Old and New Drugs for Chronic Lymphocytic Leukemia: Lights and Shadows of Real-World Evidence
Abstract
:1. Introduction
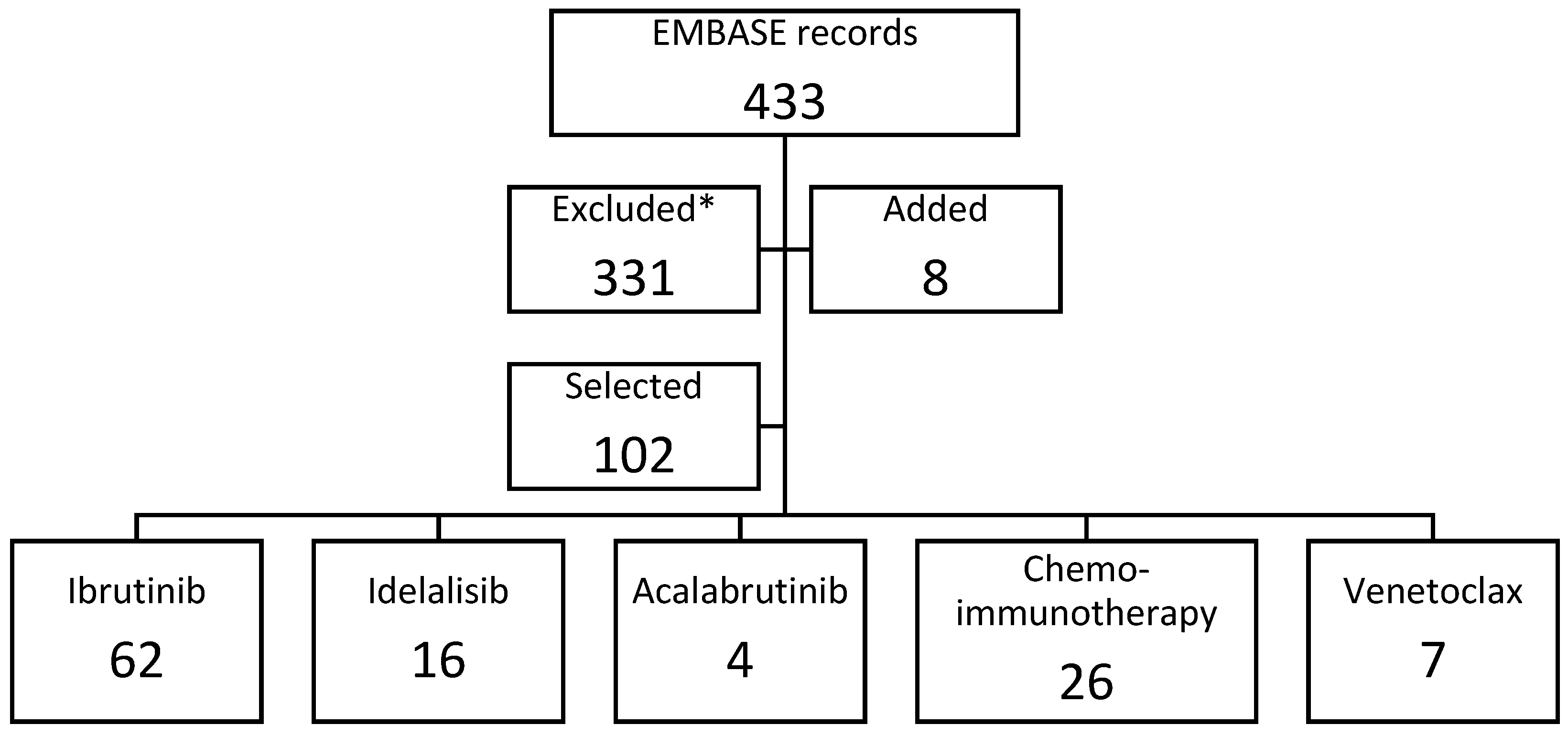
2. Methods
- (a)
- international,
- (b)
- national extensive,
- (c)
- national multi-site,
- (d)
- regional multi-site,
- (e)
- single-institution
- (a)
- health databases,
- (b)
- existing registry,
- (c)
- newly developed registry,
- (d)
- retrospective chart review,
- (e)
- phase IV study
- (a)
- geographic,
- (b)
- treatment,
- (c)
- sample availability,
- (d)
- frailty,
- (e)
- number of lines
- (a)
- <50,
- (b)
- ≥50
- (a)
- practice patterns,
- (b)
- survival,
- (c)
- prognostic yield of biomarkers,
- (d)
- prognostic yield of a score,
- (e)
- patient-related outcomes (PRO),
- (f)
- health care resource consumption and costs,
- (g)
- adverse events and discontinuation
3. Results
3.1. Ibrutinib
3.2. Acalabrutinib
3.3. Venetoclax
3.4. Chemoimmunotherapy
3.5. Idelalisib
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Author Names | Title | Source |
|---|---|---|
| Aarup K., Enggaard L., Pedersen R.S., Thomsen R.H., Bergmann O.J., Frederiksen M., Christiansen I., Nielsen T., Frederiksen H., Niemann C.U., Andersen M.A. | Real-world outcomes for 205 Danish patients with chronic lymphocytic leukemia treated with ibrutinib | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Aarup K., Rotbain E.C., Enggaard L., Pedersen R.S., Bergmann O.J., Thomsen R.H., Frederiksen M., Frederiksen H., Nielsen T., Christiansen I., Andersen M.A., Niemann C.U. | Real-world outcomes for 205 patients with chronic lymphocytic leukemia treated with ibrutinib | European Journal of Haematology (2020) 105:5 (646–654). Date of Publication: 1 Nov 2020 |
| Abrisqueta Costa D.P., Loscertales D.J., Terol D.M.J., Ramírez Payer D.Á., Ortiz D.M., Pérez D.I., Moreno D.C., Fernández De La Mata D.M., Rodríguez D.A., Lario D.A., Delgado D.J., Godoy D.A., Arguiñano Pérez D.J.M., Berruezo D.M.J., Oliveira D.A., Hernández Rivas D.J.Á., García D.L., Medina D.Á., García Martin D.P., Osorio D.S., Baltasar D.P., Fernández D.M., Marco D.F., Vidal Manceñido D.M.J., Smucler Simonovich A., López Rubio M., Jarque D.I., Suarez D.A., Fernández Álvarez D.R., Lancharro Anchel D.A., Ríos D.E., Losada Castillo D.M.D.C., Pérez Persona D.E., García Muñoz D.R., Ramos D.R., Yáñez D.L., Luis Bello D.J., Villanueva D.M. | Retrospective observational study of the treatment of chronic lymphocytic leukemia (CLL) with ibrutinib in routine clinical practice in spain | HemaSphere (2020) 4 Supplement 1 (311–312). Date of Publication: 1 Jun 2020 |
| Akhtar O.S., Attwood K., Lund I., Hare R., Hernandez-Ilizaliturri F.J., Torka P. | Dose reductions in ibrutinib therapy are not associated with inferior outcomes in patients with chronic lymphocytic leukemia (CLL) | Leukemia and Lymphoma (2019) 60:7 (1650–1655). Date of Publication: 7 Jun 2019 |
| Akhtar O.S., Torka P., Bhat S.A., Hare R., Sait S.N.J., Block A.W., Hernandez-Ilizaliturri F.J. | Disease progression on ibrutinib therapy is associated with a poor clinical outcome in chronic lymphocytic leukemia (CLL) patients managed in standard clinical practice | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Allouchery M., Delaunay P., Fourrier-Réglat A., Lafay-Chebassier C., Pérault-Pochat M.C. | Patterns of use and safety of ibrutinib in a real life practice: Preliminary results of an observational cohort study | Fundamental and Clinical Pharmacology (2019) 33 Supplement 1 (6). Date of Publication: 1 Jun 2019 |
| Autore F., Innocenti I., Corrente F., Del Principe M.I., Rosati S., Falcucci P., Fresa A., Conte E., Limongiello M.A., Renzi D., De Padua L., Andriani A., Pisani F., Cimino G., Tafuri A., Montanaro M., Mauro F.R., Del Poeta G., Laurenti L. | Front-Line Therapy for Elderly Chronic Lymphocytic Leukemia Patients: Bendamustine Plus Rituximab or Chlorambucil Plus Rituximab? Real-Life Retrospective Multicenter Study in the Lazio Region | Frontiers in Oncology (2020) 10 Article Number: 848. Date of Publication: 10 Jun 2020 |
| Beiggi S., Banerji V., Deneka A., Griffith J., Gibson S.B., Johnston J.B. | Comparison of outcome of patients with CLL who are referred or nonreferred to a specialized CLL clinic: a Canadian population-based study | Cancer Medicine (2016) 5:6 (971–979). Date of Publication: 1 Jun 2016 |
| Bird S.T., Tian F., Flowers N., Przepiorka D., Wang R., Jung T.-H., Kessler Z., Woods C., Kim B., Miller B.W., Wernecke M., Kim C., McKean S., Gelperin K., MacUrdy T.E., Kelman J.A., Graham D.J. | Idelalisib for Treatment of Relapsed Follicular Lymphoma and Chronic Lymphocytic Leukemia: A Comparison of Treatment Outcomes in Clinical Trial Participants vs Medicare Beneficiaries | JAMA Oncology (2020) 6:2 (248–254). Date of Publication: 1 Feb 2020 |
| Bouclet F., Calleja A., Dilhuydy M.-S., Amorim S., Cymbalista F., Herbaux C., De Guibert S., Roos-Weil D., Hivert B., Aurran T., Dupuis J., Blouet A., Tchernonog E., Laribi K., Dmytruk N., Morel P., Michallet A.-S., Dartigeas C., Farnault L., Lavaud A., Plantier I., Bay J.-O., Tournilhac O., Delmer A.J., Feugier P., Ysebaert L., Guieze R. | Outcome of patients receiving venetoclax for chronic lymphocytic leukemia (CLL) in real-life clinical practice: Results of the French ATU program on behalf of the Filo Group | Blood (2018) 132 Suppl. 1. Date of Publication: 1 Nov 2018 |
| Brander D.M., Rhodes J., Pagel J.M., Nabhan C., Tam C.S., Jacobs R., Hill B.T., Lamanna N., Lansigan F., Shadman M., Ujjani C.S., Skarbnik A.P., Cheson B.D., Pu J.J., Sehgal A.R., Barr P.M., Allan J.N., Beach D.F., Patel B., Pickens P.V., Nasta S.D., Kennard K., Tuncer H.H., Koch B., Furman R.R., Mato A.R. | Applicability of the chronic lymphocytic leukemia (CLL)-IPI on patients treated with front-line ibrutinib in the real world: The case for new prognostic models | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Cassin R., Visentin A., Noto A., Giannarelli D., Mauro F.R., Baldini L., Trentin L., Reda G. | Hypogammaglobulinaemia and infections in ibrutinib treated chronic lymphocytic leukemia (CLL) pazients: A prospective study | HemaSphere (2020) 4 Supplement 1 (308–309). Date of Publication: 1 Jun 2020 |
| Chien H.-C., Patil V., Rasmussen K.M., Yong C., Biondo J.M.L., Halloran M., Shapouri S., Wu M., Burningham Z.R., Sauer B.C., Halwani A.S. | Discontinuation patterns in patients receiving novel oral agents for chronic lymphocytic leukemia in the veterans health administration | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Cuneo A., Follows G., Rigolin G.M., Piciocchi A., Tedeschi A., Trentin L., Perez A.M., Coscia M., Laurenti L., Musuraca G., Farina L., Delgado A.R., Orlandi E.M., Galieni P., Mauro F.R., Visco C., Amendola A., Billio A., Marasca R., Chiarenza A., Meneghini V., Ilariucci F., Marchetti M., Molica S., Re F., Gaidano G., Gonzalez M., Forconi F., Ciolli S., Cortelezzi A., Montillo M., Smolej L., Schuh A., Eyre T.A., Kennedy B., Bowles K.M., Vignetti M., De La Serna J., Moreno C., Foà R., Ghia P. | Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: A GIMEMA, ERIC and UK CLL FORUM study | Haematologica (2018) 103:7 (1209–1217). Date of Publication: 3 Jul 2018 |
| Cuneo A., Mato A.R., Rigolin G.M., Piciocchi A., Gentile M., Laurenti L., Allan J.N., Pagel J.M., Brander D.M., Hill B.T., Winter A., Lamanna N., Tam C.S., Jacobs R., Lansigan F., Barr P.M., Shadman M., Skarbnik A.P., Pu J.J., Sehgal A.R., Schuster S.J., Shah N.N., Ujjani C.S., Roeker L., Orlandi E.M., Billio A., Trentin L., Spacek M., Marchetti M., Tedeschi A., Ilariucci F., Gaidano G., Doubek M., Farina L., Molica S., Di Raimondo F., Coscia M., Mauro F.R., de la Serna J., Medina Perez A., Ferrarini I., Cimino G., Cavallari M., Cucci R., Vignetti M., Foà R., Ghia P. | Efficacy of bendamustine and rituximab in unfit patients with previously untreated chronic lymphocytic leukemia. Indirect comparison with ibrutinib in a real-world setting: A GIMEMA-ERIC and US study | Cancer Medicine (2020) 9:22 (8468–8479). Date of Publication: 1 Nov 2020 |
| DaCosta Byfield S., Blauer-Peterson C., Dawson K., Masaquel A. | What are the health care utilization and costs associated with patients newly initiating anti-cancer systemic therapy for chronic lymphocytic leukemia? | Journal of Managed Care and Specialty Pharmacy (2018) 24 4-A SUPPL. (S29). Date of Publication: 1 Apr 2018 |
| Dartigeas C., Feugier P., Ysebaert L., Dilhuydy M.-S., Delmer A., Tardy S., Anglaret B., Voillat L., Le Dû K., Slama B., Albrecht C., Jenayah L., Beauclair S., Wapenaar R., Kavanagh C., Leblond V. | French ibrutinib observational study (FIRE): Real-world study of ibrutinib treatment for chronic lymphocytic leukemia (CLL) in france | HemaSphere (2019) 3 Supplement 1 (145–146). Date of Publication: 1 Jun 2019 |
| Del Poeta G., Biagi A., Laurenti L., Chiarenza A., Pozzo F., Innocenti I., Postorino M., Rossi F.M., Del Principe M.I., Bomben R., de Fabritiis P., Bruno A., Cantonetti M., Di Raimondo F., Zucchetto A., Gattei V. | Impaired nodal shrinkage and apoptosis define the independent adverse outcome of NOTCH1 mutated patients under ibrutinib therapy in chronic lymphocytic leukaemia | Haematologica (2020). Date of Publication: 30 Jul 2020 |
| Dimou M., Iliakis T., Pardalis V., Bitsani C., Vassilakopoulos T.P., Angelopoulou M., Tsaftaridis P., Papaioannou P., Koudouna A., Kalyva S., Kyrtsonis M.-C., Panayiotidis P. | Safety and efficacy analysis of long-term follow up real-world data with ibrutinib monotherapy in 58 patients with CLL treated in a single-center in Greece | Leukemia and Lymphoma (2019) 60:12 (2939–2945). Date of Publication: 15 Oct 2019 |
| Diop F., Moia R., Favini C., Spaccarotella E., De Paoli L., Bruscaggin A., Spina V., Cerri M., Deambrogi C., Kodipad A.A., Favini S., Sagiraju S., Jabangwe C., Mauro F.R., Del Giudice I., Forconi F., Cortelezzi A., Zaja F., Visco C., Chiarenza A., Rigolin G.M., Marasca R., Coscia M., Perbellini O., Tedeschi A., Laurenti L., Motta M., Del Poeta G., Cuneo A., Gattei V., Foa R., Gaidano G., Rossi D. | BRAF and BIRC3 mutations stratify a poor prognostic subgroup in FCR treated chronic lymphocytic leukemia | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Emole J.N., Viganego F., Schabath M.B., Shah B.D., Chavez J.C., Walko C.M., McLeod H.L., Pinilla-Ibarz J., Fradley M.G. | Incidence and risk factors for ibrutinib associated atrial fibrillation | Journal of Clinical Oncology (2016) 34 Supplement 15. Date of Publication: 1 May 2016 |
| Ferra C.M., Encinas M.P., Jimenez J.L., Ortiz M., Osorio-Prendes S., Cordoba R., Payer A.R., González-Barca E., Sánchez G.M., Diaz M.G., Sanchez M.J., Fernandez M., Tello P.B., Amutio E., García-Malo M.-D., MacEñido M.J.V., Fernandez P., Loscertales J., Rodríguez J.N., Alaez C., Ramroth H., Palla M. | Retrospective non-interventional assessment of the use of idelalisib in relapsed/refractory chronic lymphocytic leukemia patients in Spain | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Finnes H.D., Chaffee K.G., Call T.G., Ding W., Bowen D.A., Conte M., McCullough K.B., Merten J.A., Bartoo G.T., Smith M.D., Schwager S.M., Slager S.L., Kay N.E., Shanafelt T.D., Parikh S.A. | The importance of pharmacovigilance during ibrutinib therapy for chronic lymphocytic leukemia (CLL) in routine clinical practice | Blood (2015) 126:23 (717). Date of Publication: 3 Dec 2015 |
| Frei M., Aitken S.L., Jain N., Thompson P., Wierda W.G., Kontoyiannis D.P., DiPippo A. | Incidence and characterization of invasive fungal infections (IFIS) in patients with chronic lymphocytic leukemia (CLL) treated with ibrutinib (IBR) | Open Forum Infectious Diseases (2019) 6 Supplement 2 (S630). Date of Publication: 1 Oct 2019 |
| Gentile M., Morabito F., Del Poeta G., Mauro F.R., Reda G., Sportoletti P., Laurenti L., Coscia M., Herishanu Y., Recchia A.G., Varettoni M., Murru R., Chiarenza A., Condoluci A., Moia R., Pietrasanta D., Loseto G., Consoli U., Scortechini I., Rossi F.M., Zucchetto A., Fraticelli V., Vigna E., Botta C., Tripepi G., Arrigo G.D., Rago A., Angeletti I., Biagi A., Del Giudice I., Bomben R., Rigolin G.M., Rossi D., Di Raimondo F., Gaidano G., Polliack A., Cuneo A., Foà R., Gattei V. | Survival risk score for real-life relapsed/refractory chronic lymphocytic leukemia patients receiving ibrutinib. A campus CLL study | Leukemia (2020). Date of Publication: 2020 |
| Gentile M., Morabito F., Del Poeta G., Mauro F.R., Reda G., Sportoletti P., Laurenti L., Coscia M., Herishanu Y., Recchia A.G., Varettoni M., Murru R., Chiarenza A., Condoluci A., Moia R., Pietrasanta D., Loseto G., Consoli U., Scortechini I., Rossi F.M., Zucchetto A., Vigna E., Tripepi G., Rago A., Angeletti I., Biagi A., Del Giudice I., Bomben R., Rigolin G.M., Rossi D., Di Raimondo F., Gaidano G., Polliack A., Cuneo A., Foà R., Gattei V. | External validation of a novel risk model (BALL Score) in real-world relapsed/refractory chronic lymphocytic leukemia patients receiving ibrutinib: a campus cll study | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Georgantopoulos P., Yang H., Norris L.B., Bennett C.L. | Major hemorrhage in chronic lymphocytic leukemia patients in the U.S.Veterans Health Administration system in the pre-ibrutinib era: Incidence and risk factors | Cancer Medicine (2019) 8:5 (2233–2240). Date of Publication: 1 May 2019 |
| Goyal R.K., Nagar S.P., Kabadi S.M., Davis K.L., Le H., Kaye J.A. | Overall survival adverse events, and economic burden in medicare patients with chronic lymphocytic leukemia receiving cancer-directed therapy | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Halwani A.S., Rasmussen K., Patil V., Burningham Z., Sauer B.C. | Incidence of atrial fibrillation and bleeding in CLL patients treated with ibrutinib: Evidence from the veterans health administration | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Hansson L., Winqvist M., Asklid A., Andersson P.-O., Karlsson K., Karlsson C., Lauri B., Lundin J., Mattsson M., Norin S., Sandstedt A., Österborg A. | Real-world results on ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia (CLL): Data from 97 swedish patients treated in a compassionate use program | Blood (2015) 126:23 (1745). Date of Publication: 3 Dec 2015 |
| Herishanu Y., Goldschmidt N., Bairey O., Ruchlemer R., Fineman R., Rahimi-Levene N., Shvidel L., Tadmor T., Ariel A., Braester A., Shapiro M., Joffe E., Polliack A. | Efficacy and safety of front-line therapy with fludarabine-cyclophosphamide-rituximab regimen for chronic lymphocytic leukemia outside clinical trials: The Israeli CLL study group experience | Haematologica (2015) 100:5 (662–669). Date of Publication: 2015 |
| Herishanu Y., Shaulov A., Fineman R., Bašić-Kinda S., Aviv A., Wasik-Szczepanek E., Jaksic O., Zdrenghea M., Greenbaum U., Mandac I., Simkovic M., Morawska M., Benjamini O., Spacek M., Nemets A., Bairey O., Trentin L., Ruchlemer R., Laurenti L., Stanca Ciocan O., Doubek M., Shvidel L., Dali N., Mirás F., De Meûter A., Dimou M., Mauro F.R., Coscia M., Bumbea H., Szász R., Tadmor T., Gutwein O., Gentile M., Scarfò L., Tedeschi A., Sportoletti P., Gimeno Vázquez E., Marquet J., Assouline S., Papaioannou M., Braester A., Levato L., Gregor M., Rigolin G.M., Loscertales J., Medina Perez A., Nijziel M.R., Popov V.M., Collado R., Slavutsky I., Itchaki G., Ringelstein S., Goldschmidt N., Perry C., Levi S., Polliack A., Ghia P. | Frontline treatment with the combination obinutuzumab ± chlorambucil for chronic lymphocytic leukemia outside clinical trials: Results of a multinational, multicenter study by ERIC and the Israeli CLL study group | American Journal of Hematology (2020) 95:6 (604–611). Date of Publication: 1 Jun 2020 |
| Hillmen P., Diels J., Healy N., Iraqi W., Aschan J., Wildgust M. | Real-world experience of ibrutinib in >2900 chronic lymphocytic leukemia patients: Data from a global named patient program | Haematologica (2016) 101 Supplement 1 (51). Date of Publication: 1 Jun 2016 |
| Huang Q., Borra S., Li J., Wang L., Shrestha S., Sundaram M., Janjan N. | Time to next treatment, health care resource utilization, and costs associated with ibrutinib use among u.s. veterans with chronic lymphocytic leukemia/small lymphocytic lymphoma: A real-world retrospective analysis | Journal of Managed Care and Specialty Pharmacy (2020) 26:10 (1266–1275). Date of Publication: 1 Oct 2020 |
| Huang Q., Ellis L., Wang L., Shrestha S., Sundaram M. | Real-world evidence of ibrutinib use among patients with chronic lymphocytic leukemia and/or small lymphocytic lymphoma in the u.s. veterans health administration population | Journal of Managed Care and Specialty Pharmacy (2019) 25 10-A SUPPL. (S36). Date of Publication: 1 Oct 2019 |
| Huang Q., Ellis L., Wang L., Shrestha S., Sundaram M. | First-line therapy in chronic lymphocytic leukemia: A Swedish nation-wide real-world study on 1053 consecutive patients treated between 2007 and 2013 | Clinical Lymphoma, Myeloma and Leukemia (2019) 19 Supplement 1 (S285). Date of Publication: 1 Sep 2019 |
| Huang Q., Emond B., Lafeuille M.H., Gupta D., Lefebvre P., Sundaram M., Mato A. | PCN124 real-world healthcare resource utilization and total cost of care among us medicare patients with chronic lymphocytic leukemia receiving first-line ibrutinib vs. chemoimmunotherapy | Value in Health (2020) 23 Supplement 1 (S44-S45). Date of Publication: 1 May 2020 |
| Huang S.J., Lee L.J., Gerrie A.S., Gillan T.L., Bruyere H., Hrynchak M., Smith A.C., Karsan A., Ramadan K.M., Jayasundara K.S., Toze C.L. | Characterization of treatment and outcomes in a population-based cohort of patients with chronic lymphocytic leukemia referred for cytogenetic testing in British Columbia, Canada | Leukemia Research (2017) 55 (79–90). Date of Publication: 1 Apr 2017 |
| Huntington S.F., Soulos P.R., Barr P.M., Jacobs R., Lansigan F., Odejide O.O., Schwartzberg L.S., Davidoff A.J., Gross C.P. | Utilization and early discontinuation of first-line ibrutinib for patients with chronic lymphocytic leukemia treated in the community oncology setting in the United States | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Irwin D., Szabo E., Pathak A., Tang B. | Length of stay for hospitalized patients treated with ibrutinib or bendamustine first-line therapy for treatment of chronic lymphocytic leukemia | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Irwin D., Wilson K. | Characteristics and treatment patterns of patients receiving oral ibrutinib therapy for treatment of chronic lymphocytic leukemia | Journal of Managed Care and Specialty Pharmacy (2017) 23 3-A SUPPL. (S34). Date of Publication: 1 Mar 2017 |
| Iskierka-Jazdzewska E., Hus M., Giannopoulos K., Madro E., Hołojda J., Piotrowska M., Zaucha J.M., Piszczek W., Szeremet A., Wojciechowska M., Steckiewicz P., Knopińska-Posłuszny W., Osowiecki M., Drozd-Sokołowska J., Kumiega B., Kyrcz-Krzemień S., Hałka J., Dudziński M., Wieszczy P., Warzocha K., Jamroziak K. | Results of a prospective observational trial of polish adult leukemia group (PALG) on ibrutinib compassionate use in relapsed or refractory chronic lymphocytic leukaemia (CLL) in poland | Haematologica (2016) 101 Supplement 1 (439–440). Date of Publication: 1 Jun 2016 |
| Iyengar R., Gutierrez M., Ghosh N., Barrientos J., Brander D., Kadish K., Tomlinson B., Mato A., Sharman J., Ipe D., Han J., Amaya-Chanaga C., Sundaram M. | Treatment patterns, clinical outcomes, and healthcare resource utilization for previously untreated and relapsed/ refractory patients with chronic lymphocytic leukemia in the era of novel agents: Interim analysis from the informcll registry | Journal of Managed Care and Specialty Pharmacy (2019) 25 10-A SUPPL. (S35). Date of Publication: 1 Oct 2019 |
| Iyengar R., Malangone-Monaco E., Sugg C., Amaya-Chanaga C., McMorrow D., Shukrun N., Balakrishnan C., Giafis N. | Comparison of healthcare resource utilization and total direct costs for chronic lymphocytic leukemia patients treated with ibrutinib or chemoimmunotherapy | Journal of Managed Care and Specialty Pharmacy (2019) 25 10-A SUPPL. (S36). Date of Publication: 1 Oct 2019 |
| Janssens A., André M., Berneman Z., Snauwaert S., De Beleyr B., Smet A., Van Bogaert C., Wapenaar R., Bron D. | Effectiveness and safety of ibrutinib for chronic lymphocytic leukemia (CLL) in routine clinical practice: Interim analysis (IA) of the belgian ibrutinib real-world data (BiRD) study | HemaSphere (2019) 3 Supplement 1 (144). Date of Publication: 1 Jun 2019 |
| Kleeberg U.R., Linde H., Günther G., Tessen H.-W., Kersting M. | Bendamustin-rituximab combination is a safe and effective, ambulatory treatment for elderly patients with chronic lymphocytic leukemia: Retrospective real-world analysis by age from a German registry and review of the literature | Anticancer Research (2016) 36:6 (2827–2838). Date of Publication: 1 Jun 2016 |
| Knauf W., Hoechstetter M., Eissmann P., Hucke N., Van Troostenburg A., Ramroth H., Rummel M. | Prospective real world data of an ongoing post-authorization safety study on idelalisib in patients with CLL and refractory FL | HemaSphere (2018) 2 Supplement 2 (852–853). Date of Publication: 1 Jun 2018 |
| Kunk P.R., Mock J., Devitt M.E., Palkimas S., Sen J., Portell C.A., Williams M.E. | Major bleeding with ibrutinib: More than expected | Blood (2016) 128:22. Date of Publication: 2 Dec 2016 |
| Kuranz S., Stacey J., Luciano S. | Early discontinuation (ED) of therapy and treatment patterns among chronic lymphocytic leukemia (CLL) patients: Findings from a linked claims and electronic health record (EHR) dataset | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Lee L.J., Toze C.L., Huang S.J.T., Gillan T.L., Connors J.M., Sehn L.H., Bruyere H., Leitch H., Ramadan K.M., Gerrie A.S. | Improved survival outcomes with the addition of rituximab to initial therapy for chronic lymphocytic leukemia: a comparative effectiveness analysis in the province of British Columbia, Canada | Leukemia and Lymphoma (2018) 59:6 (1356–1363). Date of Publication: 3 Jun 2018 |
| Lenartova A., Johannesen T.B., Tjønnfjord G.E. | Chronic lymphocytic leukemia and secondary hematological malignancies: A nation-wide cancer registry study | European Journal of Haematology (2020) 104:6 (546–553). Date of Publication: 1 Jun 2020 |
| Li J.J., Yong A.S., Justicia J.L., Smith C., Delgado J. | Idelalisib in combination with rituximab in chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma (SLL): Real-world experience through an early access program in Europe and Australia | Haematologica (2016) 101 Supplement 1 (229). Date of Publication: 1 Jun 2016 |
| Mato A., Nabhan C., Lamanna N., Kay N.E., Grinblatt D.L., Flowers C.R., Farber C.M., Davids M.S., Swern A.S., Sullivan K., Dawn Flick E., Gressett Ussery S.M., Gharibo M., Kiselev P., Sharman J.P. | The Connect CLL Registry: Final analysis of 1494 patients with chronic lymphocytic leukemia across 199 US sites | Blood Advances (2020) 4:7 (1407–1418). Date of Publication: 14 Apr 2020 |
| Mato A.R., Allan J.N., Pagel J.M., Brander D.M., Hill B.T., Cheson B.D., Furman R.R., Lamanna N., Tam C.S., Jacobs R., Lansigan F., Barr P.M., Shadman M., Skarbnik A.P., Beach D.F., Pu J.J., Sehgal A.R., Winter A.M., Zent C.S., Tuncer H.H., Singavi A.K., Schuster S.J., Pickens P.V., Shah N.N., Williams A., Howlett C., Weissbrot H., Ali N., Patel B., Isaac K., Rhodes J., Hughes M.E., Khajavian S., Chatburn E.T., Sitlinger A., Tranchito E., Bhavsar E.B., Bailey N., Burns T.F., Yacur M., Malhotra M., Handunnetti S., Kennard K., Nabhan C., Ujjani C.S. | Front-line ibrutinib therapy for chronic lymphocytic leukemia (CLL) in the real world: Responses, toxicity, outcomes, and subsequent therapies | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Mato A.R., Barrientos J.C., Ghosh N., Pagel J.M., Brander D.M., Gutierrez M., Kadish K., Tomlinson B., Iyengar R., Ipe D., Upasani S., Amaya-Chanaga C.I., Sundaram M., Han J., Giafis N., Sharman J.P. | Prognostic Testing and Treatment Patterns in Chronic Lymphocytic Leukemia in the Era of Novel Targeted Therapies: Results From the informCLL Registry | Clinical Lymphoma, Myeloma and Leukemia (2020) 20:3 (174–183.e3). Date of Publication: 1 Mar 2020 |
| Mato A.R., Hill B.T., Lamanna N., Barr P.M., Ujjani C.S., Brander D.M., Howlett C., Skarbnik A.P., Cheson B.D., Zent C.S., Pu J.J., Kiselev P., Foon K., Lenhart J., Henick Bachow S., Winter A.M., Cruz A.-L., Claxton D.F., Goy A., Daniel C., Isaac K., Kennard K.H., Timlin C., Fanning M., Gashonia L., Yacur M., Svoboda J., Schuster S.J., Nabhan C. | Optimal sequencing of ibrutinib, idelalisib, and venetoclax in chronic lymphocytic leukemia: results from a multicenter study of 683 patients | Annals of oncology: official journal of the European Society for Medical Oncology (2017) 28:5 (1050–1056). Date of Publication: 1 May 2017 |
| Mato A.R., Nabhan C., Barr P.M., Ujjani C.S., Hill B.T., Lamanna N., Skarbnik A.P., Howlett C., Pu J.J., Sehgal A.R., Strelec L.E., Vandegrift A., Fitzpatrick D.M., Zent C.S., Feldman T., Goy A., Claxton D.F., Bachow S.H., Kaur G., Svoboda J., Nasta S.D., Porter D., Landsburg D.J., Schuster S.J., Cheson B.D., Kiselev P., Evens A.M. | Outcomes of CLL patients treated with sequential kinase inhibitor therapy: A real-world experience | Blood (2016) 128:18 (2199–2205). Date of Publication: 3 Nov 2016 |
| Mato A.R., Nabhan C., Thompson M.C., Lamanna N., Brander D.M., Hill B., Howlett C., Skarbnik A., Cheson B.D., Zent C., Pu J., Kiselev P., Goy A., Claxton D., Isaac K., Kennard K.H., Timlin C., Landsburg D., Winter A., Nasta S.D., Bachow S.H., Schuster S.J., Dorsey C., Svoboda J., Barr P., Ujjani C.S. | Toxicities and outcomes of 616 ibrutinib-treated patients in the united states: A real-world analysis | Haematologica (2018) 103:5 (874–879). Date of Publication: 30 Apr 2018 |
| Mato A.R., Sail K., Yazdy M.S., Hill B.T., Shadman M., Manzoor B.S., Tuncer H.H., Allan J.N., Ujjani C.S., Sharmokh S., Jiang D.D., Pena G., Marshall T., Nielsen J., Barr P.M., Brown J.R., Schuh A., Eyre T.A., Wierda W.G., Skarbnik A., Roeker L.E., Bannerji R., Pauff J.M., Schuster S.J., Follows G.A., Cheson B.D., Eichhorst B.F., Brander D.M., Pivneva I., Lamanna N. | Treatment sequences and outcomes of patients with CLL treated with venetoclax and other novel agents post introduction of novel therapies | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Mato A.R., Thompson M., Allan J.N., Brander D.M., Pagel J.M., Ujjani C.S., Hill B.T., Lamanna N., Lansigan F., Jacobs R., Shadman M., Skarbnik A.P., Pu J.J., Barr P.M., Sehgal A.R., Cheson B.D., Zent C.S., Tuncer H.H., Schuster S.J., Pickens P.V., Shah N.N., Goy A., Winter A.M., Garcia C., Kennard K., Isaac K., Dorsey C., Gashonia L.M., Singavi A.K., Roeker L.E., Zelenetz A., Williams A., Howlett C., Weissbrot H., Ali N., Khajavian S., Sitlinger A., Tranchito E., Rhodes J., Felsenfeld J., Bailey N., Patel B., Burns T.F., Yacur M., Malhotra M., Svoboda J., Furman R.R., Nabhan C. | Real-world outcomes and management strategies for venetoclax-treated chronic lymphocytic leukemia patients in the United States | Haematologica (2018) 103:9 (1511–1517). Date of Publication: 31 Aug 2018 |
| Mauro F.R., Giannarelli D., Visentin A., Reda G., Coscia M., Tedeschi A., Sportoletti P., Chiarenza A., Ciolli S., Levato L., Gentile M., Laurenti L., Rigolin G.M., Vitale C., Giordano A.M., Murru R., De Paoli L., Del Poeta G., Rosati S., Riemma C., Cassin R., Frustaci A.M., Stelitano C., Cuneo A., Molica S., Girmenia C., Foà R., Trentin L. | Severe infections and pneumonia in patients with chronic lymphocytic leukemia (CLL) treated with Kinase inhibitors (KIS) ibrutinib or idelalisib in the real world | Haematologica (2019) 104 Supplement 2 (100). Date of Publication: 1 Oct 2019 |
| Mauro F.R., Tedeschi A., Piciocchi A., Motta M., Iannella E., Farina L., Scarfo L., Marasca R., Coscia M., Cortelezzi A., Laurenti L., Melpignano A., Zinzani P.L., Molica S., Re F., Andriani A., Vincelli D.I., Visco C., Gozzetti A., Orlandi E.M., Trentin L., Tani M., Califano C., Tagariello G., Ghia P., Caputo M.D., Salaroli A., Innocenti I., Frustaci A., Vitale C., Petullà M., De Fabritiis P., Vignetti M., Fazi P., Foà R. | Outcome of patients with re lapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and/or 17p deletion/TP53 mutations treated with ibrutinib according to a named patient program (NPP) in Italy: Preliminary analysis of a real-life retrospective study | Blood (2016) 128:22. Date of Publication: 2 Dec 2016 |
| Michallet A.-S., Campidelli A., Lequeu H., Dilhuydy M.-S., Tournilhac O., Fornecker L.-M., Dupuis J., Cymbalista F., De Guibert S., Delmer A., Vilque J.-P., Ghez D., Leblond V., Subtil F., Feugier P., Ysebaert L. | Ibrutinib in very elderly patients with relapsed/refractory chronic lymphocytic leukemia: A real-world experience of 71 patients treated in France: A study from the French Innovative Leukemia Organization (FILO) group | American Journal of Hematology (2017) 92:6 (E105-E107). Date of Publication: 1 Jun 2017 |
| Morelli F., Tomasso A., Bacchiarri F., Gozzetti A., Laurenti L., Ciolli S. | CLL-251, Venetoclax for CLL Patients Outside Clinical Trials: An Italian Real-Life Experience | Clinical Lymphoma, Myeloma and Leukemia (2020) 20 Supplement 1 (S225-S226). Date of Publication: 1 Sep 2020 |
| Nabhan C., Chung J., Mato A.R., Kish J., Nero D. | Comparison of costs and health care resource utilization (HRU) in chronic lymphocytic leukemia (CLL) patients treated with front-line ibrutinib or chemoimmunotherapy | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Nadali G., Marchesini G., Facchinelli D., Farina F., Tisi M.C., Lessi F., Marchesi F., Cattaneo C., Candoni A., Fanci R., Prezioso L., Verga L., Laurenti L., Trastulli F., Picardi M., Del Principe M.I., Silva R., Busca A. | Infections in patients with lymphoproliferative diseases treated with target therapy. Italian multicentric retrospective study seifem 2017 | Blood (2018) 132 Suppl. 1. Date of Publication: 1 Nov 2018 |
| Nero D., Chung J., Kish J., Nabhan C. | Comparative study of healthcare resource utilization (HRU) outcomes between chronic lymphocytic leukemia (CLL) patients treated with ibrutinib versus non-ibrutinib treated patients | Value in Health (2017) 20:9 (A414). Date of Publication: 1 Oct 2017 |
| Nero D., Chung J., Mato A.R., Kish J., Nabhan C. | Comparative study of cardiovascular co-morbidities between ibrutinib and non-ibrutinib-treated chronic lymphocytic leukemia (CLL) patients | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Nohria A., Rosenblatt L., Pan X., Sharma A. | Evaluation of the risk of atrial fibrillation/flutter among patients initiating ibrutinib | Blood (2018) 132 Suppl. 1. Date of Publication: 1 Nov 2018 |
| Olszewski A.J., Davids M.S., Yakirevich I., Egan P.C. | Early adoption and outcomes of ibrutinib as treatment for older patients with chronic lymphocytic leukemia (CLL): A population-based study | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Panovská A., Němcová L., Nekvindová L., Špaček M., Šimkovič M., Papajík T., Brejcha M., Lysák D., Zuchnická J., Novák J., Starostka D., Poul H., Vrbacký F., Vodárek P., Urbanová R., Plevová K., Pospíšilová Š., Mašlejová S., Brychtová Y., Koriťáková E., Smolej L., Doubek M. | Real-world data on efficacy and safety of obinutuzumab plus chlorambucil, rituximab plus chlorambucil, and rituximab plus bendamustine in the frontline treatment of chronic lymphocytic leukemia: The GO-CLLEAR Study by the Czech CLL Study Group | Hematological Oncology (2020) 38:4 (509–516). Date of Publication: 1 Oct 2020 |
| Puła B., Budziszewska B.K., Rybka J., Gil L., Subocz E., Długosz-Danecka M., Zawirska D., Waszczuk-Gajda A., Iskierka-Jażdżewska E., Kopacz A., Szymczyk A., Czyz J., Lech-Marańda E., Warzocha K., Jamroziak K. | Comparable efficacy of idelalisib plus rituximab and ibrutinib in relapsed/refractory chronic lymphocytic leukemia: A retrospective case matched study of the polish adult leukemia group (PALG) | Anticancer Research (2018) 38:5 (3025–3030). Date of Publication: 1 May 2018 |
| Pula B., Iskierka-Jazdzewska E., Dlugosz-Danecka M., Szymczyk A., Hus M., Szeremet A., Drozd-Sokolowska J., Waszczuk-Gajda A., Zaucha J.M., Holojda J., Piszczek W., Steckiewicz P., Wojciechowska M., Osowiecki M., Knopinska-Posluszny W., Dudzinski M., Zawirska D., Subocz E., Halka J., Pluta A., Wichary R., Kumiega B., Budziszewska B.K., Jurczak W., Lech-Maranda E., Giannopoulos K., Robak T., Jamroziak K. | Long-term efficacy of ibrutinib in relapsed or refractory chronic lymphocytic leukemia: Results of the polish adult leukemia study group observational study | Anticancer Research (2020) 40:7 (4059–4066). Date of Publication: 1 Jul 2020 |
| Puła B., Iskierka-Jazdzewska E., Długosz-Danecka M., Szymczyk A., Hus M., Szeremet A., Drozd-Sokołowska J., Waszczuk-Gajda A., Zaucha J.M., Hołojda J., Piszczek W., Steckiewicz P., Wojciechowska M., Osowiecki M., Knopińska-Posłuszny W., Dudziński M., Zawirska D., Subocz E., Hałka J., Pluta A., Wichary R., Kumiega B., Budziszewska B.K., Jurczak W., Lech-Marańda E., Giannopoulos K., Robak T., Jamroziak K. | Long-term real-world clinical outcomes for ibrutinib monotherapy treatment in relapsed refractory chronic lymphocytic leukemia-observational study of the polish adult leukemia study group (PALG) | HemaSphere (2020) 4 Supplement 1 (867–868). Date of Publication: 1 Jun 2020 |
| Puła B., Iskierka-Jazdzewska E., Długosz-Danecka M., Szymczyk A., Hus M., Szeremet A., Rybka J., Drozd-Sokołowska J., Waszczuk-Gajda A., Zaucha J.M., Hołojda J., Piszczek W., Steckiewicz P., Wojciechowska M., Osowiecki M., Knopińska-Posłuszny W., Kopacz A., Dudziński M., Zawirska D., Piotrowska M., Subocz E., Hałka J., Kumiega B., Gil L., Szukalski L., Wichary R., Budziszewska B.K., Lech-Maranda E., Jurczak W., Giannopoulos K., Robak T., Warzocha K., Jamroziak K. | Second cancers in chronic lymphocytic leukemia patients treated with BCR inhibitors:-Retrospective analysis of the polish adult leukemia study group (PALG) | HemaSphere (2018) 2 Supplement 2 (851–852). Date of Publication: 1 Jun 2018 |
| Rotbain E.C., Frederiksen H., Hjalgrim H., Rostgaard K., Egholm G.J., Zahedi B., Poulsen C.B., Enggard L., Da Cunha-Bang C., Niemann C.U. | IGHV mutational status and outcome for patients with chronic lymphocytic leukemia upon treatment: A danish nationwide population-based study | Haematologica (2020) 105:6 (1621–1629). Date of Publication: 1 Jun 2020 |
| Ryan K., Burudpakdee C., Zhao X., Le H., Near A. | Characteristics of mantle cell lymphoma (MCL) and chronic lymphocytic leukemia (CLL) patients treated with acalabrutinib in a real world setting in the United States | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Sabrie N., Leong D., Prica A., Austin P., Pang A., Fang J., Calvillo-Arguelles O., Lee D., Thavendiranathan P., Abdel-Qadir H. | INCREASED RISK OF ADVERSE CARDIOVASCULAR EVENTS ASSOCIATED WITH IBRUTINIB USE IN CHRONIC LYMPHOCYTIC LEUKEMIA: A PROPENSITY-MATCHED POPULATION-BASED COHORT STUDY | Journal of the American College of Cardiology (2020) 75:11 (414). Date of Publication: 24 Mar 2020 |
| Sandoval-Sus J.D., Chavez J.C., Dalia S., Bello C.M., Shah B.D., Ho V.Q., Nodzon L., Kharfan-Dabaja M.A., Sotomayor E.M., Sokol L., Pinilla-Ibarz J. | Outcomes of patients with relapsed/refractory chronic lymphocytic leukemia after ibrutinib discontinuation outside clinical trials: A single institution experience | Blood (2015) 126:23 (2945). Date of Publication: 3 Dec 2015 |
| Scalzulli P.R., Guarini A., Loseto G., Specchia G., Giordano A.M., Pastore D., Quintana G., Mazza P., Maggi A., Di Renzo N., De Paolis M.R., Tarantini G., De Santis G., Pavone V., Greco A., Valvano M.R., Cascavilla N. | Ibrutinib, single agent BTK inhibitor, for treatment of Naïve (TN) and relapsed/refractory (R/R) chronic lymphocytic leukemia: A real-life experience from rete ematologica pugliese (REP) | Blood (2018) 132 Suppl. 1. Date of Publication: 1 Nov 2018 |
| Schetelig J., Chevallier P., van Gelder M., Hoek J., Hermine O., Chakraverty R., Browne P., Milpied N., Malagola M., Socié G., Delgado J., Deconinck E., Damaj G., Maury S., Beelen D., Quoc S.N., Shankara P., Brecht A., Mayer J., Hunault-Berger M., Bittenbring J., Thieblemont C., Lepretre S., Baldauf H., de Wreede L.C., Tournilhac O., Yakoub-Agha I., Kröger N., Dreger P. | Idelalisib treatment prior to allogeneic stem cell transplantation for patients with chronic lymphocytic leukemia: a report from the EBMT chronic malignancies working party | Bone Marrow Transplantation (2020). Date of Publication: 2020 |
| Seymour E.K., Ruterbusch J.J., Beebe-Dimmer J.L., Schiffer C.A. | Real-world testing and treatment patterns in chronic lymphocytic leukemia: A SEER patterns of care analysis | Cancer (2019) 125:1 (135–143). Date of Publication: 1 Jan 2019 |
| Shadman M., Sail K., Manzoor B.S., Yazdy M.S., Hill B.T., Tuncer H.H., Allan J.N., Ujjani C.S., Emechebe N., Kamalakar R., Sharmokh S., Jiang D.D., Pena G., Marshall T., Nielsen J., Barr P.M., Brown J.R., Schuh A., Eyre T.A., Lamanna N., Wierda W.G., Skarbnik A., Roeker L.E., Bannerji R., Pauff J.M., Schuster S.J., Follows G.A., Cheson B.D., Eichhorst B.F., Brander D.M., Pivneva I., Guerin A., Mato A.R. | Treatment discontinuation patterns for patients with CLL in the real-world settings: Results from a multi-center study | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Sharman J.P., Black-Shinn J.L., Clark J., Bitman B. | Understanding ibrutinib treatment discontinuation patterns for chronic lymphocytic leukemia | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Silva S., Espada E., Melo J.A., Lima M.P., Ionita A., Carda J.P., Andrade J., Neves M., Cabral R., Mendes T., Gaspar C., Alves D., Pina F., Botelho De Sousa A., Coelho H., Montalvão A., Vitória H., Lima F., Coutinho J., Lúcio P., Guimarães J.E., Ribeiro M.L., Gomes Da Silva M., Raposo J. | Portuguese real-life experience with ibrutinib outside clinical trials: - A multicenter analysis | Hematological Oncology (2017) 35 Supplement 2 (383–384). Date of Publication: 1 Jun 2017 |
| Sylvan S.E., Asklid A., Johansson H., Klintman J., Bjellvi J., Tolvgård S., Kimby E., Norin S., Andersson P.-O., Karlsson C., Karlsson K., Lauri B., Mattsson M., Sandstedt A.B., Strandberg M., Österborg A., Hansson L. | First-line therapy in chronic lymphocytic leukemia: A swedish nation-wide real-world study on 1053 consecutive patients treated between 2007 and 2013 | Haematologica (2019) 104:4 (797–804). Date of Publication: 31 Mar 2019 |
| Tam C., Kuss B., Opat S., Puig A., Acar M., Zhou C., Mulligan S. | Real world treatment persistence of australian ibrutinib patients in a named patient program | HemaSphere (2018) 2 Supplement 2 (129–130). Date of Publication: 1 Jun 2018 |
| Tejaswi V., Lad D.P., Jindal N., Prakash G., Malhotra P., Khadwal A., Jain A., Sreedharanunni S., Singh Sachdeva M., Naseem S., Varma N., Varma S. | Chronic Lymphocytic Leukemia: Real-World Data from India | JCO Global Oncology (2020):6 (866–872). Date of Publication: 24 Jun 2020 |
| Tombak A., Tanrikulu F.P., Durusoy S.S., Gurkan E., Kaya E., Umit E.G., Yavasoglu I., Mehtap Ö., Deveci B., Ozcan M.A., Terzi H., Okay M., Sayinalp N., Yilmaz M., Okan V., Kizikli A., Ozcan O., Çetin G., Demircioglu S., Aydogdu I., Saydam G., Davulcu E.A., Ilhan G., Ucar M.A., Ozet G., Akpinar S., Turgut B., Berber I., Kurtoglu E., Sönmez M., Batur D.S., Yildirim R., Ozkocaman V., Gunes A.K., Sahip B., Ertop S., Akay M., Basturk A., Dogu M.H., Akdeniz A., Unal A., Seyhanli A., Ferhanoglu B. | Efficacy and safety of ibrutinib use in patients with chronic lymphocytic leukemia in real-world experiences: Results of a prospective multicenter study | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Uminski K., Brown K., Bucher O., Hibbert I., Dhaliwal D.H., Johnston J.B., Geirnaert M., Dawe D.E., Banerji V. | Descriptive analysis of dosing and outcomes for patients with ibrutinib-treated relapsed or refractory chronic lymphocytic leukemia in a Canadian center | Current Oncology (2019) 26:5 (e610-e617). Date of Publication: 2019 |
| van der Straten L., Kater A.P., Doorduijn J.K., van den Broek E.C., Posthuma E.F.M., Dinmohamed A.G., Levin M.-D. | Possible hampered effectiveness of second-line treatment with rituximab-containing chemotherapy without signs of rituximab resistance: a population-based study among patients with chronic lymphocytic leukemia | Annals of Hematology (2020) 99:5 (1081–1091). Date of Publication: 1 May 2020 |
| Van Der Straten L., Levin M.-D., Visser O., Blijlevens N.M., Cornelissen J.J., Doorduijn J.K., Kater A.P., Dinmohamed A.G. | Effectiveness of ibrutinib for the treatment of chronic lymphocytic leukemia in daily practice in the netherlands: A nationwide population-based cohort study | HemaSphere (2018) 2 Supplement 2 (131). Date of Publication: 1 Jun 2018 |
| Vanderveer E., Huang S.J.T., Bruyere H., Gillan T., Li C.H., Ramadan K., Villa D., Scott D.W., Savage K.J., Connors J.M., Sehn L.H., Toze C.L., Gerrie A.S. | Oral fludarabine and intravenous rituximab (FR) for chronic lymphocytic leukemia (CLL): Long term outcomes and secondary malignancies in 673 patients treated in British Columbia (BC) | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Vitale C., Salvetti C., Griggio V., Scamuffa M.C., Zamprogna G., Visentin A., Cassin R., Laurenti L., Murru R., Rivela P., Marchetti M., Gentile M., Pennese E., Reda G., Trentin L., Tedeschi A., Mauro F.R., Foà R., Boccadoro M., Coscia M. | Pre-existing and treatment-emergent autoimmune cytopenias in patients with chronic lymphocytic leukemia treated with targeted drugs | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Wang S., Emond B., Romdhani H., Lefebvre P., Sundaram M., Mato A. | Ibrutinib treatment is associated with lower healthcare resource utilization and net cost savings compared to chemoimmunotherapy in front-line cll oncology care model episodes | Journal of Managed Care and Specialty Pharmacy (2018) 24:10 A (S28-S29). Date of Publication: 1 Oct 2018 |
| Wang S., Emond B., Romdhani H., Lefebvre P., Sundaram M., Mato A.R. | Front-line ibrutinib treatment is associated with longer time to next treatment, net total cost reduction, and lower healthcare resource utilization compared to chemoimmunotherapy in patients with chronic lymphocytic leukemia | Blood (2018) 132 Suppl. 1. Date of Publication: 1 Nov 2018 |
| Weinkove R., Doocey R., Henderson R., Islam S., Smith M., Puig A., Pateman G., Acar M., Amaya-Chanaga C., Simpson D. | Real world treatment persistence of New Zealand ibrutinib chronic lymphocytic leukemia patients in a named patient program | HemaSphere (2019) 3 Supplement 1 (861–862). Date of Publication: 1 Jun 2019 |
| Winqvist M., Andersson P.-O., Asklid A., Karlsson K., Karlsson C., Lauri B., Lundin J., Mattsson M., Norin S., Sandstedt A., Rosenquist R., Späth F., Hansson L., Österborg A. | Long-term real-world results of ibrutinib therapy in patients with relapsed or refractory chronic lymphocytic leukemia: A 30-month follow up of the swedish compassionate use cohort | Haematologica (2019) 104:5 (e208-e210). Date of Publication: 30 Apr 2019 |
| Winqvist M., Andersson P.-O., Asklid A., Karlsson K., Karlsson C., Lauri B., Lundin J., Mattsson M., Norin S., Sandstedt A.B., Hansson L., Österborg A. | Real-world results on ibrutinib in relapsed/refractory CLL: A 21-month follow-up of 95 swedish patients treated in a compassionate use program | Blood (2017) 130 Supplement 1. Date of Publication: 1 Dec 2017 |
| Yazdy M.S., Mato A.R., Roeker L.E., Jarral U., Ujjani C.S., Shadman M., Skarbnik A., Whitaker K.J., Deonarine I., Kabel C.C., Stump S.E., Goodfriend J., Pagel J.M., Bailey N., Patel K., Jacobs R., Feldman T.A., Leslie L.A., Goy A., Coombs C.C., Muluneh B., Khajavian S., Lamanna N., Weissbrot H., Weiss J., Cheson B.D. | Toxicities and outcomes of acalabrutinib-treated patients with chronic lymphocytic leukemia: A retrospective analysis of real-world patients | Blood (2019) 134 Supplement 1. Date of Publication: 1 Nov 2019 |
| Ysebaert L., Aurran-Schleinitz T., Dartigeas C., Dilhuydy M.S., Feugier P., Michallet A.S., Tournilhac O., Dupuis J., Sinet P., Albrecht C., Cymbalista F. | Large scale, real-world results on ibrutinib for 428 french patients with 17p deletion or relapsed/refractory chronic lymphocytic leukemia included in a temporary authorization for use in the (ATU) program | Haematologica (2016) 101 Supplement 1 (57–58). Date of Publication: 1 Jun 2016 |
| Zaja F., Mian M., Volpetti S., Visco C., Sissa C., Nichele I., Castelli M., Ambrosetti A., Puglisi S., Fanin R., Cortelazzo S., Pizzolo G., Trentin L., Rodeghiero F., Paolini R., Vivaldi P., Sancetta R., Isola M., Semenzato G. | Bendamustine in chronic lymphocytic leukemia: Outcome according to different clinical and biological prognostic factors in the everyday clinical practice | American Journal of Hematology (2013) 88:11 (955–960). Date of Publication: November 2013 |
| Authors | Title | Source |
|---|---|---|
| Gentile M., et al. | Validation of a survival-risk score (SRS) in relapsed/refractory CLL patients treated with idelalisib–rituximab | Blood Cancer Journal (2020) 10:9 Article Number 92 |
| Morelli F., et al. | Treatment of chronic lymphocytic leukemia in the new drugs era: The state of art in the Italian landscape | HemaSphere (2020) 4 Supplement 1 (315–316) |
| Ysebaert L., et al. | Non-interventional retrospective multicenter study evaluating real-word idelalisib use in CLL and INHL patients enrolled in the French early access program (EAP) | HemaSphere (2019) 3 Supplement 1 (862–863) |
| Salles G, et al. | Single-agent ibrutinib in RESONATE-2™ and RESONATE™ versus treatments in the real-world PHEDRA databases for patients with chronic lymphocytic leukemia | Annals Hematology (2019) Dec; 98(12): 2749–2760. |
| Azali L, et al. | Evaluation of the incidence and risk factors associated with major cardiovascular events in patients receivingacalabrutinib therapy | ASH (2020) Abstract 2223 |
| Farrukh T. Awan, et al. | Acalabrutinib monotherapy in patients with chronic lymphocytic leukemia who are intolerant to ibrutinib | Blood Advances (2019) 3:1553–62 |
| Innocenti et al. | Venetoclax in CLL patients who progress after B-cell Receptor inhibitor treatment: A retrospective multicenter Italian experience | British Journal of Haematology (2019) Oct; 187(1): e8–e11. |
| Alsina et al. | Role of age, fitness and concomitant medications in CLL patients treated with venetoclax | Abstract ASH (2020) Blood, Blood (2020) 136 (Supplement 1): 25–26. |
References
- Brugiatelli, M.; Bandini, G.; Barosi, G.; Lauria, F.; Liso, V.; Marchetti, M.; Mauro, F.R.; Meloni, G.; Zinzani, P.L.; Tura, S.; et al. Management of chronic lymphocytic leukemia: Practice guidelines from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Trans-plantation. Haematologica 2006, 91, 1662–1673. [Google Scholar] [PubMed]
- Mauro, F.R.; Bandini, G.; Barosi, G.; Billio, A.; Brugiatelli, M.; Cuneo, A.; Lauria, F.; Liso, V.; Marchetti, M.; Meloni, G.; et al. SIE, SIES, GITMO updated clinical recommendations for the management of chronic lymphocytic leukemia. Leuk. Res. 2012, 36, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Cuneo, A.; Marchetti, M.; Barosi, G.; Billio, A.; Brugiatelli, M.; Ciolli, S.; Laurenti, L.; Mauro, F.R.; Molica, S.; Montillo, M.; et al. Appropriate use of bendamustine in first-line therapy of chronic lymphocytic leukemia. Recommendations from SIE, SIES, GITMO Group. Leuk. Res. 2014, 38, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Collins, R.; Bowman, L.; Landray, M.; Peto, R. The Magic of Randomization versus the Myth of Real-World Evidence. N. Engl. J. Med. 2020, 382, 674–678. [Google Scholar] [CrossRef]
- Mwamburi, M.; Dalal, H.; Gala, S. Trends in Research Using Observational Methodologies in Chronic Lymphocytic Leukemia (CLL): A Systematic Literature Review. Value Health 2016, 19, A760. [Google Scholar] [CrossRef]
- Islam, P.; Mato, A.R. Utilizing Real-World Evidence (RWE) to Improve Care in Chronic Lymphocytic Leukemia: Challenges and Opportunities. Curr. Hematol. Malign-Rep. 2020, 15, 254–260. [Google Scholar] [CrossRef]
- Moreno, C.; Montillo, M.; Panayiotidis, P.; Dimou, M.; Bloor, A.; Dupuis, J.; Schuh, A.; Norin, S.; Geisler, C.; Hillmen, P.; et al. Ofatumumab in poor-prognosis chronic lymphocytic leukemia: A Phase IV, non-interventional, observational study from the European Research Initiative on Chronic Lymphocytic Leukemia. Haematologica 2015, 100, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Vitale, C.; Griggio, V.; Todaro, M.; Salvetti, C.; Boccadoro, M.; Coscia, M. Magic pills: New oral drugs to treat chronic lym-phocytic leukemia. Expert Opin. Pharmacother. 2017, 18, 411–425. [Google Scholar] [CrossRef]
- Munir, T.; Brown, J.R.; O’Brien, S.; Barrientos, J.C.; Barr, P.M.; Reddy, N.M.; Coutre, S.; Tam, C.S.; Mulligan, S.P.; Jaeger, U.; et al. Final analysis from RESONATE: Up to six years of follow-up on ibrutinib in patients with previously treated chronic lymphocytic leukemia or small lymphocytic lymphoma. Am. J. Hematol. 2019, 94, 1353–1363. [Google Scholar] [CrossRef] [Green Version]
- Burger, J.A.; Barr, P.M.; Robak, T.; Owen, C.; Ghia, P.; Tedeschi, A.; Bairey, O.; Hillmen, P.; Coutre, S.E.; Devereux, S.; et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020, 34, 787–798. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.; Greil, R.; Demirkan, F.; Tedeschi, A.; Anz, B.; Larratt, L.; Simkovic, M.; Novak, J.; Strugov, V.; Gill, D.; et al. First-line treatment of chronic lymphocytic leukemia with ibrutinib plus obinutuzumab versus chlorambucil plus obinutuzumab: Final analysis of the randomized, phase 3 iLLUMINATE trial. Haematologica 2022. Epub ahead of print. [Google Scholar] [CrossRef]
- Woyach, J.A.; Ruppert, A.S.; Heerema, N.A.; Zhao, W.; Booth, A.M.; Ding, W.; Bartlett, N.; Brander, D.M.; Barr, P.M.; Rogers, K.A.; et al. Ibrutinib Regimens versus Chemoimmunotherapy in Older Patients with Untreated CLL. N. Engl. J. Med. 2018, 379, 2517–2528. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, T.D.; Wang, X.V.; Kay, N.E.; Hanson, C.A.; O’Brien, S.; Barrientos, J.; Jelinek, D.F.; Braggio, E.; Leis, J.F.; Zhang, C.C.; et al. Ibrutinib-Rituximab or chemoimmunotherapy for chronic lymphocytic leukemia. N. Engl. J. Med. 2019, 381, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Gibbons, J.L.; Ferrajoli, A. Targeted Treatment of Chronic Lymphocytic Leukemia: Clinical Utility of Acalabrutinib. OncoTargets Ther. 2021, 14, 5507–5519. [Google Scholar] [CrossRef] [PubMed]
- Byrd, J.C.; Harrington, B.; O’brien, S.; Jones, J.A.; Schuh, A.; Devereux, S.; Chaves, J.; Wierda, W.G.; Awan, F.T.; Brown, J.R.; et al. Acalabrutinib (ACP-196) in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 323–332. [Google Scholar] [CrossRef] [Green Version]
- Dickerson, T.; Wiczer, T.; Waller, A.; Philippon, J.; Porter, K.; Haddad, D.; Guha, A.; Rogers, K.A.; Bhat, S.; Byrd, J.C.; et al. Hypertension and incident cardiovascular events following ibrutinib initiation. Blood 2019, 134, 1919–1928. [Google Scholar] [CrossRef]
- Kumar, P.S.; Wiczer, T.; Rosen, L.; Pollauf, A.J.; Zheng, A.; Palettas, M.; Azali, L.; Bhat, S.A.; Byrd, J.C.; Rogers, K.; et al. Evaluation of the incidence and risk factors associated with bleeding events in pa-tients receiving acalabrutinib therapy. Blood 2021, 138 (Suppl. S1), 3729. [Google Scholar] [CrossRef]
- Roberts, A.W.; Davids, M.S.; Pagel, J.M.; Kahl, B.S.; Puvvada, S.D.; Gerecitano, J.F.; Kipps, T.J.; Anderson, M.A.; Brown, J.R.; Gressick, L.; et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N. Engl. J. Med. 2016, 374, 311–322. [Google Scholar] [CrossRef]
- Kater, A.P.; Wu, J.Q.; Kipps, T.; Eichhorst, B.; Hillmen, P.; D’rozario, J.; Assouline, S.; Owen, C.; Robak, T.; de la Serna, J.; et al. Venetoclax Plus Rituximab in Relapsed Chronic Lymphocytic Leukemia: 4-Year Results and Evaluation of Impact of Genomic Complexity and Gene Mutations from the MURANO Phase III Study. J. Clin. Oncol. 2020, 38, 4042–4054. [Google Scholar] [CrossRef]
- Al-Sawaf, O.; Zhang, C.; Tandon, M.; Sinha, A.; Fink, A.M.; Robrecht, S.; Samoylova, O.; Liberati, A.M.; Pinilla-Ibarz, J.; Opat, S.; et al. Venetoclax plus obinutuzumab versus chlorambucil plus obinutuzumab for pre-viously untreated chronic lymphocytic leukaemia (CLL14): Follow-up results from a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1188–1200. [Google Scholar] [CrossRef]
- Goede, V.; Fischer, K.; Busch, R.; Engelke, A.; Eichhorst, B.; Wendtner, C.-M.; Chagorova, T.; DE LA Serna, J.; Dilhuydy, M.-S.; Illmer, T.; et al. Obinutuzumab plus Chlorambucil in Patients with CLL and Coexisting Conditions. N. Engl. J. Med. 2014, 370, 1101–1110. [Google Scholar] [CrossRef] [Green Version]
- Goede, V.; Fischer, K.; Engelke, A.; Schlag, R.; Lepretre, S.; Montero, L.F.C.; Montillo, M.; Fegan, C.; Asikanius, E.; Humphrey, K.; et al. Obinutuzumab as frontline treatment of chronic lymphocytic leukemia: Updated results of the CLL11 study. Leukemia 2015, 29, 1602–1604. [Google Scholar] [CrossRef] [PubMed]
- Sharman, J.P.; Coutre, S.E.; Furman, R.R.; Cheson, B.D.; Pagel, J.M.; Hillmen, P.; Barrientos, J.C.; Zelenetz, A.D.; Kipps, T.J.; Flinn, I.W.; et al. Final Results of a Randomized, Phase III Study of Rituximab with or without Idelalisib Followed by Open-Label Idelalisib in Patients with Relapsed Chronic Lymphocytic Leukemia. J. Clin. Oncol. 2019, 37, 1391–1402. [Google Scholar] [CrossRef] [PubMed]
- Bird, S.T.; Tian, F.; Flowers, N.; Przepiorka, D.; Wang, R.; Jung, T.-H.; Kessler, Z.; Woods, C.; Kim, B.; Miller, B.W.; et al. Idelalisib for treatment of relapsed follicular lymphoma and chronic lymphocytic leuke-mia: A comparison of treatment outcomes in clinical trial participants vs Medicare beneficiaries. JAMA Oncol. 2020, 6, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Eyre, T.A.; Osborne, W.L.; Gallop-Evans, E.; Ardeshna, K.M.; Kassam, S.; Sadullah, S.; Sidra, G.; Culligan, D.; Arumainathan, A.; Shankara, P.; et al. Results of a multicenter UK-wide compassionate use programme evaluating the efficacy of idelalisib monotherapy in relapsed, refractory follicular lymphoma. Br. J. Haematol. 2018, 181, 555–559. [Google Scholar] [CrossRef]
- Schetlelig, J.; Chevallier, P.; van Gelder, M.; Hoek, J.; Hermine, O.; Chakraverty, R.; Browne, P.; Milpied, N.; Malagola, M.; Socié, G.; et al. Idelalisib treatment prior to allogeneic stem cell transplantation for patients with chronic lymphocytic leukemia: A report from the EBMT chronic malignancies working party. Bone Marrow Transplant. 2021, 56, 605–613. [Google Scholar] [CrossRef]
- Vitale, C.; Salvetti, C.; Griggio, V.; Scamuffa, M.C.; Zamprogna, G.; Visentin, A.; Cassin, R.; Laurenti, L.; Murru, R.; Rivela, P.; et al. Pre-existing and treatment-emergent autoimmune cytopenias in patients with chron-ic lymphocytic leukemia treated with targeted drugs. Blood 2021, 137, 3507–3517. [Google Scholar] [CrossRef]
- Pula, B.; Budziszewska, B.K.; Rybka, J.; Gil, L.; Subocz, E.; Długosz-Danecka, M.; Zawirska, D.; Waszczuk-Gajda, A.; Iskierka-Jażdżewska, E.; Kopacz, A.; et al. Comparable efficacy of idelalisib plus rituximab and ibrutinib in relapsed/refractory chronic lymphocytic leukemia: A retrospective case matched study of the Polish Adult Leukemia Group (PALG). Anticancer. Res. 2018, 38, 3025–3030. [Google Scholar]
- Zinzani, P.L.; Rambaldi, A.; Gaidano, G.; Girmenia, C.; Marchetti, M.; Pane, F.; Tura, S.; Barosi, G. Infection control in patients candidate to treatment with ibrutinib or idelalisib in chronic lymphocytic leukemia: Recommendations from Italian Society of Hematology. Leuk. Res. 2019, 81, 88–94. [Google Scholar] [CrossRef]
- Cuneo, A.; Follows, G.; Rigolin, G.M.; Piciocchi, A.; Tedeschi, A.; Trentin, L.; Perez, A.M.; Coscia, M.; Laurenti, L.; Musuraca, G.; et al. GIMEMA, European Research Initiative on CLL (ERIC) and UK CLL forum. Effi-cacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: A GIMEMA, ERIC and UK CLL FORUM study. Haematologica 2018, 103, 1209–1217. [Google Scholar] [CrossRef] [Green Version]
- Cuneo, A.; Mato, A.R.; Rigolin, G.M.; Piciocchi, A.; Gentile, M.; Laurenti, L.; Allan, J.N.; Pagel, J.M.; Brander, D.M.; Hill, B.T.; et al. Efficacy of bendamustine and rituximab in unfit patients with previously untreated chronic lymphocytic leukemia. Indirect comparison with ibrutinib in a real-world setting. A GIMEMA-ERIC and US study. Cancer Med. 2020, 9, 8468–8479. [Google Scholar] [CrossRef]
- Rigolin, G.M.; Cavazzini, F.; Piciocchi, A.; Arena, V.; Visentin, A.; Reda, G.; Zamprogna, G.; Cibien, F.; Vitagliano, O.; Coscia, M.; et al. Efficacy of idelalisib and Rituximab in relapsed/refractory chronic lympho-cytic leukemia treated outside of clinical trials. A report of the GIMEMA Working Group. Hematol. Oncol. 2021, 39, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Chatzikonstantinou, T.; Scarfo, L.; Demonsthenous, C.; Kotaskova, J.; Iacoboni, G.; Minga, E.; Chammou, D.; Karakatsoulis, G.; Albi, E.; Alcoceba, M.; et al. Real-world evidence on therapeutic strategies and treatment-sequencing in patients with chronic lymphocytic leukemia: An international study of ERIC, the European Research Initiative on CLL. Blood 2021, 138, 2635. [Google Scholar] [CrossRef]
- Scarfo, L.; Albi, E.; Quaglia, F.M.; Marasca, R.; Sanna, A.; Murru, R.; Laurenti, L.; Gaidano, G.; Mannina, D.; Gentile, M.; et al. An observational study on patients with relapsed/refractory chronic lymphocytic leu-kemia treated with venetoclax-based regimens outside clinical trials in Italy (GIMEMA CLL1920). Blood 2021, 138, 3746. [Google Scholar] [CrossRef]
- Pacheco-Paez, T.; Conte, C.; Rousseau, V.; Chebane, L.; Ysebaert, L.; Levy, V.; Montastruc, J.; Despas, F. Cardiovascular adverse drug reactions of ibrutinib, idelalisib, acalabrutinib, and venetoclax used in chronic lymphocytic leukemia: Systematic review, meta-analysis and Signal detection by dispropor-tionality analysis from VigiBase®. Fundament. Clin. Pharmacol. 2021, 35 (Suppl. S1), 38–39. [Google Scholar]
- Morabito, F.; Tripepi, G.; Del Poeta, G.; Mauro, F.R.; Reda, G.; Sportoletti, P.; Laurenti, L.; Coscia, M.; Herishanu, Y.; Bossio, S.; et al. Comparison of ibrutinib and idelalisib plus rituximab in real-life relapsed/refractory chronic lymphocytic leukemia cases. Eur. J. Haematol. 2021, 106, 493–499. [Google Scholar] [CrossRef]
- Weymann, D.; Costa, S.; Regier, D.A. Validation of a Cyclic Algorithm to Proxy Number of Lines of Systemic Cancer Therapy Using Administrative Data. JCO Clin. Cancer Inform. 2019, 3, 1–10. [Google Scholar] [CrossRef]
| Purposes of RWS before Drug Authorization |
|---|
| To describe the characteristics of the target population |
| To record the incidence of disease outcomes in the clinical practice |
| To identify the determinants of disease outcomes in the clinical practice |
| To provide information on standards of care |
| Purposes of RWS after Drug Authorization |
| To confirm safety and effectiveness in the target population (i.e., phase IV studies) |
| To confirm safety in subpopulations (i.e., comorbid patients) |
| To survey modified patterns of care and health-care resource consumption |
| All | Ibrutinib | Idelalisib | Venetoclax | CIT | Acalabrutinib | |
|---|---|---|---|---|---|---|
| Study number | 117 | 62 | 16 | 7 | 28 ^ | 4 |
| Registry | 34 (29%) | 18 | 3 | 0 | 13 | - |
| NPP/EAP | 13 (11%) | 10 | 2 | 1 | - | - |
| Electronic record database | 33 (28%) | 28 | 2 | 0 | 2 | 1 |
| Retrospective data collection | 70 (60%) | 45 | 5 | 6 | 11 | 3 |
| Multicenter | 52 (44%) | 21 | 10 | 5 | 15 | 1 |
| Europe | 40 (45%) | 25 | 11 | 4 | na | - |
| US/international | 41 (46%) | 29 | 5 | 3 | na | 4 |
| No explicit patient selection | 52 (63%) | 37 | 12 | na | na | 3 |
| Selectively naiive patients | 27 (23%) | 8 | 0 | 0 | 18 | 1 |
| Full papers | 40 (34%) | 15 | 5 | 2 | 17 | 1 |
| Studies Reporting: | All | Ibrutinib | Acalabrutinib | Venetoclax | Idelalisib | CIT |
|---|---|---|---|---|---|---|
| Number of treated patients | 113 (96%) | 58 (93%) | 4 (100%) | 7 (100%) | 16 (100%) | 28 (100%) |
| Patients’ age | 90 (77%) | 48 (77%) | 4 (100%) | 5 (71%) | 10 (62%) | 25 (89%) |
| Number of treatment lines | 61 (52%) | 21 (34%) | 2 (50%) | 5 (71%) | 9 (56%) | 24 (86%) |
| Rai/Binet stage | 44 (37%) | 17 (27%) | 1 (25%) | 2 (28%) | 6 (37%) | 18 (64%) |
| Median time from diagnosis | 11 (9%) | 8 (13%) | 1 (25%) | 0 | 2 (12%) | na |
| Comorbidity | 23 (20%) | 7 (11%) | 2 (50%) | 1 (14%) | 2 (12%) | 11 (39%) |
| TP53 status | 67 (57%) | 32 (52%) | 2 (50%) | 5 (71%) | 7 (44%) | 21 (75%) |
| Other high-risk molecular or cytogenetic features | 61 (52%) | 33 (53%) | 2 (50% | 5 (71%) | 2 (12%) | 19 (67%) |
| Median follow-up | 78 (66%) | 38 (61%) | 2 (50%) | 4 (57%) | 8 (50%) | 26 (93%) |
| Discontinuation rate | 31 (26%) | 13 (21%) | 2 (50%) | 2 (28%) | 9 (56%) | 5 (18%) |
| Response rate | 47 (40%) | 20 (32%) | 2 (50%) | 6 (86%) | 6 (37%) | 13 (46%) |
| Richter transformation | 10 (8%) | 6 (10%) | 0 | 2 (28%) | 2 (12%) | na |
| PFS | 52 (44%) | 22 (35%) | 1 (25%) | 4 (57%) | 4 (25%) | 21 (75%) |
| OS | 61 (52%) | 25 (40%) | 1 (25%) | 5 (71%) | 8 (50%) | 22 (78%) |
| TFS or TTNT | 16 (14%) | 2 (3%) | 0 | 0 | 2 (12%) | 12 (43%) |
| SPM | 12 (10%) | 2 (3%) | 1 (25%) | 0 | 1 (6%) | 8 (28%) |
| Specific adverse events ^ | 12 (10%) | 4 (6%) | 1 (25%) | 3 (57%) | 4 (25%) | 0 |
| Ibrutinib | Idelalisib | Venetoclax | CIT | Acalabrutinib | |
|---|---|---|---|---|---|
| Patient number: Mean, median, inter-quartile range | 486 179 89–554 | 89 74 54–104 | 107 76 67–149 | 532 277 174–817 | 149 136 33–290 |
| Age: Median years, inter-quartile range | 69 65–70 | 72 67–74 | 68 67–69 | 70 68–75 | 68 64–71 |
| Number of prior treatment lines: Median, inter-quartile range | 2 0–3 | 3 1–4 | 3.5 3–4 | 0 0–1 | 4 (1 study) |
| Follow-up (mo): Median, inter-quartile range | 16 9–21 | 16 7–19 | 13 9–17 | 37 25–57 | 12 5–19 |
| Discontinuation rate: Range | 23–41% | 63–100% | 7–27% | 2–30% | 19–30% |
| Overall Response Rate: Median, inter-quartile range | 77% 73–84% | 79% 65–86% | 74% 72–75% | 83% 76–91% | 61% 60–62% |
| Complete Response Rate: Median, Inter-quartile range | 17% 11–18% | 14% 1 study | 25% 23–28% | 30% 19–55% | 6% 3–9% |
| PFS: Median, range, | 38 na | na 10–36 | na na | 42 28–51 | na na |
| OS | na * | >36 | na | 74 | na |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, M.; Vitale, C.; Rigolin, G.M.; Vasile, A.; Visentin, A.; Scarfò, L.; Coscia, M.; Cuneo, A. Old and New Drugs for Chronic Lymphocytic Leukemia: Lights and Shadows of Real-World Evidence. J. Clin. Med. 2022, 11, 2076. https://doi.org/10.3390/jcm11082076
Marchetti M, Vitale C, Rigolin GM, Vasile A, Visentin A, Scarfò L, Coscia M, Cuneo A. Old and New Drugs for Chronic Lymphocytic Leukemia: Lights and Shadows of Real-World Evidence. Journal of Clinical Medicine. 2022; 11(8):2076. https://doi.org/10.3390/jcm11082076
Chicago/Turabian StyleMarchetti, Monia, Candida Vitale, Gian Matteo Rigolin, Alessandra Vasile, Andrea Visentin, Lydia Scarfò, Marta Coscia, and Antonio Cuneo. 2022. "Old and New Drugs for Chronic Lymphocytic Leukemia: Lights and Shadows of Real-World Evidence" Journal of Clinical Medicine 11, no. 8: 2076. https://doi.org/10.3390/jcm11082076
APA StyleMarchetti, M., Vitale, C., Rigolin, G. M., Vasile, A., Visentin, A., Scarfò, L., Coscia, M., & Cuneo, A. (2022). Old and New Drugs for Chronic Lymphocytic Leukemia: Lights and Shadows of Real-World Evidence. Journal of Clinical Medicine, 11(8), 2076. https://doi.org/10.3390/jcm11082076










