Comparative Clinical Value of Pharmacologic Therapies for B-Cell Chronic Lymphocytic Leukemia: An Umbrella Analysis
Abstract
1. Introduction
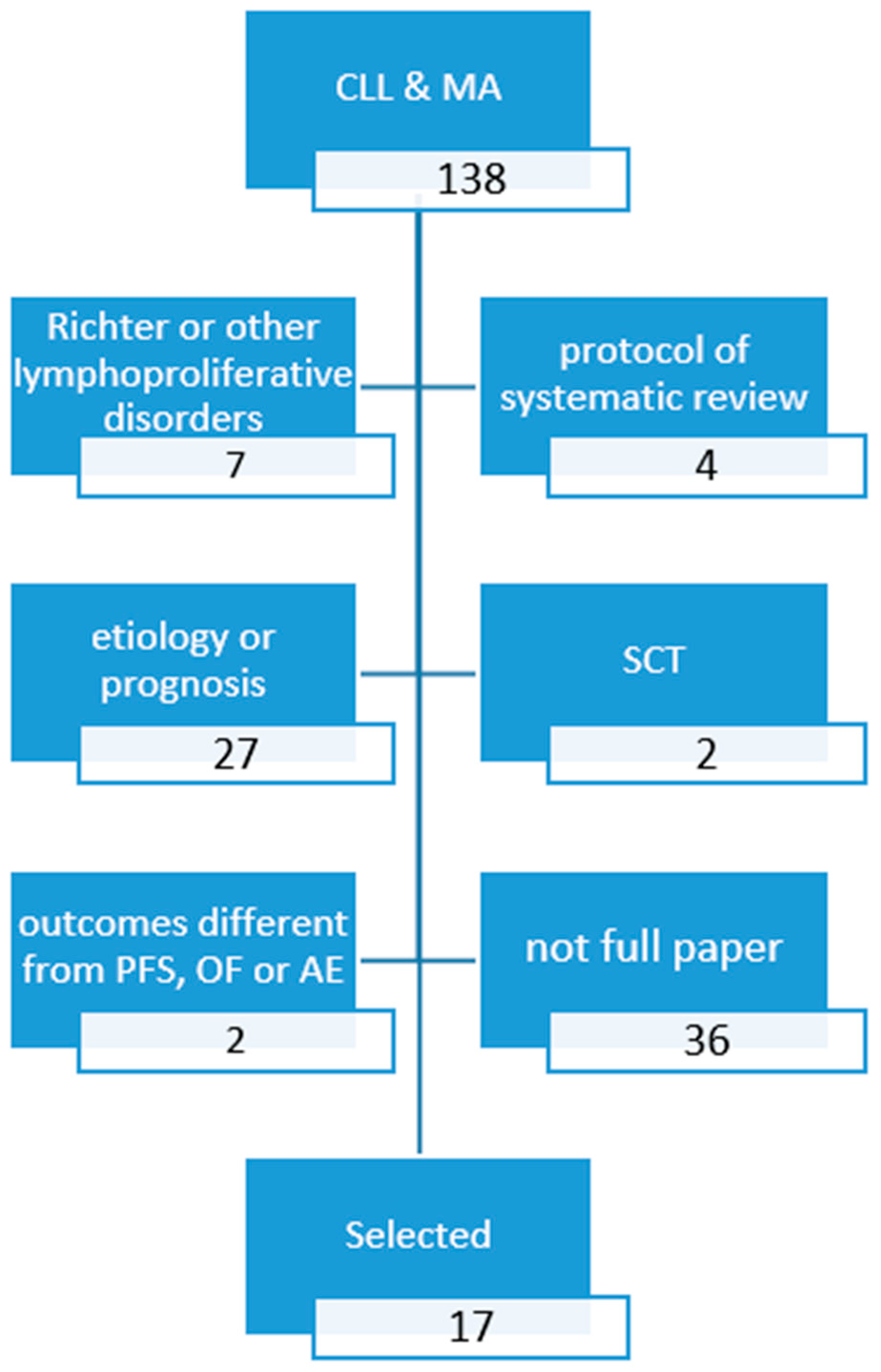
2. Methods
3. Results
3.1. Relative Survival Benefits Associated with Novel Drugs in Naïve CLL Patients
3.2. Relative Survival Benefits Associated with Novel Drugs in Refractory/Relapsed CLL Patients
3.3. Safety of Novel Drugs in CLL Patients
3.4. Partially Reported Meta-Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Weide, R.; Feiten, S.; Chakupurakal, G.; Friesenhahn, V.; Kleboth, K.; Köppler, H.; Lutschkin, J.; van Roye, C.; Thomalla, J.; Heymanns, J. Survival improvement of patients with chronic lymphocytic leukemia (CLL) in routine care 1995–2007. Leuk. Lymphoma 2020, 61, 557–566. [Google Scholar] [CrossRef]
- Lichtenberg, F.R. How many life-years have new drugs saved? A three-way fixed-effects analysis of 66 diseases in 27 countries, 2000–2013. Int. Health 2019, 11, 403–416. [Google Scholar] [CrossRef]
- NCCN Guidelines for Professionals. Chronic Lymphoytic Leukemia/Small Lymphocytic Lymphoma. Vers 2.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf (accessed on 27 January 2022).
- Singh, M.; Mealing, S.; Baculea, S.; Cote, S.; Whelan, J. Impact of novel agents on patient-relevant outcomes in patients with previously untreated chronic lymphocytic leukemia who are not eligible for fludarabine-based therapy. J. Med. Econ. 2017, 20, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Mow, E.; Keech, J.; Naipaul, R.; Beca, J.M.; Gavura, S.; Kouroukis, C.T. Impact of novel chronic lymphocytic leukemia drugs on public spending. J. Clin. Oncol. 2018, 36 (Suppl. S1), 103. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid.-Based. Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Davids, M.S.; Waweru, C.; le Nouveau, P.; Padhiar, A.; Gautamjeet, S.; Adhyankar, S.; Leblod, V. Comparative efficacy of acalabrutinib in frontline treatment of chronic lymphocytic leukemia: A systematic review and network meta-analysis. Clin. Ther. 2020, 42, 1955–1974. [Google Scholar] [CrossRef]
- Molica, S.; Giannarelli, D.; Montserrat, E. Comparison between Venetoclax-based and Bruton Tyrosine Kinase Inhibitor-based Therapy as Upfront Treatment of Chronic Lymphocytic Leukemia (CLL): A Systematic Review and Network Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 21, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Song, S.; Yu, M.; Zhu, H.; Gao, A.; Gao, W.; Ran, X.; Huo, D. Comparison of acalabrutinib plus obinutuzumab, ibrutinib plus obinutuzumab and venetoclax plus obinutuzumab for untreated CLL: A network meta-analysis. Leuk. Lymphoma 2020, 61, 3432–3439. [Google Scholar] [CrossRef]
- Städler, N.; Shang, A.; Bosch, F.; Briggs, A.; Goede, V.; Berthier, A.; Renaudin, C.; Leblond, V. A Systematic Review and Network Meta-Analysis to Evaluate the Comparative Efficacy of Interventions for Unfit Patients with Chronic Lymphocytic Leukemia. Adv. Ther. 2016, 33, 1814–1830. [Google Scholar] [CrossRef]
- Xu, Y.; Fahrbach, K.; Dorman, E.; Baculea, S.; Côté, S.; van Sanden, S.; Diels, J. Front-line treatment of patients with chronic lymphocytic leukemia: A systematic review and network meta-analysis. J. Comp. Eff. Res. 2018, 7, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Baumann, T.; Montserrat, E. Ibrutinib as initial therapy in chronic lymphocytic leukemia: A systematic review and meta-analysis. Eur. J. Haematol. 2020, 104, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Shapouri, S.; Manzoor, B.S.; Ravelo, A.; Sail, K.; Qendri, V.; van de Wetering, G.; Davids, M.S. Cost-effectiveness of a 12-month fixed-duration venetoclax treatment in combination with obinutuzumab in first-line, unfit chronic lymphocytic leukemia in the United States. J. Manag. Care Spéc. Pharm. 2021, 27, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Ho, C.-L.; Lin, C.; Wu, Y.-Y.; Huang, T.-C.; Tu, Y.-K.; Lee, C.-H. Treatment Outcomes of Novel Targeted Agents in Relapse/Refractory Chronic Lymphocytic Leukemia: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2019, 8, 737. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, P.-H.; Lin, C.; Wang, C.-Y.; Ho, C.-L. A network meta-analysis of maintenance therapy in chronic lymphocytic leukemia. PLoS ONE 2020, 15, e0226879. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Shanafelt, T.D. Comparison of venetoclax plus rituximab with B-cell receptor inhibitors in patients with relapsed/refractory chronic lymphocytic leukemia: A systematic review and network Meta-analysis. Leuk. Lymphoma 2019, 61, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Mirabelli, R.; Levato, L.; Shanafelt, T.D. The magnitude of improvement in progression-free survival with targeted therapy in relapsed/refractory chronic lymphocytic leukemia based on prognostic risk category: A systematic review and meta-analysis. Leuk. Lymphoma 2018, 60, 1644–1649. [Google Scholar] [CrossRef]
- Puła, A.; Stawiski, K.; Braun, M.; Iskierka-Jażdżewska, E.; Robak, T. Efficacy and safety of B-cell receptor signaling pathway inhibitors in relapsed/refractory chronic lymphocytic leukemia: A systematic review and meta-analysis of randomized clinical trials. Leuk. Lymphoma 2017, 59, 1084–1094. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Gu, Y.; Xia, J.; Kong, X.; Qian, Q.; Hong, Y. Safety and efficacy of Ofatumumab in chronic lymphocytic leukemia: A systematic review and meta-analysis. Hematology 2017, 22, 578–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ball, S.; Das, A.; Vutthikraivit, W.; Edwards, P.J.; Hardwicke, F.; Short, N.J.; Borthakur, G.; Maiti, A. Risk of Infection Associated with Ibrutinib in Patients with B-Cell Malignancies: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Lymphoma Myeloma Leuk. 2020, 20, 87–97.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, H.; Yang, M.; Xu, C. Adverse drug events associated with ibrutinib for the treatment of elderly patients with chronic lymphocytic leukemia. A systematic review and meta-analysis of randomized trials. Medicine 2019, 98, e16915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, A.; Zhou, H.; Zhu, J.; Niu, T. Risk of Bleeding Associated with Ibrutinib in Patients with B-Cell Malignancies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef]
- Pacheco-Paez, T.; Conte, C.; Rousseau, V.; Chebane, L.; Ysebaert, L.; Levy, V.; Montastruc, J.L.; Despas, F. Cardiovascular adverse drug reactions of ibrutinib, idelalisib, acalabrutinib, and venetoclax used in chronic lymphocytic leukemia: Systematic review-meta-analysis and Signal detection by disproportionality analysis from VigiBase®. Fund. Clin. Pharmacol. 2021, 35 (Suppl. 1), 38–39. [Google Scholar]
- Hilal, T.; Hillegass, W.B.; Gonzalez-Velez, M.; Leis, J.F.; Rosenthal, A.C. Adverse Events in Clinical Trials of Ibrutinib and Acalabrutinib for B-Cell Lymphoproliferative Disorders: A Systematic Review and Network Meta-Analysis. Blood 2020, 136, 23. [Google Scholar] [CrossRef]
- Coll Bastus, N.; Bavids, M.S.; Huntington, S.F.; Moreno, C.; Follows, G.; Cuneo, A.; Humpphrey, K.; Schary, W.; Sail, K.; Song, Y.; et al. Indirect treatment comparison analysis of venetoclax + obinutuzumab with standard front-line regimens for chronic lymphocytic leukaemia. Br. J. Haematol. 2020, 189 (Suppl. 1), 219–220. [Google Scholar]
- Khalid, Y.; Dasu, N.; Dasu, K.; Fradley, M.; Shah, A. Ventricular arrhythmias with ibrutinib use a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2021, 77, 3333. [Google Scholar] [CrossRef]
- A Haddad, P.; Ganey, N.; Gallagher, K.M. Comparative Efficacy of First-Line Chemotherapy-Free Combinations in Chronic Lymphocytic Leukemia (CLL): A Network Meta-Analysis. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Sudhapalli, P.; Piena, M.; Palaka, A.; Mato, A.; van de Wetering, G.; Manzoor, B.; Sail, K. Systematic literature review and network meta-analysis comparing therapies for treatment naive patients with chronic lymphocytic leukemia. HemaSphere 2020, 4 (Suppl. 1), 320. [Google Scholar]
- Tang, X.; Zou, W.; Peng, P.; Bai, Y. Venetoclax alone or in combination with other regimens treatment achieve deep and sustained remission of relapsed/refractory chronic lymphocytic leukemia: A meta-analysis. Clin. Exp. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Habib RAiman, W.; Garg, I.; Niaz, R.; Butt, S.K.; Saeed, M.; Zubair, H.; Kashif, H.; Farrukh, M.; Naveed MTahir, N.; Fatima, A.; et al. Efficacy and safety of chimeric antigen receptor T cell therapy in chronic lymphocytic leukemia: A systematic review. Blood 2021, 138 (Suppl. 1), 4822. [Google Scholar]
- Cuneo, A.; Follows, G.; Rigolin, G.M.; Piciocchi, A.; Tedeschi, A.; Trentin, L.; Perez, A.M.; Coscia, M.; Laurenti, L.; Musuraca, G.; et al. Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: A GIMEMA, ERIC and UK CLL FORUM study. Haematologica 2018, 103, 1209–1217. [Google Scholar] [CrossRef]
- Marchetti, M.; Carobbio, A.; Capitoni, E.; Barbui, T. Lymphoproliferative disorders in patients with chronic myeloproliferative neoplasms. Am. J. Hematol. 2018, 93, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Salvetti, C.; Griggio, V.; Porrazzo, M.; Schiattone, L.; Zamprogna, G.; Visentin, A.; Vassallo, F.; Cassin, R.; Rigolin, G.M.; et al. Preexisting and treatment-emergent autoimmune cytopenias in patients with CLL treated with targeted drugs. Blood 2021, 137, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Brugiatelli, M.; Bandini, G.; Barosi, G.; Lauria, F.; Liso, V.; Marchetti, M.; Mauro, F.R.; Meloni, G.; Zinzani, P.L.; Tura, S. Management of chronic lymphocytic leukemia: Practice guidelines from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 2006, 91, 1662–1673. [Google Scholar]
- Zinzani, P.L.; Rambaldi, A.; Gaidano, G.; Girmenia, C.; Marchetti, M.; Pane, F.; Tura, S.; Barosi, G. Infection control in patients candidate to treatment with ibrutinib or idelalisib in chronic lymphocytic leukemia: Recommendations from Italian Society of Hematology. Leuk. Res. 2019, 81, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Barosi, G.; Liberato, L.N. Fludarabine for chronic lymphocytic leukemia. N. Engl. J. Med. 2001, 344, 1166–1167. [Google Scholar]
- Marchetti, M.; Montillo, M.; Cuneo, A.; Mauro, F.R.; Martelli, E.; Pedone, M.P. Cost-effectiveness of idelalisib-rituximab for the treatment of relapsed-refractory chronic lymphocytic leukemia. Hematol. Int. J. 2017, 1, 000106. [Google Scholar] [CrossRef]
- Marchetti, M. Cost-effectiveness of kinase inhibitors for hematologic malignancies: A systematic and critical review. Expert Rev. Pharm. Outcomes Res. 2017, 17, 469–480. [Google Scholar] [CrossRef] [PubMed]
| Author, Year | NMA | Sponsored | N Studies (Patients) | Intervention | Comparator | Hazard Ratio or Risk Ratio (Confidence or Credible Intervals) |
|---|---|---|---|---|---|---|
| PFS | ||||||
| Stadler, 2016 [10] | Yes | Yes | 5 (2882) | O-Chl | Chl F Ofa-Chl R-Chl RB FCR-lite | 0.19 (0.14–0.25) 0.20 (0.13–0.49) 0.33 (0.23–0.49) 0.43 (0.34–0.54) 0.81 (0.49–1.33) 0.88 (0.51–1.52) |
| Xu, 2018 [11] | Yes | Yes | 15 (5300) | I | Chl Flud O-Chl FC FCR B R-Chl Ofa-Chl RB | 0.16 (0.08, 0.31) 0.19 (0.09, 0.38) 0.82 (0.35, 1.88) 0.38 (0.18, 0.78) 0.72 (0.32, 1.61) 0.71 (0.31, 1.65) 0.33 (0.15, 0.71) 0.28 (0.13, 0.63) 0.55 (0.24, 1.28) |
| Sheng, 2020 [9] | Yes | No | 3 (1017) | OA | OI OV | 0.43 (0.22–0.87) 0.46 (0.22–0.96) § 0.30 (0.15–0.59) 0.34 (0.17–0.68) § |
| Davids, 2020 [7] | Yes | Yes | 8 (3778) | A | I OI IR OV BR Chl O-Chl Ofa-Chl R-Chl | 0.35 (0.18–0.66) 0.61 (0.32–1.15) ^ 0.87 (0.46–1.63) 0.63 (0.32–1.27) ^ 0.37 (0.18–0.75) 0.56 (0.27–1.14) ^ 0.60 (0.33–1.11) 0.47 (0.24–0.89) ^ 0.15 (0.08–0.27) 0.19 (0.10–0.35) ^ 0.04 (0.02–0.07) 0.03 (0.02–0.06) ^ 0.20 (0.13–0.31) 0.16 (0.10–0.27) ^ 0.07 (0.04–0.12) 0.06 (0.03–0.10) ^ 0.08 (0.05–0.14) 0.07 (0.04–0.13) ^ |
| OA | I OI IR OV BR Chl O-Chl Ofa-Chl R-Chl | 0.19 (0.09–0.38) 0.46 (0.23–0.92)^ 0.46 (0.23–0.94) 0.48 (0.23–1.01) ^ 0.20 (0.09–0.44) 0.43 (0.20–0.91) ^ 0.32 (0.16–0.64) 0.36 (0.18–0.71) ^ 0.08 (0.04–0.16) 0.14 (0.07–0.28) ^ 0.02 (0.01–0.04) 0.02 (0.01–0.05) ^ 0.11 (0.06–0.18) 0.12 (0.07–0.22) ^ 0.04 (0.02–0.07) 0.04 (0.02–0.08) ^ 0.04 (0.02–0.08) 0.06 (0.03–0.10) ^ | ||||
| Molica, 2020 CLM [8] | Yes | No | 3 1 (1191) | A | OI OV | 0.87 (0.47–1.61) 0.57 (0.32v1.03) |
| OA | OI OV | 0.43(0.22–0.87) 0.29(0.15–0.56) | ||||
| OV | OI | 1.52 (0.82–1.81) | ||||
| Molica, 2020 EJH [12] | No | No | 4 (1574) | I +/− R/O | Mixed chemo (Chl, O-Chl, RB, FCR) | 0.331 (0.272–0.403) 0.159 (0.077–0.327) 11q- 0.178 (0.121–0.261) IGVH unmut 0.270 (0.149–0.489) IGVH mut |
| Chatterjee, 2021 [13] | Yes | Yes | 6 | A OA BR OI I IR | OV | 0.6 (0.3–1.0) 0.4 (0.2–0.8) 6.9 (3.3–13.2) 0.9 (0.5–1.6) 2.5 (1.4–4.3) 2.8 (1.2–5.4) |
| OS | ||||||
| Stadler, 2016 [10] | Yes | Yes | 5 (2882) | O-Chl | F Chl Ofa-Chl R-Chl RB | 0.35 (0.07–1.86) 0.48 (0.30–0.78) 0.53 (0.28–1.04) 0.81 (0.52–1.26) 0.81 (0.37–1.76) |
| Xu, 2018 [11] | Yes | Yes | 15 (5300) | I | Chl Flud O-Chl FC FCR B R-Chl Ofa-Chl RB | 0.16(0.04, 0.56) 0.15(0.04, 0.53) 0.41 (0.09, 1.70) 0.14(0.04, 0.52) 0.20(0.05, 0.79) 0.21(0.05, 0.80) 0.27 (0.06, 1.05) 0.18(0.04, 0.71) 0.30 (0.06, 1.29) |
| Sheng, 2020 [9] | Yes | No | 3 (1017) | OA | OI OV | 0.51 (0.18–1.44) 0.38 (0.13–1.08) |
| Davids, 2020 [7] | Yes | Yes | 8 (3778) | A | I OI IR OV BR Chl O-Chl Ofa-Chl R-Chl | 0.44 (0.16–1.27) 0.66 (0.25–1.75) ^ 0.65 (0.24–1.75) 0.45 (0.15–1.40) 0.64 (0.22–1.87) ^ 0.48 (0.18–1.30) 0.45 (0.16–1.27) 0.61 (0.23–1.60) ^ 0.23 (0.09–0.59) 0.27 (0.11–0.70) ^ 0.60 (0.28–1.26) 0.59 (0.28–1.26) ^ 0.25 (0.09–0.71) 0.30 (0.11v0.85) ^ 0.38 (0.15–0.94) 0.44 (0.18–1.07) ^ |
| AO | I OI IR OV BR Chl O-Chl Ofa-Chl R-Chl | 0.35 (0.12–1.04) 0.53 (0.19–1.45) ^ 0.51 (0.18–1.45) 0.36 (0.11–1.15) 0.51 (0.17–1.54) ^ 0.38 (0.13–1.08) 0.36 (0.12–1.05) 0.48 (0.17–1.34) ^ 0.18 (0.07–0.48) 0.22 (0.08–0.58) ^ 0.47 (0.21–1.06) 0.20 (0.07–0.59) 0.24 (0.08–0.71) ^ 0.30 (0.12–0.78) 0.35 (0.14–0.88) ^ | ||||
| Molica, 2020 EJH [12] | No | No | 3 (1027) | I +/− R I +/− O | Mixed chemo +/− R/O | 0.289 (0.07–1.175) |
| Chatterjee, 2021 [13] | Yes | Yes | 6 | A OA BR OI I IR | OV | 0.6 (0.3–1.2) 0.5 (0.1–1.1) 1.2 (0.5–2.4) 1.0 (0.4–2.1) 1.2 (0.5–2.3) 1.2 (0.4–2.6) |
| Author, Year | NMA | Sponsored | N Studies (Patients) | Intervention | Comparator | Hazard Ratio or Risk Ratio (Confidence or Credible Intervals) |
|---|---|---|---|---|---|---|
| PFS | ||||||
| Wu, 2017 [19] | No | No | 13 (2314) | Ofa-based | Non-Ofa-based | 0.88 (0.47–1.63) |
| Pula, 2018 [18] | No | No | 5 (1866) | BTK inhibitors | Non-BTK inhibitors | 0.24(0.19–0.30) |
| Chen, 2019 [14] | Yes | No | 7 (2514) | RV I | Ofa | 0.10(0.05–0.21) 0.10(0.07–0.17) |
| Lee, 2020 [15] | No | Yes | 6 (1615) | Lenalidomide (maint) R (maint) Ofa (maint) | No maintenance | 0.37(0.27–0.50) 0.50(0.38–0.66) 0.52(0.41–0.66) |
| Molica, 2019 [17] | No | No | 7 (2409) | I or A or V +/− R/O | No BTK inhibitor nor venetoclax | 0.187 (0.126–0.279) non 17p- 0.240 (0.185–0.311) TP53wt 0.239 (0.166–0.344) IGVH mutated 0.208 (0.168–0.59) non 11q- 0.206 (0.108–0.392) 17p- 0.231 (0.137–0.390) TP53 mutated 0.172 (0.109–0.272) IGVH unmutated 0.081 (0.054–0.121) 11q- |
| OS | ||||||
| Wu, 2017 [19] | No | No | 13 (2314) | Ofa-based | Non-Ofa-based | 0.97 (0.70–1.36) |
| Pula, 2018 [18] | No | No | 5 (1866) | BCR-inhibitors | Non BCR-inhibitors | 0.58(0.46–0.73) |
| Chen, 2019 [14] | Yes | No | 7 (2514) | RV I | Ofa | 0.33(0.11–0.99) 0.36(0.21–0.63) |
| Molica, 2020 LL [16] | Yes | No | 3 (1383) | RV RB + I RB + idelalisib | RB | 0.17(0.11–0.25) 0.20(0.15–0.28) 0.33(0.25–0.44) |
| Lee, 2020 [15] | No | Yes | 6 (1615) | Lenalidomide, R, or Ofa maintenance | No maintenance | 0.89 (0.70–1.14) |
| Author, Year | NMA | Sponsored | N Studies (Patients) | Population | Intervention | Comparator | Outcome | Hazard Ratio or Risk Ratio (Confidence or Credible Intervals) |
|---|---|---|---|---|---|---|---|---|
| Wu, 2017 [19] | No | No | 13 (2314) | R/R | Ofa-based | Non-Ofa-based | AE | Infections more frequent Thrombocytopenia & anemia less frequent |
| Pula, 2018 [18] | No | No | 5 (1866) | R/R | BTK inhibitors | Non BTK inhibitors | AE HG AE disc AE death | 1.25 (1.08–1.44) 1.26 (0.88–1.81) 1.06 (0.72–1.57) |
| Xu, 2018 [11] | Yes | Yes | 15 (5300) | Naïve | I | Chl O-Chl R-Chl Ofa-Chl B | AE disc | 0.32 (0.08–1.18) 0.31 (0.05–2.00) 0.66 (0.1–4.31) 0.31 (0.05–1.90) 0.08 (0.1–0.6) |
| Naïve, FI | I | Chl O-Chl R-Chl Ofa-Chl B | AE disc | 0.23(0.15–0.63) 0.31(0.11–0.80) 0.65 (0.23–1.81) 0.31(0.12–0.77) 0.08(0.02–0.7) | ||||
| Zhou 2019 [21] | No | No | 5 (2456) | Naïve, R/R | I | Mixed | Anemia Thrombocytopenia Neutropenia Febrile neutropenia Respiratory tract infections Abdominal AE Arthralgia | 0.90 (0.67–1.21) 0.61 (0.32–1.14) 0.50 (0.25–1.00) 0.89 (0.32–2.49) 1.01 (0.78–1.30) 2.14 (1.44–3.17) 1.86 (1.10–3.15) |
| Caldeira, 2019 [23] | No | No | 8 (2580) | CLL & | I-based therapy | Mixed | Arterial hypertension Atrial fibrillation | 2.82(1.52–5.23) 4.69(2.17–7.64) |
| Wang 2020 [22] | 11 (4288) | CLL & | I | Mixed | Bleeding Major bleed | 3.08(2.07–4.58) 2.46(1.37–4.41) | ||
| Ball, 2020 [20] | No | No | 5 (1739) | Naïve, R/R | I | Mixed | Infections HG | 1.24(1.02–1.50) |
| Lee, 2020 [15] | No | Yes | 6 (1615) | R/R | Lenalidomide (maint) R (maint) Ofa (maint) | No maintenance | AE | 1.84 (0.98–3.43) 1.11 (0.69–1.79) 2.11 (0.92–4.81) |
| Sheng, 2020 [9] | Yes | No | 3 (1017) | Naïve | OA | OI | AE disc Grade 3–4 AE Any AE | 0.64 (0.11–1.86) 1.10 (0.52–2.32) 0.48 (0.01–48.20) |
| OA | OV | AE disc Grade 3–4 AE Any AE | 0.68 (0.26–1.81) 5.28 (0.03–831.44) 0.89 (0.45–1.77) | |||||
| Molica, 2020 CLM [8] | Yes | No | 3 (1027) | Naïve | VO A A | IO IO VO | Grade 3–4 AE | 1.05 (0.64–1.73) 0.73 (0.43–1.24) 0.69 (0.44–1.09) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, M.; Rivela, P.; Bertassello, C.; Canicattì, M. Comparative Clinical Value of Pharmacologic Therapies for B-Cell Chronic Lymphocytic Leukemia: An Umbrella Analysis. J. Clin. Med. 2022, 11, 1868. https://doi.org/10.3390/jcm11071868
Marchetti M, Rivela P, Bertassello C, Canicattì M. Comparative Clinical Value of Pharmacologic Therapies for B-Cell Chronic Lymphocytic Leukemia: An Umbrella Analysis. Journal of Clinical Medicine. 2022; 11(7):1868. https://doi.org/10.3390/jcm11071868
Chicago/Turabian StyleMarchetti, Monia, Paolo Rivela, Claudia Bertassello, and Manuela Canicattì. 2022. "Comparative Clinical Value of Pharmacologic Therapies for B-Cell Chronic Lymphocytic Leukemia: An Umbrella Analysis" Journal of Clinical Medicine 11, no. 7: 1868. https://doi.org/10.3390/jcm11071868
APA StyleMarchetti, M., Rivela, P., Bertassello, C., & Canicattì, M. (2022). Comparative Clinical Value of Pharmacologic Therapies for B-Cell Chronic Lymphocytic Leukemia: An Umbrella Analysis. Journal of Clinical Medicine, 11(7), 1868. https://doi.org/10.3390/jcm11071868







