Sensory Thresholds and Peripheral Nerve Responses in Chronic Tension-Type Headache and Neuropsychological Correlation
Abstract
1. Introduction
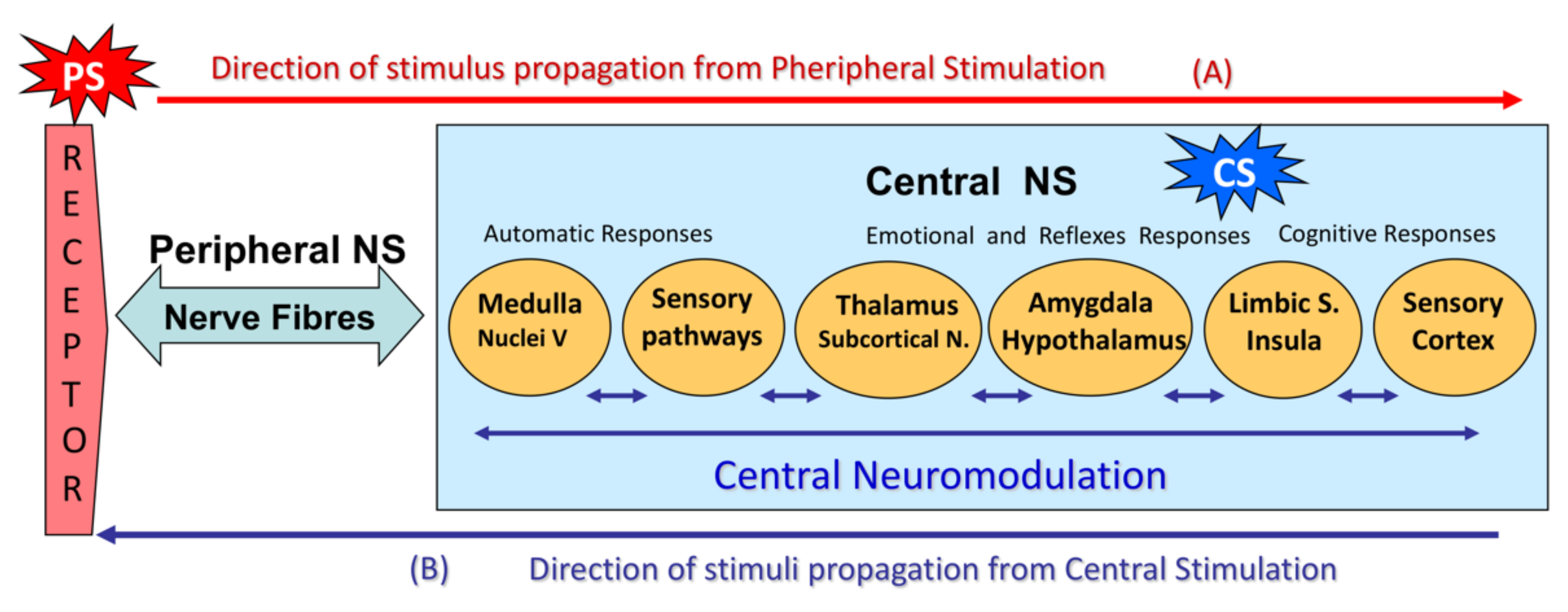
- (A)
- A peripheral stimulus (PS) activates external (skin) or internal (fascia, muscles, viscera) neuroreceptors that stimulate nerve fibers. Large-diameter, myelinated nerve fibers (Aα and Aβ) are more easily excitable than small nerve fibers (Aδ and C).The nerve fibers carry the stimulus to the CNS. Large-diameter, myelinated nerve fibers (Aβ) conduct signals more rapidly than small nerve fibers (Aδ and C).The first stimuli to reach the spinal cord come from large-diameter, myelinated nerve fibers, which then control the input from the remaining fibers in the dorsal horn of the spinal cord (“gate control”).If signals from the small fibers are particularly intense (intense stimulus) and/or selective, they will override the gate-control mechanism.Once in the spinal cord (CNS), the nerve impulses travel along the lemniscal pathways or posterior funiculi to suprasegmental structures.In the head and neck, sensory stimuli travel directly to the trigeminal sensory nucleus in the brain stem.The impulses reach subcortical structures (thalamus, subcortical nuclei) and then the amygdala and hypothalamus, triggering the emotional reaction and its vegetative response, which in turn is a sensory modulation mechanism.From the thalamus, impulses travel to cortical structures, such as the limbic cortex, insula cortex, and somatosensory association cortex, generating the sensory cognitive response that perceives the sensation. The sensation is in turn appraised and linked to a prior experience, and this may generate a conditioned responseAll these CNS structures are interrelated and constitute the central neuromodulation mechanism.
- (B)
- The circuit can be reversed: an impulse (central stimulus, CS) can be generated primarily in sensory-related CNS structures (central sensory nuclei, psycho-emotional regulation circuits, sensory cortex, etc.), which will trigger perception.Central hyperstimulation due to functional alterations in pain perception circuits would lead not only to an abnormal, exaggerated perception of pain but also to peripheral tissue hypersensitivity. This stimulates nerve fibers (peripheral hypersensitization), which in turn feed back to the CNS to generate secondary central hypersensitization.
2. Materials and Methods
2.1. Participants
2.2. Materials
2.2.1. Electrophysiology Study
2.2.2. Questionnaires
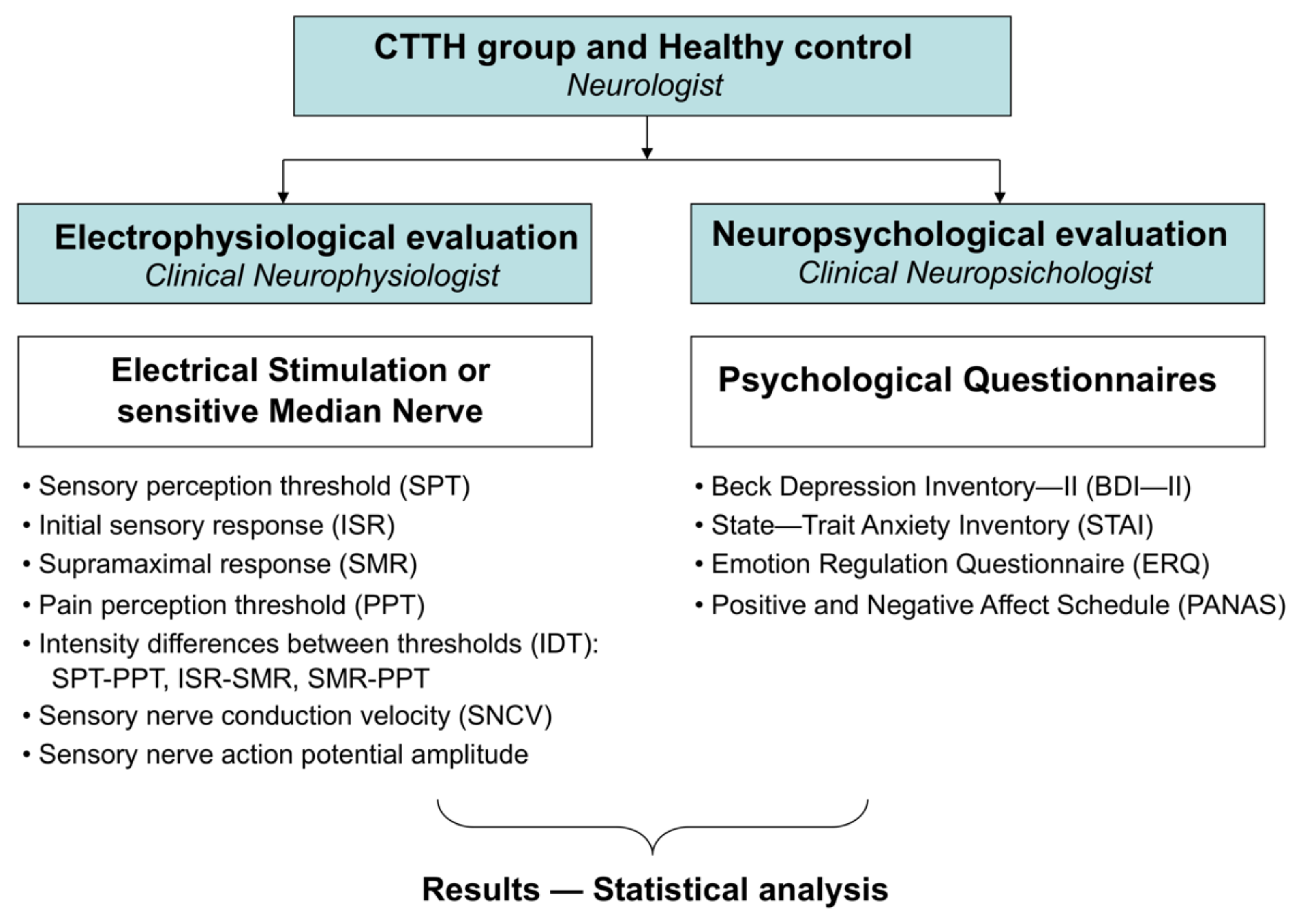
2.3. Methods
2.4. Statistical Analysis
3. Results
3.1. Differences in Subjective and Objective Electrophysiological Responses between Subjects with Chronic Tension-Type Headache and Healthy Controls
3.2. Psychological Differences between Subjects with Chronic Tension-Type Headache and Healthy Controls
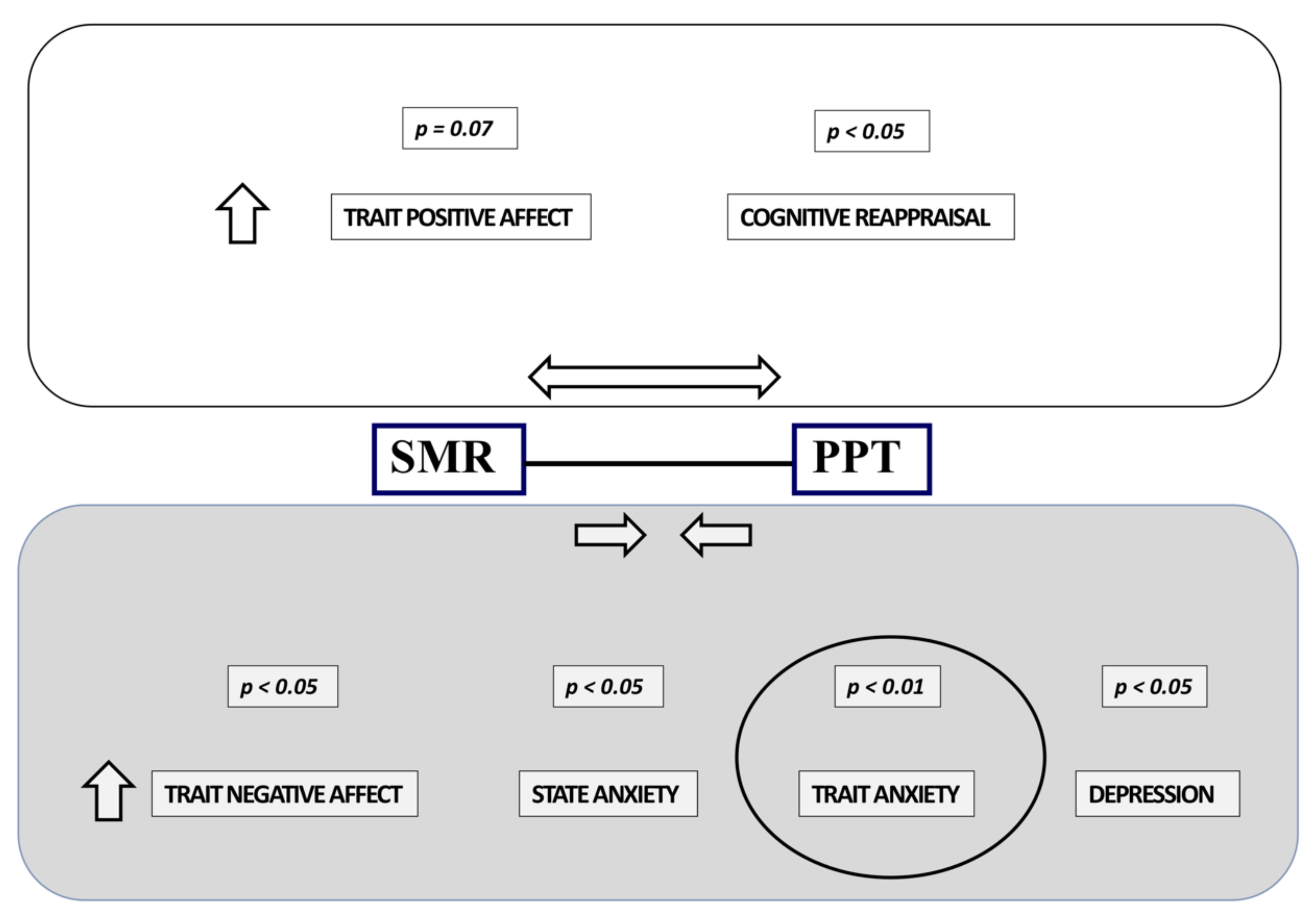
3.3. Correlations between Electrophysiological and Psychological Variables
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Bendtsen, L.; Jensen, R. Tension-type headache: The most common, but also the most neglected, headache disorder. Curr. Opin. Neurol. 2006, 19, 305–309. [Google Scholar] [CrossRef] [PubMed]
- Linde, M.; Gustavsson, A.; Stovner, L.J.; Steiner, T.J.; Barré, J.; Katsarava, Z.; Lainez, J.M.; Lampl, C.; Lantéri-Minet, M.; Rastenyte, D.; et al. The cost of headache disorders in Europe: The Eurolight project. Eur. J. Neurol. 2012, 19, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Ashina, S.; Buse, D.C.; Bjorner, J.B.; Bendtsen, L.; Lyngberg, A.C.; Jensen, R.H.; Lipton, R.B. Health-related quality of life in tension-type headache: A population-based. Scand. J. Pain 2021, 21, 778–787. [Google Scholar] [CrossRef]
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Ashina, S.; Bendtsen, L.; Ashina, M.; Magerl, W.; Jensen, R. Generalized hyperalgesia in patients with chronic tension-type headache. Cephalalgia 2006, 26, 940–948. [Google Scholar] [CrossRef]
- Fernández-de-Las-Peñas, C.; Plaza-Manzano, G.; Navarro-Santana, M.J.; Olesen, J.; Jensen, R.H.; Bendtsen, L. Evidence of localized and widespread pressure pain hypersensitivity in patients with tension-type headache: A systematic review and meta-analysis. Cephalalgia 2021, 41, 256–273. [Google Scholar] [CrossRef]
- Gatchel, R.J.; Peng, Y.B.; Peters, M.L.; Fuchs, P.N.; Turk, D.C. The biopsychosocial approach to chronic pain: Scientific advances and future directions. Psychol. Bull. 2007, 133, 581–624. [Google Scholar] [CrossRef]
- Salomons, T.V.; Nusslock, R.; Detloff, A.; Johnstone, T.; Davidson, R.J. Neural emotion regulation circuitry underlying anxiolytic effects of perceived control over pain. J. Cogn. Neurosci. 2015, 27, 222–233. [Google Scholar] [CrossRef]
- Caamaño-Barrios, L.H.; Galán-Del-Río, F.; Fernández-de-Las-Peñas, C.; Plaza- Manzano, G.; Arendt-Nielsen, L.; Ortega-Santiago, R. Widespread Pressure Pain Sensitivity over Nerve Trunk Areas in Women with Frequent Episodic Tension-Type Headache as a Sign of Central Sensitization. Pain Med. 2020, 21, 1408–1414. [Google Scholar] [CrossRef]
- Sang, C.N.; Max, M.B.; Gracely, R.H. Stability and Reliability of Detection Thresholds for Human A-Beta and A-Delta Sensory Afferents Determined by Cutaneous Electrical Stimulation. J. Pain Symptom Manag. 2003, 25, 64–73. [Google Scholar] [CrossRef]
- Chichorro, J.G.; Porreca, F.; Sessle, B. Mechanisms of craniofacial pain. Cephalalgia 2017, 37, 613–626. [Google Scholar] [CrossRef]
- Burke, D.; Kiernan, M.C.; Bostock, H. Excitability of human axons. Clin. Neurophysiol. 2001, 112, 1575–1585. [Google Scholar] [CrossRef]
- Wilhelmsen, I. Biological sensitisation and psychological amplification: Gateways to subjective health complaints and somatoform disorders. Psychoneuroendocrinology 2005, 30, 990–995. [Google Scholar] [CrossRef]
- Pasluosta, C.; Kiele, P.; Stieglitz, T. Paradigms for restoration of somatosensory feedback via stimulation of the peripheral nervous system. Clin. Neurophysiol. 2018, 129, 851–862. [Google Scholar] [CrossRef]
- Valls-Solé, J. Electromiografía y Electrodiagnóstico Neurológico: Fundamentos, Consideraciones Anatómicas y Fisiológicas. In Manual de Electromiografía Clínica, 3rd ed.; Gutiérrez-Rivas, E., Jiménez, M.D., Pardo, J., Romero-Acebal, M., Eds.; Ergon: Madrid, Spain, 2021; pp. 9–18. [Google Scholar]
- Bostock, H.; Cikurel, K.; Burke, D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve 1998, 21, 137–158. [Google Scholar] [CrossRef]
- Woolf, C.J. What is this thing called pain? J. Clin. Investig. 2010, 120, 3742–3744. [Google Scholar] [CrossRef]
- Ossipov, M.H.; Dussor, G.O.; Porreca, F. Central modulation of pain. J. Clin. Investig. 2010, 120, 3779–3787. [Google Scholar] [CrossRef]
- Bechara, A.; Damasio, A.R. The somatic marker hypothesis: A neural theory of economic decision. Games Econ. Behav. 2005, 52, 336–372. [Google Scholar] [CrossRef]
- Fernández-de-las-Peñas, C.; Schoenen, J. Chronic tension-type headache: What is new? Curr. Opin. Neurol. 2009, 22, 254–261. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Uncovering the relation between pain and plasticity. Anesthesiology 2007, 106, 864–867. [Google Scholar] [CrossRef]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef]
- Damasio, A.R. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos. Trans. R. Soc. Lond. B Biol. Sci. 1996, 351, 1413–1420. [Google Scholar] [CrossRef]
- Raja, S.N.; Carr, D.B.; Cohen, M.; Finnerup, N.B.; Flor, H.; Gibson, S.; Keefe, F.J.; Mogil, J.S.; Ringkamp, M.; Sluka, K.A.; et al. The revised International Association for the Study of Pain definition of pain: Concepts, challenges, and compromises. Pain 2020, 161, 1976–1982. [Google Scholar] [CrossRef]
- Tursky, B.; Watson, P.D. Controlled physical and subjective intensities of electric shock. Psychophysiology 1964, 1, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Sanz, J.; Perdigón, A.L.; Vázquez, C. The spanish adaptation of Beck’s Depression Inventory-II (BDI-II): 2. Psychometric properties in the general population. Clín. Y Salud 2003, 14, 249–280. [Google Scholar]
- Spielberger, C.; Gorsuch, R.; Lushene, R. STAI Manual for the State-Trait Anxiety Inventory; Consulting Psychologist Press: Palo Alto, CA, USA, 1970. [Google Scholar]
- Spielberger, C.; Gorsuch, R.; Lushene, R. Cuestionario de Ansiedad Estado-Rasgo Manual, 4th ed.; TEA Ediciones SA: Madrid, Spain, 1993. [Google Scholar]
- Gross, J.J.; John, O.P. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J. Pers. Soc. Psychol. 2003, 85, 348–362. [Google Scholar] [CrossRef]
- Cabello, R.; Salguero, J.M.; Fernández-Berrocal, P.; Gross, J.J. A Spanish adaptation of the Emotion Regulation Questionnaire. Eur. J. Psychol. Assess. 2013, 29, 234–240. [Google Scholar] [CrossRef]
- Watson, D.; Clark, L.A.; Tellegen, A. Development and validation of brief measures of positive and negative affect: The PANAS scales. J. Pers. Soc. Psychol. 1988, 54, 1063–1070. [Google Scholar] [CrossRef]
- Serafini, K.; Malin-Mayor, B.; Nich, C.; Hunkele, K.; Carroll, K.M. Psychometric properties of the Positive and Negative Affect Schedule (PANAS) in a heterogeneous sample of substance users. Am. J. Drug Alcohol Abus. 2016, 42, 203–212. [Google Scholar] [CrossRef]
- Basser, P.J.; Roth, B.J. New currents in electrical stimulation of excitable tissues. Annu. Rev. Biomed. Eng. 2000, 2, 377–397. [Google Scholar] [CrossRef]
- Rattay, F. The basic mechanism for the electrical stimulation of the nervous system. Neuroscience 1999, 89, 335–346. [Google Scholar] [CrossRef]
- Rubinstein, J.T. Analytical theory for extracellular electrical stimulation of nerve with focal electrodes. II. Passive myelinated axon. Biophys. J. 1991, 60, 538–555. [Google Scholar] [CrossRef][Green Version]
- Gracely, R.H. Pain measurement. Acta Anaesthesiol. Scand. 1999, 43, 897–908. [Google Scholar] [CrossRef]
- Sekuler, R.; Nash, D.; Armstrong, R. Sensitive, objective procedure for evaluating response to light touch. Neurology 1973, 23, 1282–1291. [Google Scholar] [CrossRef]
- Schepelmann, K.; Dannhausen, M.; Kotter, I.; Schabet, M.; Dichgans, J. Exteroceptive suppression of temporalis muscle activity in patients with fibromyalgia, tension-type headache, and normal controls. Electroencephalogr. Clin. Neurophysiol. 1998, 107, 196–199. [Google Scholar] [CrossRef]
- Tataroglu, C.; Kanik, A.; Sahin, G.; Ozge, A.; Yalcinkaya, D.; Idiman, F. Exteroceptive suppression patterns of masseter and temporalis muscles in central and peripheral headache disorders. Cephalalgia 2002, 22, 444–452. [Google Scholar] [CrossRef]
- Le Bars, D.; Dickenson, A.H.; Besson, J.M. Diffuse noxious inhibitory controls (DNIC). I. Effects on dorsal horn convergent neurons in the rat. Pain 1979, 6, 283–304. [Google Scholar] [CrossRef]
- Le Bars, D.; Dickenson, A.H.; Besson, J.M. Diffuse noxious inhibitory controls (DNIC): II. Lack of effects on non-convergent neurons, supraspinal involvement and theoretical implications. Pain 1979, 6, 305–327. [Google Scholar] [CrossRef]
- Pielsticker, A.; Haag, G.; Zaudig, M.; Lautenbacher, S. Impairment of pain inhibition in chronic tension-type headache. Pain 2005, 118, 215–223. [Google Scholar] [CrossRef]
- Romero-Godoy, R.; Romero-Godoy, J.; Gitiérrez-Gutiérrez, G.; Romero-Acebal, M. Fibras nerviosas finas y sistema nervioso autónomo. In Manual de Electromiografía Clínica, 3rd ed.; Gutiérrez-Rivas, E., Jiménez, M.D., Pardo, J., Romero-Acebal, M., Eds.; Ergon: Madrid, Spain, 2021; pp. 359–369. [Google Scholar]
- Flor, H.; Diers, M.; Birbaumer, N. Peripheral and electrocortical responses to painful and non-painful stimulation in chronic pain patients, tension headache patients and healthy controls. Neurosci. Lett. 2004, 361, 147–150. [Google Scholar] [CrossRef]
- Lautenbacher, S.; Rollman, G.B. Possible deficiences of pain modulation in fibromyalgia. Clin. J. Pain 1997, 13, 189–196. [Google Scholar] [CrossRef]
- Kosek, E.; Hansson, P. Modulatory influence on somatosensory perception from vibration and heterotopic noxious conditioning stimulation (HNCS) in fibromyalgia patients and healthy subjects. Pain 1997, 70, 41–51. [Google Scholar] [CrossRef]
- Leffler, A.S.; Hansson, P.; Kosek, E. Somatosensory perception in a remote pain-free area and function of diffuse noxious inhibitory controls (DNIC) in patients suffering from long-term trapezius myalgia. Eur. J. Pain 2002, 6, 149–159. [Google Scholar] [CrossRef]
- Jensen, R. Pathophysiological mechanisms of tension-type headache: A review of epidemiological and experimental studies. Cephalalgia 1999, 19, 602–621. [Google Scholar] [CrossRef]
- Schoenen, J.; Bottin, D.; Hardy, F.; Gerard, P. Cephalic and extracephalic pressure pain thresholds in chronic tension-type headache. Pain 1991, 47, 145–149. [Google Scholar] [CrossRef]
- Langemark, M.; Jensen, K.; Jensen, T.S.; Olesen, J. Pressure pain thresholds and thermal nociceptive thresholds in chronic tension-type headache. Pain 1989, 38, 203–210. [Google Scholar] [CrossRef]
- Bendtsen, L.; Jensen, R.; Olesen, J. Decreased pain detection and tolerance thresholds in chronic tension-type headache. Arch. Neurol. 1996, 53, 373–376. [Google Scholar] [CrossRef]
- Hole, K.; Berge, O.G. Regulation of pain sensitivity in the central nervous system. Cephalalgia 1981, 1, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Pielsticker, A.; Lautenbacher, S. Disturbances in pain perception in primary headaches (migraine, tension-type headache and cluster headache). In The Pathophysiology of Pain Perception; Lautenbacher, S., Fillingim, R.B., Eds.; Plenum: New York, NY, USA, 2004; pp. 43–57. [Google Scholar]
- Milanov, I.; Bogdanova, D. Trigemino-cervical reflex in patients with headache. Cephalalgia 2003, 23, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Cuadrado, M.L.; Arendt-Nielsen, L.; Simons, D.G.; Pareja, J.A. Myofascial trigger points and sensitization: An updated pain model for tension-type headache. Cephalalgia 2007, 27, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-Las-Peñas, C.; Ge, H.Y.; Arendt-Nielsen, L.; Cuadrado, M.L.; Pareja, J.A. The local and referred pain from myofascial trigger points in the temporalis muscle contributes to pain profile in chronic tension-type headache. Clin. J. Pain 2007, 23, 786–792. [Google Scholar] [CrossRef]
- Do, T.P.; Heldarskard, G.F.; Kolding, L.T.; Hvedstrup, J.; Schytz, H.W. Myofascial trigger points in migraine and tension-type headache. J. Headache Pain 2018, 19, 84. [Google Scholar] [CrossRef]
- Torelli, P.; Abrignani, G.; Castellini, P.; Lambru, G.; Manzoni, G.C. Human psyche and headache: Tension-type headache. Neurol. Sci. 2008, 29, S93–S95. [Google Scholar] [CrossRef]
- Aaseth, K.; Grande, R.B.; Leiknes, K.A.; Benth, J.Š.; Lundqvist, C.; Russell, M.B. Personality traits and psychological distress in persons with chronic tension-type headache. The Akershus study of chronic headache. Acta Neurol. Scand. 2011, 124, 375–382. [Google Scholar] [CrossRef]
- Haratian, A.; Amjadi, M.M.; Ghandehari, K.; Hatamian, H.; Kiani, S.; Habibi, M; : Aghababaei, Z; Ataei, M. Emotion Regulation Difficulties and Repetitive Negative Thinking in Patients With Tension Headaches and Migraine. Casp. J. Neurol. Sci. 2020, 6, 147–155. [Google Scholar] [CrossRef]
- Zebenholzer, K.; Lechner, A.; Broessner, G.; Lampl, C.; Luthringshausen, G.; Wuschitz, A.; Obmann, S.M.; Berek, K.; Wöber, C. Impact of depression and anxiety on burden and management of episodic and chronic headaches—A cross-sectional multicentre study in eight Austrian headache centres. J. Headache Pain 2016, 17, 15. [Google Scholar] [CrossRef]
- Everaert, J.; Joormann, J. Emotion regulation difficulties related to depression and anxiety: A network approach to model relations among symptoms, positive reappraisal, and repetitive negative thinking. Clin. Psychol. Sci. 2019, 7, 1304–1318. [Google Scholar] [CrossRef]
- Cooney, R.E.; Joormann, J.; Eugène, F.; Dennis, E.L.; Gotlib, I.H. Neural correlates of rumination in depression. Cogn. Affect. Behav. Neurosci. 2010, 10, 470–478. [Google Scholar] [CrossRef]
- Chuen Yee Lo, B.; Lau, S.; Cheung, S.H.; Allen, N.B. The impact of rumination on internal attention switching. Cogn. Emot. 2012, 26, 209–223. [Google Scholar] [CrossRef]

| Healthy Controls (in mA) | CTTH (in mA) | p | |||
|---|---|---|---|---|---|
| MD ± SD | Median | MD ± SD | Median | ||
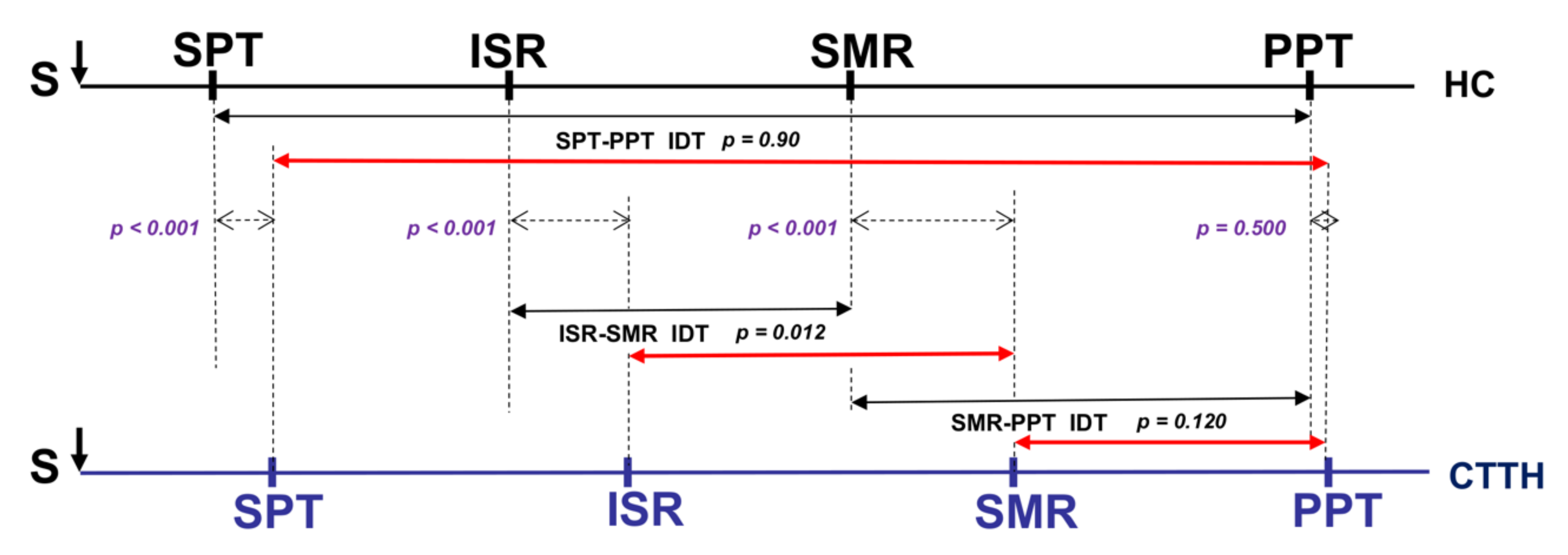
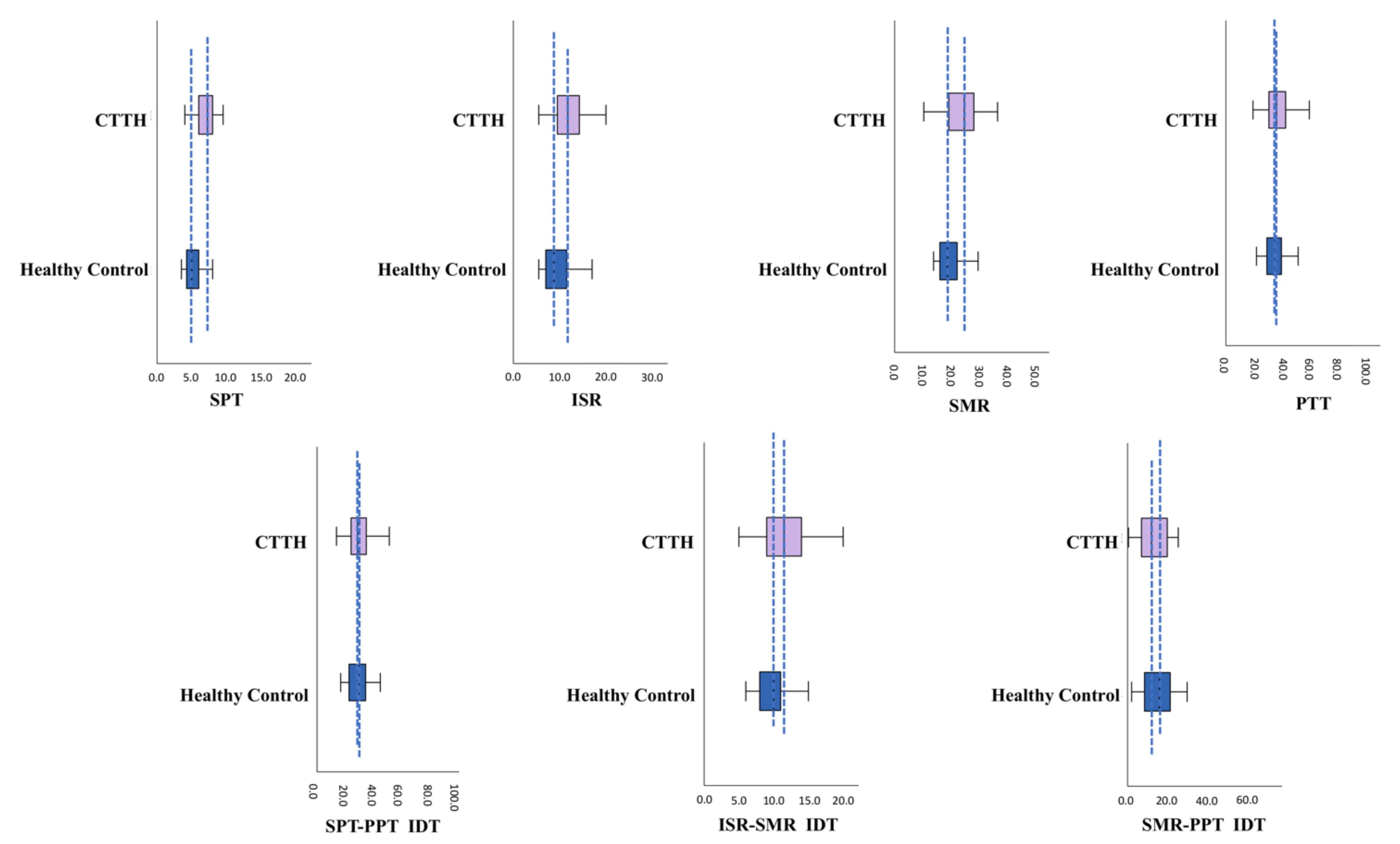
| SPT ** | 5.38 ± 1.34 | 5.00 | 7.49 ± 2.45 | 7.25 | <0.001 b |
| ISR ** | 9.70 ± 3.33 | 8.75 | 13.19 ± 5.15 | 11.75 | <0.001 a |
| SMR ** | 19.66 ± 4.08 | 19.00 | 24.93 ± 3.33 | 25.00 | <0.001 b |
| PPT | 36.65 ± 10.92 | 35.50 | 39.24 ± 14.58 | 36.50 | 0.372 a |
| SPT-PPT IDT | 31.28 ± 10.94 | 30.00 | 31.75 ± 13.36 | 29.25 | 0.090 a |
| ISR-SMR IDT ** | 9.96 ± 2.11 | 10.00 | 11.74 ± 3.59 | 11.50 | <0.01 b |
| SMR-PPT IDT | 16.99 ± 10.93 | 16.00 | 14.31 ± 12.08 | 12.00 | 0.302 a |
| ISR | SMR | PPT | SPT-PPT IDT | ISR-SMR IDT | SMR-PPT IDT | ||
|---|---|---|---|---|---|---|---|
| SPT | HC | 0.602 ** | 0.644 ** | ||||
| CTTH | 0.575 ** | 0.557 ** | 0.558 ** | 0.426 ** | 0.324 ** | 0.332 * | |
| ISR | HC | 0.856 ** | 0.453 ** | ||||
| CTTH | 0.897 ** | 0.418 ** | 0.351 * | 0.415 ** | |||
| SMR | HC | 0.580 ** | |||||
| CTTH | 0.562 ** | 0.511 ** | 0.774 ** | ||||
| PPT | HC | 0.992 ** | 0.930 ** | ||||
| CTTH | 0.988 ** | 0.559 ** | 0.862 ** | ||||
| SPT-PPT IDT | HC | 0.952 ** | |||||
| CTTH | 0.551 ** | 0.879 ** | |||||
| Healthy Controls (n = 40) | CTTH (n = 40) | p | |
|---|---|---|---|
| State anxiety ** | 20.20 ± 11.90 | 35.80 ± 14.25 | <0.001 a |
| Trait anxiety ** | 19.33 ± 9.21 | 30.90 ± 11.61 | <0.001 a |
| Depression ** | 7.53 ± 5.42 | 16.13 ± 9.58 | <0.001 a |
| State positive affect ** | 31.58 ± 7.19 | 25.18 ± 7.23 | <0.001 a |
| Trait positive affect * | 33.00 ± 6.01 | 29.25 ± 7.97 | 0.020 a |
| State negative affect ** | 18.08 ± 5.99 | 25.40 ± 8.70 | <0.001 a |
| Trait negative affect | 18.58 ± 5.88 | 21.35 ± 6.84 | 0.055 a |
| Cognitive reappraisal * | 4.71 ± 1.38 | 4.05 ± 1.33 | 0.033 a |
| Expressive suppression | 3.26 ± 1.42 | 3.93 ± 1.64 | 0.055 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Godoy, R.; Romero-Godoy, S.R.; Romero-Acebal, M.; Gutiérrez-Bedmar, M. Sensory Thresholds and Peripheral Nerve Responses in Chronic Tension-Type Headache and Neuropsychological Correlation. J. Clin. Med. 2022, 11, 1905. https://doi.org/10.3390/jcm11071905
Romero-Godoy R, Romero-Godoy SR, Romero-Acebal M, Gutiérrez-Bedmar M. Sensory Thresholds and Peripheral Nerve Responses in Chronic Tension-Type Headache and Neuropsychological Correlation. Journal of Clinical Medicine. 2022; 11(7):1905. https://doi.org/10.3390/jcm11071905
Chicago/Turabian StyleRomero-Godoy, Rosalinda, Sara Raquel Romero-Godoy, Manuel Romero-Acebal, and Mario Gutiérrez-Bedmar. 2022. "Sensory Thresholds and Peripheral Nerve Responses in Chronic Tension-Type Headache and Neuropsychological Correlation" Journal of Clinical Medicine 11, no. 7: 1905. https://doi.org/10.3390/jcm11071905
APA StyleRomero-Godoy, R., Romero-Godoy, S. R., Romero-Acebal, M., & Gutiérrez-Bedmar, M. (2022). Sensory Thresholds and Peripheral Nerve Responses in Chronic Tension-Type Headache and Neuropsychological Correlation. Journal of Clinical Medicine, 11(7), 1905. https://doi.org/10.3390/jcm11071905






