulin Concentrations by Delaying Intestinal Glucose Absorption. J. Clin. Endocrinol. Metab. 2019, 105, e1235–e1249. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Pitt, B.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Inzucchi, S.E.; Kosiborod, M.N.; et al. Sotagliflozin in Patients with Diabetes and Chronic Kidney Disease. N. Engl. J. Med. 2020, 384, 129–139. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2020, 384, 117–128. [Google Scholar] [CrossRef]
- Kato, E.T.; Silverman, M.G.; Mosenzon, O.; Zelniker, T.A.; Cahn, A.; Furtado, R.H.M.; Kuder, J.; Murphy, S.A.; Bhatt, D.L.; Leiter, L.A.; et al. Effect of Dapagliflozin on Heart Failure and Mortality in Type 2 Diabetes Mellitus. Circulation 2019, 139, 2528–2536. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Rocca, H.-P.B.-L.; Choi, D.-J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Nassif, M.E.; Windsor, S.L.; Borlaug, B.A.; Kitzman, D.W.; Shah, S.J.; Tang, F.; Khariton, Y.; Malik, A.O.; Khumri, T.; Umpierrez, G.; et al. The SGLT2 Inhibitor Dapagliflozin in Heart Failure with Preserved Ejection Fraction: A Multicenter Randomized Trial. Nat. Med. 2021, 27, 1954–1960. [Google Scholar] [CrossRef] [PubMed]
- Butler, J.; Filippatos, G.; Siddiqi, T.J.; Brueckmann, M.; Böhm, M.; Chopra, V.; Ferreira, J.P.; Januzzi, J.L.; Kaul, S.; Piña, I.L.; et al. Empagliflozin, Health Status, and Quality of Life in Patients with Heart Failure and Preserved Ejection Fraction: The EMPEROR-Preserved Trial. Circulation 2021, 145, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; Shah, S.J.; Lindholm, D.; et al. Dapagliflozin in Heart Failure with Preserved and Mildly Reduced Ejection Fraction: Rationale and Design of the DELIVER Trial. Eur. J. Heart Fail. 2021, 23, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Damman, K.; Beusekamp, J.C.; Boorsma, E.M.; Swart, H.P.; Smilde, T.D.J.; Elvan, A.; Eck, J.W.M.; Heerspink, H.J.L.; Voors, A.A. Randomized, Double-blind, Placebo-controlled, Multicentre Pilot Study on the Effects of Empagliflozin on Clinical Outcomes in Patients with Acute Decompensated Heart Failure (EMPA-RESPONSE-AHF). Eur. J. Heart Fail. 2020, 22, 713–722. [Google Scholar] [CrossRef] [Green Version]
- Voors, A.A.; Angermann, C.E.; Teerlink, J.R.; Collins, S.P.; Kosiborod, M.; Biegus, J.; Ferreira, J.P.; Nassif, M.E.; Psotka, M.A.; Tromp, J.; et al. The SGLT2 Inhibitor Empagliflozin in Patients Hospitalized for Acute Heart Failure: A Multinational Randomized Trial. Nat. Med. 2022, 28, 568–574. [Google Scholar] [CrossRef]
- Wanner, C.; Inzucchi, S.E.; Lachin, J.M.; Fitchett, D.; Eynatten, M.; von Mattheus, M.; Johansen, O.E.; Woerle, H.J.; Broedl, U.C.; Zinman, B.; et al. Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 323–334. [Google Scholar] [CrossRef]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- McGill, J.B.; Subramanian, S. Safety of Sodium-Glucose Co-Transporter 2 Inhibitors. Am. J. Cardiol. 2019, 124, S45–S52. [Google Scholar] [CrossRef] [Green Version]
- Fadini, G.P.; Bonora, B.M.; Avogaro, A. SGLT2 Inhibitors and Diabetic Ketoacidosis: Data from the FDA Adverse Event Reporting System. Diabetologia 2017, 60, 1385–1389. [Google Scholar] [CrossRef]
- Association, D. Addendum. 10. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2021. Diabetes Care 2020, 44, S125–S150. [Google Scholar] [CrossRef]
- Committee, W.; Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Januzzi, J.L.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.; Morris, P.B.; et al. 2020 Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2020, 76, 1117–1145. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the Diagnosis and Treatment of Acute and Chronic Heart FailureDeveloped by the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC) with the Special Contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Committee, W.; Maddox, T.M.; Januzzi, J.L.; Allen, L.A.; Breathett, K.; Butler, J.; Davis, L.L.; Fonarow, G.C.; Ibrahim, N.E.; Lindenfeld, J.; et al. 2021 Update to the 2017 ACC Expert Consensus Decision Pathway for Optimization of Heart Failure Treatment: Answers to 10 Pivotal Issues About Heart Failure with Reduced Ejection Fraction: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2021, 71, 201–230. [Google Scholar] [CrossRef]
- American Diabetes Association. 3. Foundations of Care and Comprehensive Medical Evaluation. Diabetes Care 2015, 39, S23–S35. [Google Scholar] [CrossRef] [Green Version]
- Association, A.D. 6. Obesity Management for the Treatment of Type 2 Diabetes. Diabetes Care 2015, 39, S47–S51. [Google Scholar] [CrossRef] [Green Version]
- Jarvie, J.L.; Whooley, M.A.; Regan, M.C.; Sin, N.L.; Cohen, B.E. Effect of Physical Activity Level on Biomarkers of Inflammation and Insulin Resistance Over 5 Years in Outpatients with Coronary Heart Disease (from the Heart and Soul Study). Am. J. Cardiol. 2014, 114, 1192–1197. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-Induced Reduction in Obesity and Insulin Resistance in Women: A Randomized Controlled Trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef]
- Ståhle, A.; Mattsson, E.; Rydén, L.; Unden, A.-L.; Nordlander, R. Improved Physical Fitness and Quality of Life Following Training of Elderly Patients after Acute Coronary Events. A 1 Year Follow-up Randomized Controlled Study. Eur. Heart J. 1999, 20, 1475–1484. [Google Scholar] [CrossRef] [Green Version]
- Sattelmair, J.; Pertman, J.; Ding, E.L.; Kohl, H.W., III; Haskell, W.; Lee, I.-M. Dose Response Between Physical Activity and Risk of Coronary Heart Disease. Circulation 2011, 124, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Wei, M.; Kampert, J.B.; Barlow, C.E.; Nichaman, M.Z.; Gibbons, L.W.; Paffenbarger, J.R.S.; Blair, S.N. Relationship Between Low Cardiorespiratory Fitness and Mortality in Normal-Weight, Overweight, and Obese Men. JAMA 1999, 282, 1547–1553. [Google Scholar] [CrossRef]
- Shah, R.V.; Murthy, V.L.; Colangelo, L.A.; Reis, J.; Venkatesh, B.A.; Sharma, R.; Abbasi, S.A.; Goff, D.C.; Carr, J.J.; Rana, J.S.; et al. Association of Fitness in Young Adulthood with Survival and Cardiovascular Risk: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Int. Med. 2015, 176, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Garg, S.; Khunger, M.; Darden, D.; Ayers, C.; Kumbhani, D.J.; Mayo, H.G.; de Lemos, J.A.; Berry, J.D. Dose–Response Relationship Between Physical Activity and Risk of Heart Failure. Circulation 2015, 132, 1786–1794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, D.H.; Lingvay, I.; Colhoun, H.M.; Deanfield, J.; Emerson, S.S.; Kahn, S.E.; Kushner, R.F.; Marso, S.; Plutzky, J.; Brown-Frandsen, K.; et al. Semaglutide Effects on Cardiovascular Outcomes in People with Overweight or Obesity (SELECT) Rationale and Design. Am. Heart J. 2020, 229, 61–69. [Google Scholar] [CrossRef]
- Braunwald, E. SGLT2 Inhibitors: The Statins of the 21st Century. Eur. Heart J. 2021, 43, 1029–1030. [Google Scholar] [CrossRef] [PubMed]
| Drug Class | Name of Anti-Diabetic Drug Evaluated, Study Name, Number of Patients Enrolled (N) | Patient Population | Effect on HBA1c | Effect on Weight | Effect on Blood Pressure | Renal Deterioration (Decrease in eGFR, Proteinuria and Dialysis) | Major Side Effects |
|---|---|---|---|---|---|---|---|
| GLP1-RA | Liraglutide vs. Placebo (LEADER) (16) (29) N = 9340 All diabetic Follow-up duration (Median): 3.8 years | (1) ≥50 years of age with ASCVD or HF NYHA II/III (2) ≥60 years or more with at least one cardiovascular risk factor:
| ↓ −0.40% | ↓↓ −2.3 kg | ↑ Systolic 1.2 mmHg Diastolic 0.6 mmHg | Prevent deterioration | Gastrointestinal disorders, Acute gallstone disease, ↑ Heart Rate |
| Semaglutide vs. Placebo (SUSTAIN-6) (20) (29) N = 3297 All diabetic Follow-up duration (Median): 2.1 years | (1) Age ≥ 50 years with ASCVD (2) Age ≥ 60 years of age with cardiovascular risk factors only (as above) | ↓↓ (−1.1%) to (−1.4%) | ↓↓↓ (−3.6 kg) to (−4.9 kg) | ↓ Systolic −3.4 mmHg to −5.4 mmHg /Diastolic −2.2 mm/Hg to −2.8 mmHg | Prevent deterioration | Gastrointestinal disorders, ↑ Heart Rate, Retinopathy | |
| Semaglutide (Oral) vs. Placebo (PIONEER-6) (21)(29) N = 3183 All diabetic Follow-up duration (Median): 1.4 years | ↓ ≈1.0% | ↓↓ ≈4.2 kg | ↓ Systolic −2.6 (−3.7 to −1.5) mmHg/Diastolic 0.7 (0.0 to 1.3) | Prevent deterioration | Gastrointestinal disorders, ↑ Heart Rate | ||
| Dulaglutide vs. Placebo (REWIND) (22) (29) N = 9901 All diabetic Follow-up duration (Median): 5.4 years | (1) Age ≥ 50 years with ASCVD or unstable angina or cardiac ischemia evident on imaging (2) Age ≥ 55 years with ASCVD (3) Age ≥ 60 years ASCVD + 2 of conditions: tobacco use, dyslipidaemia, hypertension, or abdominal obesity | ↓ −0.61% | ↓ −1.46 kg (1.25 to 1.67) | ↓ −1.70 mmHg (1.33 to 1.07) | Prevent deterioration | Gastrointestinal disorders, ↑ Heart Rate | |
| Albiglutide vs. Placebo (Harmony Outcomes) (24) (29) N = 9463 All diabetic Follow-up duration (Median): 1.5 years | Age ≥ 40 years with ASCVD and glycated haemoglobin concentration > 7.0% (53 mmol/mole) | ↓ −0.52% |  −0.83 kg |  Systolic −0.67 mmHg | Natural effect | Injection site reactions | |
| SGLT2i | Empagliflozin vs. Placebo (EMPA-REG) (47) N = 7020 All diabetic Follow-up duration (Median): 3.1 years | Patients with type 2 diabetes with established ASCVD | ↓ −0.54% | ↓ −2–3 kg | ↓ Systolic −(4–5) mmHg/ Diastolic −(1–2) mmHg | Prevent deterioration | Diabetic ketoacidosis, Genital infection, Urosepsis |
| Dapagliflozin vs. Placebo (DECLARE) (50) N = 17,160 All diabetic Follow-up duration (Median): 4.2 years | Age ≥ 40 years with type 2 diabetes, a glycated hemoglobin of 6.5–12.0% and eGFR > 60 mL/min with: (1) ASCVD or (2) multiple risk factors for atherosclerotic cardiovascular disease | ↓ 0.42% | ↓ 1.8 kg | ↓ Systolic −2.7 mmHg (95%/ Diastolic −0.7 mmHg | Prevent deterioration | Diabetic ketoacidosis, Genital infection, | |
| Canagliflozin vs. Placebo (CANVAS) (49) N = 10,142 All diabetic Follow-up duration (Median): 2.4 years | Age ≥ 30 years with type 2 diabetes, a glycated hemoglobin of ≥7.0% and ≤10.5% with: (1) ASCVD (2) Age > 50 years with two or more of the following: Duration of diabetes of at least 10 years, Systolic blood pressure > 140 mmHg while they were receiving one or more antihypertensive agents, Current smoking, Microalbuminuria or macroalbuminuria High-density lipoprotein cholesterol level of <1 mmol/L (38.7 mg/dL) | ↓ −0.58% | ↓ −1.60 kg | ↓ Systolic −3.93 mmHg/ Diastolic −1.39 mmHg | Prevent deterioration | Diabetic ketoacidosis, Amputation, Fractures, Infection of male genitalia, Mycotic genital infection in women | |
| Sotagliflozin vs. Placebo (SCORED) (65,66) N = 1222 All diabetic Follow-up duration (Median): 1.3 years | Glycated hemoglobin level of >7%, chronic kidney disease (eGFR, 25 to 60 mL/min/1.73 m2), with either: (1) At least one major cardiovascular risk factor (HF, LVEF ≤ 40%, LVH, CAC score > 300 or elevated hsTrop or NT-BNP) (2) Age > 55 years and at least two minor cardiovascular risk factors (BMI > 35, dyslipidemia, smoker, CAC score 100–300, hypertension despite treatment, or positive cardiac family history) | ↓ −0.60% | ↓ −1.40 K | ↓ Systolic −3.54 mmHg/ Diastolic 2.05 mmHg | Natural Effect | Diarrhea, Genital mycotic infections, Diabetic ketoacidosis |
| Drug Name | 3-Point MACE | Cardiovascular Death | Non-Fatal MI | Non-Fatal Stroke | All-Cause Mortality | Heart Failure Re-Hospitalization | |
|---|---|---|---|---|---|---|---|
| GLP1-RA | Liraglutide LEADER(16) | 0.87 (0.78–0.97) | 0.78 (0.66–0.93) | 0.88 (0.75–1.03) | 0.89 (0.72–1.11) | 0.85 (0.74–0.97) | 0.87 (0.73–1.05) |
| Semaglutide SUSTAIN-6 (20) | 0.74 (0.58–0.95) | 0.98 (0.65–1.48) | 0.74 (0.51–1.08) | 0.61 (0.38–0.99) | 1.05 (0.74–1.50) | 1.11 (0.77–1.61) | |
| Semaglutide (Oral) PIONEER-6 (21) | 0.79 (0.57–1.11) | 0.49 (0.27–0.92) | 1.18 (0.73–1.90) | 0.74 (0.35–1.57) | 0.51 (0.31–0.84) | 0.86 (0.48–1.55) | |
| Dulaglutide REWIND (22) | 0.88 (0.79–0.99) | 0.91 (0.78–1.06) | 0.96 (0.79–1.16) | 0.76 (0.61–0.95) | 0.90 (0.80–1.01) | 0.93 (0.77–1.12) | |
| Albiglutide Harmony Outcomes (24) | 0.78 (0.68–0.90) | 0.93 (0.73–1.19) | Not Reported | Not Reported | 0.95 (0.79–1.16) | Not Reported | |
| SGLT2i | Empagliflozin EMPA-REG OUTCOME (47) | 0.86 (0.74–0.99) | 0.62 (0.49–0.77) | 0.87 (0.70–1.09) | 1.24 (0.92–1.67) | 0.68 (0.57–0.82) | 0.65 (0.50–0.85) |
| Dapagliflozin DECLARE TIMI-58 (50) | 0.93 (0.84–1.03) | 0.98 (0.82–1.17) | 0.89 (0.77–1.01) | 1.01 (0.84–1.21) | 0.93 (0.82–1.04) | 0.73 (0.61–0.88) | |
| Canagliflozin CANVAS (49) | 0.86 (0.75–0.97) | 0.87 (0.72–1.06) | 0.85 (0.69–1.05) | 0.90 (0.71–1.15) | 0.87 (0.74–1.01) | 0.67 (0.52–0.87) | |
| Sotagliflozin SCORED (65) | 0.77 (0.65–0.91) | 0.90 (0.73–1.12) | Not Reported | Not Reported | 0.99 (0.83–1.18) | 0.67 (0.55–0.82) | |
| Significant (p < 0.05) | Non-significant | p value not reported |
| Drug Name | Primary End-Point | Heart Failure Hospitalization | Cardiovascular Death | All Cause Mortality | Worsening Renal Function | |
|---|---|---|---|---|---|---|
| HFrEF | ||||||
| Empagliflozin EMPEROR-reduced (62) | 0.75 (0.65 to 0.86) & | 0.69 (0.59 to 0.81) | 0.92 (0.75 to 1.12) | 0.92 (0.77 to 1.10) | 0.50 (0.32 to 0.77) | |
| Dapagliflozin DAPA-HF (63) | 0.74 (0.65 to 0.85) * | 0.70 (0.59 to 0.83) | 0.82 (0.69 to 0.98) | 0.83 (0.71 to 0.97) | 0.71 (0.44 to 1.16) | |
| Sotagliflozin $ SOLOIST-WHF (66) | 0.67 (0.52 to 0.85) # | 0.64 (0.49 to 0.83) | 0.84 (0.58 to 1.22) | 0.82 (0.59 to 1.14) | No data | |
| HFpEF | ||||||
| Empagliflozin EMPEROR-Preserved (70) | 0.79 (0.69 to 0.90) & | 0.71 (0.60 to 0.83) | 0.91 (0.76 to 1.09) | 1.00 (0.87 to 1.15) | 1.36 (1.06 to 1.66) | |
| Dapagliflozin | Ongoing, results expected mid 2022. | |||||
| Significant (p < 0.05) | p value not re-ported | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazin, I.; Chernomordik, F.; Fefer, P.; Matetzky, S.; Beigel, R. The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients. J. Clin. Med. 2022, 11, 1904. https://doi.org/10.3390/jcm11071904
Mazin I, Chernomordik F, Fefer P, Matetzky S, Beigel R. The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients. Journal of Clinical Medicine. 2022; 11(7):1904. https://doi.org/10.3390/jcm11071904
Chicago/Turabian StyleMazin, Israel, Fernando Chernomordik, Paul Fefer, Shlomi Matetzky, and Roy Beigel. 2022. "The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients" Journal of Clinical Medicine 11, no. 7: 1904. https://doi.org/10.3390/jcm11071904
APA StyleMazin, I., Chernomordik, F., Fefer, P., Matetzky, S., & Beigel, R. (2022). The Impact of Novel Anti-Diabetic Medications on CV Outcomes: A New Therapeutic Horizon for Diabetic and Non-Diabetic Cardiac Patients. Journal of Clinical Medicine, 11(7), 1904. https://doi.org/10.3390/jcm11071904






