NLRP3, NLRC4 and NLRC5 Gene Polymorphisms Associate with Susceptibility of Pulmonary Aspergillosis in Non-Neutropenic Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Selection of SNPs and Genotyping
2.3. Statistics
3. Results
3.1. Characteristics of the Patient Population
3.2. Hardy-Weinberg Equilibrium Analysis and Minor Allele Frequencies of SNPs
3.3. Association of NLRs Variants with Pulmonary Aspergillosis
3.4. Association of NLRs Variants with Different Kinds of Aspergillosis
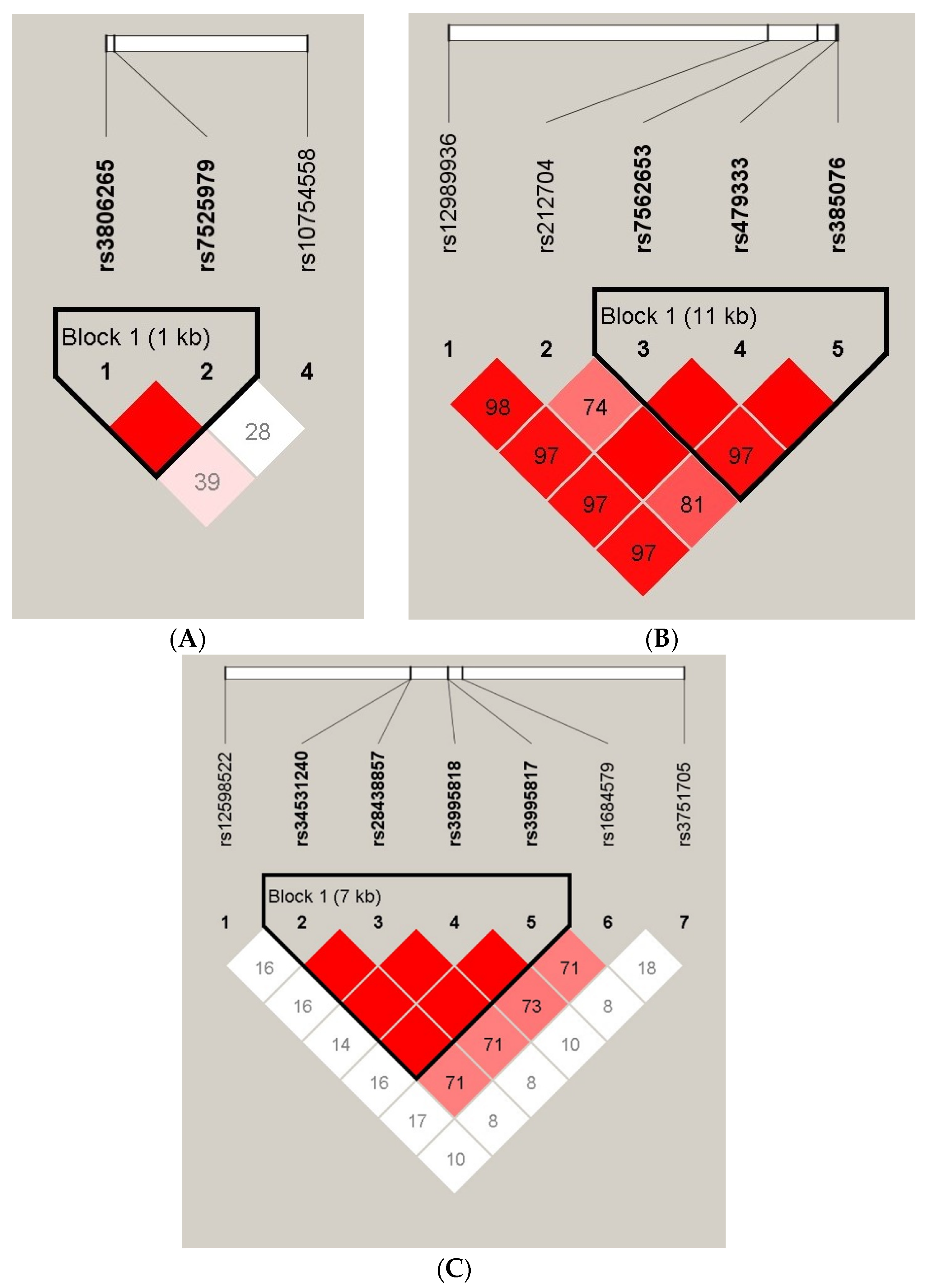
3.5. Linkage Disequilibrium and Haplotype Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Arastehfar, A.; Carvalho, A.; Houbraken, J.; Lombardi, L.; Garcia-Rubio, R.; Jenks, J.D.; Rivero-Menendez, O.; Aljohani, R.; Jacobsen, I.D.; Berman, J.; et al. Aspergillus fumigatus and aspergillosis: From basics to clinics. Stud. Mycol. 2021, 100, 100115. [Google Scholar] [CrossRef] [PubMed]
- Tekaia, F.; Latge, J.P. Aspergillus fumigatus: Saprophyte or pathogen? Curr. Opin. Microbiol. 2005, 8, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Kosmidis, C.; Denning, D.W. The clinical spectrum of pulmonary aspergillosis. Thorax 2015, 70, 270–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latge, J.P.; Chamilos, G. Aspergillus fumigatus and Aspergillosis in 2019. Clin. Microbiol. Rev. 2019, 33, e00140-18. [Google Scholar] [CrossRef] [PubMed]
- Plato, A.; Hardison, S.E.; Brown, G.D. Pattern recognition receptors in antifungal immunity. Semin. Immunopathol. 2015, 37, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, C.; Carvalho, A. Genetic defects in fungal recognition and susceptibility to invasive pulmonary aspergillosis. Med. Mycol 2019, 57 (Suppl. 2), S211–S218. [Google Scholar] [CrossRef]
- Wojtowicz, A.; Bochud, P.Y. Host genetics of invasive Aspergillus and Candida infections. Semin. Immunopathol. 2015, 37, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Bochud, P.Y.; Chien, J.W.; Marr, K.A.; Leisenring, W.M.; Upton, A.; Janer, M.; Rodrigues, S.D.; Li, S.; Hansen, J.A.; Zhao, L.P.; et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N. Engl. J. Med. 2008, 359, 1766–1777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, C.E.; Hohl, T.M.; Fan, W.; Storer, B.E.; Levine, D.M.; Zhao, L.P.; Martin, P.J.; Warren, E.H.; Boeckh, M.; Hansen, J.A. Validation of single nucleotide polymorphisms in invasive aspergillosis following hematopoietic cell transplantation. Blood 2017, 129, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Li, H.; Rui, Y.; Liu, L.; He, B.; Shi, Y.; Su, X. Pentraxin 3 Gene Polymorphisms and Pulmonary Aspergillosis in Chronic Obstructive Pulmonary Disease Patients. Clin. Infect. Dis. 2018, 66, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kale, S.D.; Ayubi, T.; Chung, D.; Tubau-Juni, N.; Leber, A.; Dang, H.X.; Karyala, S.; Hontecillas, R.; Lawrence, C.B.; Cramer, R.A.; et al. Modulation of Immune Signaling and Metabolism Highlights Host and Fungal Transcriptional Responses in Mouse Models of Invasive Pulmonary Aspergillosis. Sci. Rep. 2017, 7, 17096. [Google Scholar] [CrossRef] [PubMed]
- Mangan, M.S.J.; Olhava, E.J.; Roush, W.R.; Seidel, H.M.; Glick, G.D.; Latz, E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat. Rev. Drug Discov. 2018, 17, 588–606. [Google Scholar] [CrossRef] [PubMed]
- Swanson, K.V.; Deng, M.; Ting, J.P. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef]
- Briard, B.; Fontaine, T.; Samir, P.; Place, D.E.; Muszkieta, L.; Malireddi, R.K.S.; Karki, R.; Christgen, S.; Bomme, P.; Vogel, P.; et al. Galactosaminogalactan activates the inflammasome to provide host protection. Nature 2020, 588, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Karki, R.; Man, S.M.; Malireddi, R.K.S.; Gurung, P.; Vogel, P.; Lamkanfi, M.; Kanneganti, T.D. Concerted activation of the AIM2 and NLRP3 inflammasomes orchestrates host protection against Aspergillus infection. Cell Host Microbe 2015, 17, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Bauer, R.; Rauch, I. The NAIP/NLRC4 inflammasome in infection and pathology. Mol. Asp. Med. 2020, 76, 100863. [Google Scholar] [CrossRef]
- Meissner, T.B.; Li, A.; Biswas, A.; Lee, K.H.; Liu, Y.J.; Bayir, E.; Iliopoulos, D.; van den Elsen, P.J.; Kobayashi, K.S. NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. USA 2010, 107, 13794–13799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Downs, I.; Vijayan, S.; Sidiq, T.; Kobayashi, K.S. CITA/NLRC5: A critical transcriptional regulator of MHC class I gene expression. Biofactors 2016, 42, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Biswas, A.; Meissner, T.B.; Kawai, T.; Kobayashi, K.S. Cutting edge: Impaired MHC class I expression in mice deficient for Nlrc5/class I transactivator. J. Immunol. 2012, 189, 516–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupfer, C.R.; Stokes, K.L.; Kuriakose, T.; Kanneganti, T.D. Deficiency of the NOD-Like Receptor NLRC5 Results in Decreased CD8(+) T Cell Function and Impaired Viral Clearance. J. Virol. 2017, 91, e00377-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, T.; Ferrero, R.L.; Girardin, S.E.; Gommerman, J.L.; Philpott, D.J. NLRC5 deficiency has a moderate impact on immunodominant CD8(+) T-cell responses during rotavirus infection of adult mice. Immunol. Cell Biol. 2019, 97, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Gresnigt, M.S.; Cunha, C.; Jaeger, M.; Goncalves, S.M.; Malireddi, R.K.S.; Ammerdorffer, A.; Lubbers, R.; Oosting, M.; Rasid, O.; Jouvion, G.; et al. Genetic deficiency of NOD2 confers resistance to invasive aspergillosis. Nat. Commun. 2018, 9, 2636. [Google Scholar] [CrossRef]
- Iannitti, R.G.; Napolioni, V.; Oikonomou, V.; De Luca, A.; Galosi, C.; Pariano, M.; Massi-Benedetti, C.; Borghi, M.; Puccetti, M.; Lucidi, V.; et al. IL-1 receptor antagonist ameliorates inflammasome-dependent inflammation in murine and human cystic fibrosis. Nat. Commun. 2016, 7, 10791. [Google Scholar] [CrossRef] [PubMed]
- Patterson, T.F.; Thompson, G.R., 3rd; Denning, D.W.; Fishman, J.A.; Hadley, S.; Herbrecht, R.; Kontoyiannis, D.P.; Marr, K.A.; Morrison, V.A.; Nguyen, M.H.; et al. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 63, e1–e60. [Google Scholar] [CrossRef] [PubMed]
- Alexander, B.D.; Lamoth, F.; Heussel, C.P.; Prokop, C.S.; Desai, S.R.; Morrissey, C.O.; Baddley, J.W. Guidance on Imaging for Invasive Pulmonary Aspergillosis and Mucormycosis: From the Imaging Working Group for the Revision and Update of the Consensus Definitions of Fungal Disease from the EORTC/MSGERC. Clin. Infect. Dis. 2021, 72 (Suppl. 2), S79–S88. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.C.; Fry, B.; Maller, J.; Daly, M.J. Haploview: Analysis and visualization of LD and haplotype maps. Bioinformatics 2005, 21, 263–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigginton, J.E.; Cutler, D.J.; Abecasis, G.R. A note on exact tests of Hardy-Weinberg equilibrium. Am. J. Hum. Genet. 2005, 76, 887–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kesh, S.; Mensah, N.Y.; Peterlongo, P.; Jaffe, D.; Hsu, K.; Van Den Brink, M.; O’Reilly, R.; Pamer, E.; Satagopan, J.; Papanicolaou, G.A. TLR1 and TLR6 polymorphisms are associated with susceptibility to invasive aspergillosis after allogeneic stem cell transplantation. Ann. N. Y. Acad. Sci. 2005, 1062, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Sainz, J.; Salas-Alvarado, I.; Lopez-Fernandez, E.; Olmedo, C.; Comino, A.; Garcia, F.; Blanco, A.; Gomez-Lopera, S.; Oyonarte, S.; Bueno, P.; et al. TNFR1 mRNA expression level and TNFR1 gene polymorphisms are predictive markers for susceptibility to develop invasive pulmonary aspergillosis. Int. J. Immunopathol. Pharmacol. 2010, 23, 423–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grube, M.; Loeffler, J.; Mezger, M.; Kruger, B.; Echtenacher, B.; Hoffmann, P.; Edinger, M.; Einsele, H.; Andreesen, R.; Holler, E. TLR5 stop codon polymorphism is associated with invasive aspergillosis after allogeneic stem cell transplantation. Med. Mycol. 2013, 51, 818–825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainz, J.; Lupianez, C.B.; Segura-Catena, J.; Vazquez, L.; Rios, R.; Oyonarte, S.; Hemminki, K.; Forsti, A.; Jurado, M. Dectin-1 and DC-SIGN polymorphisms associated with invasive pulmonary Aspergillosis infection. PLoS ONE 2012, 7, e32273. [Google Scholar] [CrossRef]
- Lupianez, C.B.; Canet, L.M.; Carvalho, A.; Alcazar-Fuoli, L.; Springer, J.; Lackner, M.; Segura-Catena, J.; Comino, A.; Olmedo, C.; Rios, R.; et al. Polymorphisms in Host Immunity-Modulating Genes and Risk of Invasive Aspergillosis: Results from the AspBIOmics Consortium. Infect. Immun. 2015, 84, 643–657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, K.W.; Kim, D.H.; Sohn, S.K.; Lee, N.Y.; Chang, H.H.; Kim, S.W.; Jeon, S.B.; Baek, J.H.; Kim, J.G.; Suh, J.S.; et al. Protective role of interleukin-10 promoter gene polymorphism in the pathogenesis of invasive pulmonary aspergillosis after allogeneic stem cell transplantation. Bone Marrow Transplant. 2005, 36, 1089–1095. [Google Scholar] [CrossRef] [PubMed]
- Lupianez, C.B.; Villaescusa, M.T.; Carvalho, A.; Springer, J.; Lackner, M.; Sanchez-Maldonado, J.M.; Canet, L.M.; Cunha, C.; Segura-Catena, J.; Alcazar-Fuoli, L.; et al. Common Genetic Polymorphisms within NFkappaB-Related Genes and the Risk of Developing Invasive Aspergillosis. Front. Microbiol. 2016, 7, 1243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarrinfar, H.; Mirhendi, H.; Fata, A.; Khodadadi, H.; Kordbacheh, P. Detection of Aspergillus flavus and A. fumigatus in Bronchoalveolar Lavage Specimens of Hematopoietic Stem Cell Transplants and Hematological Malignancies Patients by Real-Time Polymerase Chain Reaction, Nested PCR and Mycological Assays. Jundishapur J. Microbiol. 2015, 8, e13744. [Google Scholar]
- Zarrinfar, H.; Makimura, K.; Satoh, K.; Khodadadi, H.; Mirhendi, H. Incidence of pulmonary aspergillosis and correlation of conventional diagnostic methods with nested PCR and real-time PCR assay using BAL fluid in intensive care unit patients. J. Clin. Lab. Anal. 2013, 27, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Elinav, E.; Strowig, T.; Henao-Mejia, J.; Flavell, R.A. Regulation of the antimicrobial response by NLR proteins. Immunity 2011, 34, 665–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schroder, K.; Tschopp, J. The inflammasomes. Cell 2010, 140, 821–832. [Google Scholar] [CrossRef] [Green Version]
- Imani, D.; Azimi, A.; Salehi, Z.; Rezaei, N.; Emamnejad, R.; Sadr, M.; Izad, M. Association of nod-like receptor protein-3 single nucleotide gene polymorphisms and expression with the susceptibility to relapsing-remitting multiple sclerosis. Int. J. Immunogenet. 2018, 45, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Bidoki, A.Z.; Harsini, S.; Sadr, M.; Soltani, S.; Mohammadzadeh, M.; Najafi, S.; Rezaei, N. NLRP3 gene polymorphisms in Iranian patients with recurrent aphthous stomatitis. J. Oral Pathol. Med. 2016, 45, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Agah, E.; Nafissi, S.; Saleh, F.; Sarraf, P.; Tafakhori, A.; Mousavi, S.V.; Saghazadeh, A.; Sadr, M.; Sinaei, F.; Mohebbi, B.; et al. Investigating the possible association between NLRP3 gene polymorphisms and myasthenia gravis. Muscle Nerve 2021, 63, 730–736. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Hao, S.; Zheng, H.; Zhao, X.; Li, Y. Association of NLRP1 and NLRP3 Polymorphisms with Psoriasis Vulgaris Risk in the Chinese Han Population. BioMed Res. Int. 2018, 2018, 4714836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.; Sun, X.; Xia, Y.; Luo, S.; Lin, J.; Xiao, Y.; Liu, Y.; Wang, Y.; Huang, G.; Li, X.; et al. Polymorphisms of the NLRC4 Gene are Associated with the Onset Age, Positive Rate of GADA and 2-h Postprandial C-Peptide in Patients with Type 1 Diabetes. Diabetes Metab. Syndr. Obes. 2020, 13, 811–818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes Torres, A.; Leite, N.; Tureck, L.V.; de Souza, R.L.R.; Titski, A.C.K.; Milano-Gai, G.E.; Lazarotto, L.; da Silva, L.R.; Furtado-Alle, L. Association between Toll-like receptors (TLR) and NOD-like receptor (NLR) polymorphisms and lipid and glucose metabolism. Gene 2019, 685, 211–221. [Google Scholar] [CrossRef]
- Zupin, L.; Navarra, C.O.; Robino, A.; Bevilacqua, L.; Di Lenarda, R.; Gasparini, P.; Crovella, S. NLRC5 polymorphism is associated with susceptibility to chronic periodontitis. Immunobiology 2017, 222, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Catalano, C.; da Silva Filho, M.I.; Frank, C.; Jiraskova, K.; Vymetalkova, V.; Levy, M.; Liska, V.; Vycital, O.; Naccarati, A.; Vodickova, L.; et al. Investigation of single and synergic effects of NLRC5 and PD-L1 variants on the risk of colorectal cancer. PLoS ONE 2018, 13, e0192385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, C.; da Silva Filho, M.I.; Jiraskova, K.; Vymetalkova, V.; Levy, M.; Liska, V.; Vycital, O.; Naccarati, A.; Vodickova, L.; Hemminki, K.; et al. Short article: Influence of regulatory NLRC5 variants on colorectal cancer survival and 5-fluorouracil-based chemotherapy. Eur. J. Gastroenterol. Hepatol. 2018, 30, 838–842. [Google Scholar] [CrossRef]
| Characteristics | Case Group (n = 73) | Control Group (n = 103) | p Value |
|---|---|---|---|
| Age | 58.48 ± 1.63 | 55.25 ± 1.038 | n.s. |
| Gender (male/female) | 41/32 | 64/39 | n.s. |
| Ethnicity | n.s. | ||
| Han | 73 (100) | 103 (100) | |
| Serum albumin, g/L | 32.23 ± 0.7314 | ||
| Comorbidities | |||
| Hypertension | 20 (27.40) | ||
| Diabetes | 12 (16.44) | ||
| COPD | 11 (15.07) | ||
| Asthma | 5 (6.85) | ||
| Bronchiectasis | 23 (31.51) | ||
| Tuberculosis | 20 (27.40) | ||
| History of smoking | 21 (28.77) | ||
| Steroid treatment | 13 (17.81) | ||
| History of hepatitis infection | 3 (4.11) | ||
| Serum albumin < 30 g/L | 27 (36.99) | ||
| Classification | |||
| IPA | 30 (41.10) | ||
| CPA | 27 (36.99) | ||
| ABPA | 16 (21.92) |
| Gene | SNP Number | Chromosome Position | Gene Location | MAF | HWE in Control Group |
|---|---|---|---|---|---|
| NLRP3 | rs3806265 | 247586336 | intron1 | 0.472 | 0.0628 |
| rs7525979 | 247587408 | synon_exon3 | 0.188 | 0.0381 | |
| rs35829419 | 247588858 | nonsynon_exon3 | 0.0 | 1.0 | |
| rs10754558 | 247612036 | 3′-UTR_exon10 | 0.469 | 0.269 | |
| NLRC4 | rs12989936 | 32268586 | 3′-flanking | 0.347 | 1.0 |
| rs212704 | 32450348 | intron8 | 0.466 | 0.7296 | |
| rs7562653 | 32478629 | intron2 | 0.415 | 0.5904 | |
| rs479333 | 32489158 | intron1 | 0.344 | 1.0 | |
| rs385076 | 32489851 | intron1 | 0.392 | 0.9022 | |
| NLRC5 | rs12598522 | 57022352 | 5′-flanking | 0.5 | 0.8838 |
| rs34531240 | 57060340 | synon_exon5 | 0.44 | 0.0882 | |
| rs28438857 | 57060353 | nonsynon_exon5 | 0.44 | 0.0882 | |
| rs3995818 | 57068106 | nonsynon_exon12 | 0.443 | 0.0882 | |
| rs3995817 | 57068107 | synon_exon12 | 0.44 | 0.0882 | |
| rs1684579 | 57071113 | synon_exon14 | 0.423 | 0.1275 | |
| rs3751705 | 57116458 | 3′-UTR_exon48 | 0.327 | 0.5313 |
| Gene | SNP | Model | Genotype | Case | Control | OR(95% CI) | p Value |
|---|---|---|---|---|---|---|---|
| NLRP3 | rs3806265 | Codominant | CC/CT/TT | 21/35/17 | 14/61/28 | 0.0451 * | |
| Dominant | CC+CT/TT | 56/17 | 75/28 | 1.23 (0.6289 to 2.394) | 0.5593 | ||
| Recessive | CC/CT+TT | 21/52 | 14/89 | 2.567 (1.239 to 5.255) | 0.0130 * | ||
| Allele | C/T | 77/69 | 89/117 | 1.467 (0.9608 to 2.254) | 0.0774 | ||
| NLRC4 | rs212704 | Codominant | TT/CT/CC | 10/41/22 | 27/49/27 | 0.1329 | |
| Dominant | TT+CT/CC | 51/22 | 76/27 | 0.8236 (0.4285 to 1.578) | 0.5672 | ||
| Recessive | TT/CT+CC | 10/63 | 27/76 | 0.4468 (0.2071 to 0.959) | 0.0447 * | ||
| Allele | T/C | 61/85 | 103/103 | 0.7176 (0.4649 to 1.097) | 0.1278 | ||
| NLRC5 | rs12598522 | Codominant | TT/CT/CC | 23/37/13 | 20/53/30 | 0.09069 | |
| Dominant | TT+CT/CC | 60/13 | 73/30 | 1.897 (0.9209 to 3.82) | 0.0851 | ||
| Recessive | TT/CT+CC | 23/50 | 20/83 | 1.909 (0.957 to 3.897) | 0.0659 | ||
| Allele | T/C | 83/63 | 93/113 | 1.601 (1.048 to 2.47) | 0.0305 * | ||
| rs1684579 | Codominant | CC/CT/TT | 12/31/30 | 17/60/26 | 0.0661 | ||
| Dominant | CC+CT/TT | 43/30 | 77/26 | 0.484 (0.2489 to 0.9213) | 0.0261 * | ||
| Recessive | CC/CT+TT | 12/61 | 17/86 | 0.9952 (0.4295 to 2.301) | 0.9907 | ||
| Allele | C/T | 55/91 | 94/112 | 0.7201 (0.4725 to 1.111) | 0.1364 |
| Gene | SNP | Model | Non-ABPA Group | Control Group | OR(95% CI) | p Value |
|---|---|---|---|---|---|---|
| NLRP3 | rs3806265 | Codominant | 17/24/16 | 14/61/28 | 0.0298 * | |
| Dominant | 41/16 | 75/28 | 0.9567 (0.4636 to 1.977) | 0.9044 | ||
| Recessive | 17/40 | 14/89 | 2.702 (1.172 to 5.924) | 0.0129 * | ||
| Allele | 58/56 | 89/117 | 1.362 (0.8541 to 2.179) | 0.1872 | ||
| NLRC5 | rs12598522 | Codominant | 17/31/9 | 20/53/30 | 0.1077 | |
| Dominant | 48/9 | 73/30 | 2.192 (0.9867 to 5.247) | 0.0599 | ||
| Recessive | 17/40 | 20/83 | 1.764 (0.829 to 3.663) | 0.1349 | ||
| Allele | 65/49 | 93/113 | 1.612 (1.012 to 2.529) | 0.0419 * | ||
| rs1684579 | Codominant | 9/21/27 | 17/60/26 | 0.0125 * | ||
| Dominant | 30/27 | 77/26 | 0.3752 (0.1889 to 0.7292) | 0.0044 ** | ||
| Recessive | 9/48 | 17/86 | 0.9485 (0.4001 to 2.237) | 0.9065 | ||
| Allele | 39/75 | 94/112 | 0.6196 (0.381 to 1.006) | 0.0471 * |
| Gene | SNP | Model | IPA Group | Control Group | OR(95% CI) | p Value |
|---|---|---|---|---|---|---|
| NLRP3 | rs3806265 | Codominant | 13/11/6 | 14/61/28 | 0.0017 ** | |
| Dominant | 24/6 | 75/28 | 1.493 (0.5538 to 3.979) | 0.4273 | ||
| Recessive | 13/17 | 14/89 | 4.861 (2.007 to 11.9) | 0.0004 *** | ||
| Allele | 37/23 | 89/117 | 2.115 (1.196 to 3.741) | 0.0117 * | ||
| NLRC5 | rs34531240 | Codominant | 4/13/13 | 17/61/25 | 0.1251 | |
| Dominant | 17/13 | 78/25 | 0.4191 (0.1796 to 1.026) | 0.0420 * | ||
| Recessive | 4/26 | 17/86 | 0.7783 (0.2655 to 2.484) | 0.8928 | ||
| Allele | 21/39 | 95/111 | 0.6291 (0.346 to 1.122) | 0.1265 | ||
| rs28438857 | Codominant | 4/13/13 | 17/61/25 | 0.1251 | ||
| Dominant | 17/13 | 78/25 | 0.4191 (0.1796 to 1.026) | 0.0420 * | ||
| Recessive | 4/26 | 17/86 | 0.7783 (0.2655 to 2.484) | 0.8928 | ||
| Allele | 21/39 | 95/111 | 0.6291 (0.346 to 1.122) | 0.1265 | ||
| rs3995818 | Codominant | 4/13/13 | 17/61/25 | 0.1251 | ||
| Dominant | 17/13 | 78/25 | 0.4191 (0.1796 to 1.026) | 0.0420 * | ||
| Recessive | 4/26 | 17/86 | 0.7783 (0.2655 to 2.484) | 0.8928 | ||
| Allele | 21/39 | 95/111 | 0.6291 (0.346 to 1.122) | 0.1265 | ||
| rs3995817 | Codominant | 4/13/13 | 17/61/25 | 0.1251 | ||
| Dominant | 17/13 | 78/25 | 0.4191 (0.1796 to 1.026) | 0.0420 * | ||
| Recessive | 4/26 | 17/86 | 0.7783 (0.2655 to 2.484) | 0.8928 | ||
| Allele | 21/39 | 95/111 | 0.6291 (0.346 to 1.122) | 0.1265 | ||
| rs1684579 | Codominant | 5/9/16 | 17/60/26 | 0.0092 ** | ||
| Dominant | 14/16 | 77/26 | 0.2955 (0.1234 to 0.6804) | 0.0036 ** | ||
| Recessive | 5/25 | 17/86 | 1.012 (0.3787 to 2.931) | 0.9833 | ||
| Allele | 19/41 | 94/112 | 0.5522 (0.3026 to 1) | 0.0541 |
| Haplotype | rs34531240 | rs28438857 | Total | Case | Control | p Value |
|---|---|---|---|---|---|---|
| H1 | T | C | 0.526 | 0.383 | 0.568 | 0.0117 * |
| H2 | C | C | 0.289 | 0.417 | 0.252 | 0.0136 * |
| H3 | C | T | 0.184 | 0.200 | 0.180 | 0.72 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhong, J.; Liu, L.; Lu, Y.; Gu, Y.; Zhao, J.; Chen, B.; Zhou, W.; Su, X. NLRP3, NLRC4 and NLRC5 Gene Polymorphisms Associate with Susceptibility of Pulmonary Aspergillosis in Non-Neutropenic Patients. J. Clin. Med. 2022, 11, 1870. https://doi.org/10.3390/jcm11071870
Zhong J, Liu L, Lu Y, Gu Y, Zhao J, Chen B, Zhou W, Su X. NLRP3, NLRC4 and NLRC5 Gene Polymorphisms Associate with Susceptibility of Pulmonary Aspergillosis in Non-Neutropenic Patients. Journal of Clinical Medicine. 2022; 11(7):1870. https://doi.org/10.3390/jcm11071870
Chicago/Turabian StyleZhong, Jinjin, Lulu Liu, Yajie Lu, Yu Gu, Jiangnan Zhao, Bilin Chen, Wei Zhou, and Xin Su. 2022. "NLRP3, NLRC4 and NLRC5 Gene Polymorphisms Associate with Susceptibility of Pulmonary Aspergillosis in Non-Neutropenic Patients" Journal of Clinical Medicine 11, no. 7: 1870. https://doi.org/10.3390/jcm11071870
APA StyleZhong, J., Liu, L., Lu, Y., Gu, Y., Zhao, J., Chen, B., Zhou, W., & Su, X. (2022). NLRP3, NLRC4 and NLRC5 Gene Polymorphisms Associate with Susceptibility of Pulmonary Aspergillosis in Non-Neutropenic Patients. Journal of Clinical Medicine, 11(7), 1870. https://doi.org/10.3390/jcm11071870





