Abstract
Several new drugs are progressively improving the life span of patients with B-cell chronic lymphocytic leukemia (CLL). However, the rapidly evolving standard of care precludes robust assessments of the incremental clinical value of further innovative drugs. Therefore, we systematically reviewed comparative evidence on newly authorized CLL drugs, as reported by standard and network meta-analyses (MA) published since 2016. Overall, 17 MAs addressed the relative survival or safety of naïve and/or refractory/relapsed (R/R) CLL patients. In R/R patients, therapies including BTK- and BCL2-inhibitors reported progression free survival (PFS) hazard ratios ranging from 0.08 to 0.24 (versus chemotherapy) and a significant advantage in overall survival (OS). In naïve patients, the PFS hazard ratios associated with four recent chemo-free therapies (obinutuzumab- and/or acalabrutinib-based) ranged from 0.11 to 0.61 versus current standard treatments (STs), without a significant OS advantage. Ten MAs addressed the risk of cardiovascular, bleeding, and infective events associated with BTK inhibitors, with some reporting a different relative safety in naïve and R/R patients. In conclusion, last-generation therapies for CLL consistently increase PFS, but not OS, and minimally decrease safety, as compared with STs. Based on available evidence, the patient-customized adoption of new therapies, rather than universal recommendations, seems desirable in CLL patients.
1. Introduction
B-cell chronic lymphocytic leukemia (CLL) is a rare neoplasm, accounting for 1.2% of overall new cancer diagnoses. It has an indolent course and affects 56 per 100,000 people (https://seer.cancer.gov/statfacts/html/clyl.html, accessed on 30 January 2022). Moreover, 5-year overall survival (OS) has increased to over 86% and mortality has decreased to 1.1/100,000 patients per year in the last 20 years [1].
It is estimated that the recent approval of several novel drugs and combination treatments can potentially save further years lost, despite the old age at which CLL usually occurs [2]. Frontline treatment with BCL2 inhibitors (venetoclax) or BTK inhibitors (BTKis), possibly associated with anti-CD20 monoclonal agents—especially obinutuzumab (O)—is currently being proposed as the standard of care for most CLL patients, despite their molecular risk [3]. As a consequence of the widespread availability of such treatments, mean overall survival (OS) is expected to exceed 10 years and quality-adjusted life expectancy to be higher than 7.6 quality-adjusted life years [4]. Despite the clinical improvements associated with novel drugs, CLL-related drug expenditures have already increased by almost 10-fold [5], questioning the competitive value-for-cost of CLL therapies, especially continuous ones, as compared with other healthcare interventions. Moreover, side effects might change the clinical value of new drugs that are currently widely accepted in the real world. Finally, even though technology assessments of newly proposed treatments take into account different standard-of-care treatments, randomized trials usually include few treatments.
Conducting a systematic review of evidence and meta-analyses (MA) can help in translating wide volumes of clinical research to assist in decision making. Network meta-analyses (NMA) are particularly useful MAs that allow the indirect comparison of treatments with head-to-head studies by using the Bayesian inferential analyses of several chained studies. However, as an overwhelming number of MAs and NMAs have been reported in the last years, a systematic review method for published MAs/NMAs has been developed. “Umbrella reviews” represent the highest level of evidence synthesis currently available [6].
The present umbrella review aims to examine published MAs and NMAs in order to estimate the incremental clinical value of novel versus standard therapies for CLL.
2. Methods
We searched Embase (Excerpta Medica database), the largest bibliographic database, selecting MAs and NMAs published in the last 5 years comparing pharmacologic therapies for CLL. The following query was used: ‘Leukemia, Lymphocytic, Chronic, B-Cell’/exp AND (eta-analysis)/lim AND (2016–2021)/py AND (English)/lim. A PRISMA (Preferred Reported Items for Systematic Reviews and Meta Analyses) diagram of the literature retrieval and selection process is shown in Figure 1. The enquiry was performed on 25 September 2021. The major results of the selected studies were reported according to the standard patient intervention comparison outcomes (PICO) format. We referred to the “recent standards of care” when ibrutinib (single-agent or associated with rituximab) or obinutuzumab–chlorambucil were the comparators in naïve patients. We referred to the “old standards of care” when bendamustine/rituximab, fludarabine-based therapies, or chlorambucil—as a single agent or in association with rituximab—were proposed as comparators in naïve patients.
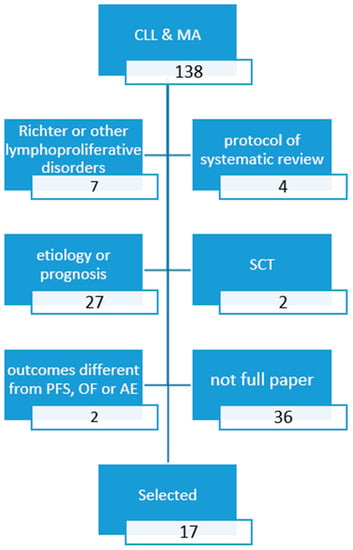
Figure 1.
PRISMA diagram search a): Fully published (article or review) meta-analyses since 2016.
From the selected studies, we retrieved data regarding OS, progression-free survival (PFS) and safety. More specifically, hazard ratios (HR) or risk ratios (RR), along with confidence or credible intervals (CI), were extracted.
3. Results
Overall, 138 records were retrieved: 13 fully published MAs reported PFS or OS (Table 1 and Table 2) [7,8,9,10,11,12,13,14,15,16,17,18,19] and 10 MAs reported safety outcomes (Table 3) [8,9,11,15,18,19,20,21,22,23].

Table 1.
Fully published MAs and NMAs since 2016: PFS and OS in naïve patients [7,8,9,10,11,12,13].

Table 2.
Fully published MAs and NMAs since 2016: PFS and OS in relapsed/refractory (R/R) patients [14,15,16,17,18,19].

Table 3.
Fully published MAs and NMAs since 2016: safety outcomes [8,9,11,15,18,19,20,21,22,23].
3.1. Relative Survival Benefits Associated with Novel Drugs in Naïve CLL Patients
Four MAs compared frontline BTKis with both the older and recent standards of care (Table 1). A sponsored NMA compared ibrutinib with nine treatments based on alkylating agents and reported the PFS HRs to be 0.16 (0.08–0.31) when compared with chlorambucil, 0.82 (0.35–1.88) when compared with obinutuzumab–chlorambucil, and 0.72 (0.32–1.61) when compared with fludarabine–cyclophosphamide–rituximab (FCR) [11]. A non-sponsored MA confirmed a favorable PFS of ibrutinib-based frontline therapies in IGVH-mutated and unmutated naïve patients, as well as in those carrying the 11q deletion [12]. A significant OS advantage was not demonstrated by the above study [12]; however, an NMA that included 15 studies reported superior OS with ibrutinib compared to single-agent alkylating agents (FCR) and the combination of chlorambucil with ofatumumab [11].
Three NMAs [7,8,9] assessed the comparative survival of acalabrutinib-based therapies versus several other treatment options; however, only one NMA [7] used current standard treatments among the comparators. As a result, acalabrutinib had a superior PFS, but not OS, when compared to obinutuzumab–chlorambucil (HR 0.26; 95% CI 0.10–0.27) and heterogeneous PFS HRs when compared to ibrutinib or ibrutinib–rituximab. In particular, PFS was not significantly ameliorated by acalabrutinib as compared to ibrutinib when cross-trial comparisons were included in the NMA.
Finally, three NMAs cross-compared the most recently proposed frontline therapies, namely acalabrutinib, acalabrutinib–obinutuzumab, ibrutinib–obinutuzumab, and venetoclax–obinutuzumab, following three randomized trials (ILLUMINATE, ELEVATE-TN, and CLL-14) [8,9,13]. The single agent acalabrutinib did not appear to significantly prolong PFS when compared to the obinutuzumab-based combinations with ibrutinib or venetoclax, while indirect comparisons consistently showed a superior expected PFS of acalabrutinib–obinutuzumab against obinutuzumab–venetoclax and obinutuzumab–ibrutinib. The acalabrutinib-based therapies showed a significant OS advantage in indirect comparison with chlorambucil and its combinations with rituximab or ofatumumab. However, the acalabrutinib-based therapies did not show an OS superior to that of the current standard treatments, namely ibrutinib or obinutuzumab–chlorambucil.
3.2. Relative Survival Benefits Associated with Novel Drugs in Refractory/Relapsed CLL Patients
Six MAs were devoted to R/R patients (Table 2); however, only two of them [17,18] compared BTK inhibitors to mixed comparators. The others either adopted the single agent ofatumumab as a comparator, which is a rarely used treatment, or addressed maintenance therapies, which are not reimbursed in most countries. Both PFS and OS were significantly enhanced by BTK inhibitors: the HR was 0.24 (0.19–0.30) for PFS and 0.58 (0.46–0.73) for OS. Rituximab–venetoclax was compared to rituximab–bendamustine or ofatumumab monotherapy in two MAs [14,16], which reported a considerable amelioration of both PFS and OS. In addition, the combinations of BTK inhibitors with rituximab and bendamustine were indirectly compared with rituximab–bendamustine and OS HRs of 0.20 (0.5–0.28) and 0.33 (0.25–0.44) were reported for ibrutinib-based and idelalisib-based combinations, respectively.
3.3. Safety of Novel Drugs in CLL Patients
Ten MAs analyzed the safety outcomes of different pharmacologic therapies for CLL patients (Table 3). Four MAs compared BTK inhibitors to mixed standards of care that did not include BTK inhibitors or BCL2 inhibitors; discontinuation due to adverse events (AE) was not significantly different. However, a considerable increased risk of infections (OR 1.24; 1.02–1.50) was reported in relapsed/refractory (R/R) patients. Conversely, a much lower risk of discontinuation was associated with ibrutinib in naïve patients that were not eligible for fludarabine therapy. The above MAs reported that in mixed populations including naïve and R/R patients, abdominal AE, arthralgia, any-grade bleeding, arterial hypertension, and atrial fibrillation, were higher with ibrutinib treatment, as compared to alkylating agents. Similarly, in two MAs with R/R CLL patients, besides a small portion of lymphomas, high-grade adverse events were higher and the risk ratio for major bleedings was 2.46 (1.37–4.41) in patients receiving ibrutinib.
No significantly lower risk of adverse events was reported when patients were given acalabrutinib or its combination with obinutuzumab versus obinutuzumab–venetoclax or obinutuzumab–ibrutinib.
3.4. Partially Reported Meta-Analyses
An additional search was performed to analyze MAs or NMAs reported at international meetings in the last two years that were not accessible as full texts. We retrieved six relevant NMAs [24,25,26,27,28,29]. Most of these meta-analyses addressed safety outcomes. In particular, the meta-analyses confirmed an increased risk of cardiovascular adverse events (a 3.7-fold increase) and ventricular arrhythmias (relative risk 8.13; 4.37–15.10) in patients treated with ibrutinib [24,27]. However, in VigiBase®, the relative risk of reporting (ROR) of cardiovascular adverse events was also higher in patients treated with venetoclax and idelalisib, and, more specifically, it was similarly high in patients treated with ibrutinib (ROR 3.06; 2.81–3.21) and acalabrutinib (ROR 2.66; 1.27–5.58) [24]. A more recent NMA that included 27 trials estimated a significant difference favoring acalabrutinib versus ibrutinib for arterial hypertension, atrial fibrillation, grade 3 arterial hypertension (OR 0.15, 95% CI 0.08–0.27; p < 0.0001), and grade 3 atrial fibrillation (OR 0.04, 95% CI 0.01–0.25; p = 0.0009) [25]. A meta-regression analysis indicated that the incidence of ventricular arrhythmias associated with ibrutinib exposure increased with a longer duration of treatment (coefficient = 0.0206, p = 0.049) and age (greater than 60 years; coefficient = 0.0237, p = 0.044) [27].
Non-cardiovascular adverse events related to ibrutinib were also analyzed [25]. Significant differences were reported in AE rates including headache (12% vs. 37%) and infections (35% vs. 57%) favoring ibrutinib versus acalabrutinib. However, an indirect NMA comparison revealed that grade 3 infections were significantly less frequent in patients treated with acalabrutinib (OR 0.62, 0.46–0.85; p = 0.003), after adjusting for age and median follow-up. Additionally, cytopenias and myalgias were reported less frequently in patients treated with acalabrutinib. Bleedings were not reported to be significantly higher in patients treated with ibrutinib versus acalabrutinib [25].
Three NMAs addressed survival outcomes by comparing obinutuzumab–acalabrutinib with other obinutuzumab-based therapies [26] and obinutuzumab–venetoclax with other therapies [26,29]. By including seven and ten studies enrolling naïve CLL patients, respectively, two NMAs reported a superior outcome of obinutuzumab–venetoclax when compared to rituximab-based chemotherapy, and also rituximab–ibrutinib in unfit patients [29]. However, an indirect comparison in naïve fit CLL patients between obinutuzumab–venetoclax and obinutuzumab–ibrutinib did not show significantly different PFS [29].
4. Discussion
Both life expectancy and quality of life in patients with B-cell chronic lymphocytic leukemia (CLL) are progressively improving owing to an increasing number of safer and more effective pharmacologic therapies. However, the speed of new drug introduction has resulted in differences in the standard of care and compromised robust technology assessments. Moreover, as the incidence of CLL is higher than 5 in 10,000 people, high-cost therapies for CLL may pose significant budget hurdles for the healthcare system.
This review aimed to systematically retrieve a comparative evidence base for the approval and adoption of novel CLL drugs. NMAs allow for an indirect comparison of treatments that are not directly compared in clinical trials, owing to Bayesian networks. Despite their limitations, NMAs have become standard tools, which have also been approved by regulatory agencies, for comparing new treatments with composite comparators. Therefore, we conducted an umbrella analysis by analyzing MAs and NMAs published since 2016 that addressed biologic therapies for CLL, and retrieved 20 meta-analyses that were fully (17) or partially (three) published. We did not take into account those studies addressing intermediate outcomes, such as clinical or molecular responses [30] or cellular therapies [31]. Several of the retrieved MAs included a large list of comparators, ranging from the single-agent chlorambucil to venetoclax- or ibrutinib-based therapies. The information mined from the MAs, however, allowed us to highlight some consistent associations. In particular, acalabrutinib as a single agent and obinutuzumab in combination with BTK or BCL-2 inhibitors demonstrated significantly longer PFS than ibrutinib, ibrutinib–rituximab, and obinutuzumab–chlorambucil, which are the current standard of treatment for naïve patients. However, a significantly superior OS of the above frontline treatments was reported by uniquely comparing acalabrutinib-based therapies with chemotherapy not including obinutuzumab.
Our umbrella analysis also addressed the safety of drugs. Treatment with BTK inhibitors, namely ibrutinib, reported significantly higher rates of arterial hypertension, atrial fibrillation, major bleedings, and arthralgia than non-BTK-based therapies. In only one partially reported NMA, acalabrutinib showed better cardiovascular and infective outcomes than ibrutinib, but similar bleeding events.
Some limitations of the present umbrella analysis should be highlighted. Firstly, several retrospective comparisons among treatments for CLL were excluded from almost all the retrieved MAs. However, real-life studies are of paramount importance, since they also include a large portion of patients who are usually excluded from clinical trials because of comorbidities or concurrent neoplasms [32,33]. Moreover, very rare adverse events, such as immune cytopenias, can rarely be tracked by randomized studies [34]. Another important limitation is that many of the retrieved MAs were sponsored by pharmaceutical companies and company employees were included among the authors. This is a major conflict of interest which may have partially biased the results and should be carefully considered.
In conclusion, novel combination therapies for CLL consistently prolong PFS when compared to the current standard of care, while OS is improved only in comparison with old therapies. The safety advantages of acalabrutinib-based therapies still need to be fully exploited in naïve patients, compared to the current standard of care; however, they are expected to be favorable. Based on the present umbrella analysis, we can remind scientific societies elaborating clinical practice guidelines to highlight critical outcomes for each recommendation, according to the current standards for evidence-based evidence [35,36]. Furthermore, we expect that regulatory agencies agree with the stakeholders that comparators and major endpoints are valuable for approving new drugs for CLL. This is a heated debate since future therapies for pluri-relapsed and multi-refractory “difficult-to-treat” CLL patients include powerful cellular therapies, such as autologous CAR-T therapy. A recent systematic review of nine published studies (208 patients overall) reported cytokine release syndrome in 15% and neurotoxicity in 13% of the treated patients; however, a complete response was achieved in 40% of the treated patients [31]. While survival data are being collected, the appropriate threshold risk/benefit and cost/benefit ratios of innovative therapies for CLL should be further investigated [37,38,39].
Author Contributions
Conceptualization, M.M. and M.C.; methodology, M.M.; validation, P.R. and C.B.; formal analysis, M.M; investigation, M.M.; data curation, P.R.; writing—original draft preparation, M.M.; writing—review and editing, M.M., P.R. and M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We would like to acknowledge Samantha Farinelli for her extensive linguistic revision of the manuscript.
Conflicts of Interest
P.R., C.B. and M.C. have no conflict of interest to declare. M.M. received consultancy fees from Gilead Sciences SRL and speaker fees from Amgen.
References
- Weide, R.; Feiten, S.; Chakupurakal, G.; Friesenhahn, V.; Kleboth, K.; Köppler, H.; Lutschkin, J.; van Roye, C.; Thomalla, J.; Heymanns, J. Survival improvement of patients with chronic lymphocytic leukemia (CLL) in routine care 1995–2007. Leuk. Lymphoma 2020, 61, 557–566. [Google Scholar] [CrossRef]
- Lichtenberg, F.R. How many life-years have new drugs saved? A three-way fixed-effects analysis of 66 diseases in 27 countries, 2000–2013. Int. Health 2019, 11, 403–416. [Google Scholar] [CrossRef]
- NCCN Guidelines for Professionals. Chronic Lymphoytic Leukemia/Small Lymphocytic Lymphoma. Vers 2.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/cll.pdf (accessed on 27 January 2022).
- Singh, M.; Mealing, S.; Baculea, S.; Cote, S.; Whelan, J. Impact of novel agents on patient-relevant outcomes in patients with previously untreated chronic lymphocytic leukemia who are not eligible for fludarabine-based therapy. J. Med. Econ. 2017, 20, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- Mow, E.; Keech, J.; Naipaul, R.; Beca, J.M.; Gavura, S.; Kouroukis, C.T. Impact of novel chronic lymphocytic leukemia drugs on public spending. J. Clin. Oncol. 2018, 36 (Suppl. S1), 103. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid.-Based. Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef]
- Davids, M.S.; Waweru, C.; le Nouveau, P.; Padhiar, A.; Gautamjeet, S.; Adhyankar, S.; Leblod, V. Comparative efficacy of acalabrutinib in frontline treatment of chronic lymphocytic leukemia: A systematic review and network meta-analysis. Clin. Ther. 2020, 42, 1955–1974. [Google Scholar] [CrossRef]
- Molica, S.; Giannarelli, D.; Montserrat, E. Comparison between Venetoclax-based and Bruton Tyrosine Kinase Inhibitor-based Therapy as Upfront Treatment of Chronic Lymphocytic Leukemia (CLL): A Systematic Review and Network Meta-analysis. Clin. Lymphoma Myeloma Leuk. 2020, 21, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.; Song, S.; Yu, M.; Zhu, H.; Gao, A.; Gao, W.; Ran, X.; Huo, D. Comparison of acalabrutinib plus obinutuzumab, ibrutinib plus obinutuzumab and venetoclax plus obinutuzumab for untreated CLL: A network meta-analysis. Leuk. Lymphoma 2020, 61, 3432–3439. [Google Scholar] [CrossRef]
- Städler, N.; Shang, A.; Bosch, F.; Briggs, A.; Goede, V.; Berthier, A.; Renaudin, C.; Leblond, V. A Systematic Review and Network Meta-Analysis to Evaluate the Comparative Efficacy of Interventions for Unfit Patients with Chronic Lymphocytic Leukemia. Adv. Ther. 2016, 33, 1814–1830. [Google Scholar] [CrossRef]
- Xu, Y.; Fahrbach, K.; Dorman, E.; Baculea, S.; Côté, S.; van Sanden, S.; Diels, J. Front-line treatment of patients with chronic lymphocytic leukemia: A systematic review and network meta-analysis. J. Comp. Eff. Res. 2018, 7, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Baumann, T.; Montserrat, E. Ibrutinib as initial therapy in chronic lymphocytic leukemia: A systematic review and meta-analysis. Eur. J. Haematol. 2020, 104, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Shapouri, S.; Manzoor, B.S.; Ravelo, A.; Sail, K.; Qendri, V.; van de Wetering, G.; Davids, M.S. Cost-effectiveness of a 12-month fixed-duration venetoclax treatment in combination with obinutuzumab in first-line, unfit chronic lymphocytic leukemia in the United States. J. Manag. Care Spéc. Pharm. 2021, 27, 1532–1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-H.; Ho, C.-L.; Lin, C.; Wu, Y.-Y.; Huang, T.-C.; Tu, Y.-K.; Lee, C.-H. Treatment Outcomes of Novel Targeted Agents in Relapse/Refractory Chronic Lymphocytic Leukemia: A Systematic Review and Network Meta-Analysis. J. Clin. Med. 2019, 8, 737. [Google Scholar] [CrossRef]
- Lee, C.-H.; Chen, P.-H.; Lin, C.; Wang, C.-Y.; Ho, C.-L. A network meta-analysis of maintenance therapy in chronic lymphocytic leukemia. PLoS ONE 2020, 15, e0226879. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Shanafelt, T.D. Comparison of venetoclax plus rituximab with B-cell receptor inhibitors in patients with relapsed/refractory chronic lymphocytic leukemia: A systematic review and network Meta-analysis. Leuk. Lymphoma 2019, 61, 955–958. [Google Scholar] [CrossRef] [PubMed]
- Molica, S.; Giannarelli, D.; Mirabelli, R.; Levato, L.; Shanafelt, T.D. The magnitude of improvement in progression-free survival with targeted therapy in relapsed/refractory chronic lymphocytic leukemia based on prognostic risk category: A systematic review and meta-analysis. Leuk. Lymphoma 2018, 60, 1644–1649. [Google Scholar] [CrossRef]
- Puła, A.; Stawiski, K.; Braun, M.; Iskierka-Jażdżewska, E.; Robak, T. Efficacy and safety of B-cell receptor signaling pathway inhibitors in relapsed/refractory chronic lymphocytic leukemia: A systematic review and meta-analysis of randomized clinical trials. Leuk. Lymphoma 2017, 59, 1084–1094. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, Y.; Gu, Y.; Xia, J.; Kong, X.; Qian, Q.; Hong, Y. Safety and efficacy of Ofatumumab in chronic lymphocytic leukemia: A systematic review and meta-analysis. Hematology 2017, 22, 578–584. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ball, S.; Das, A.; Vutthikraivit, W.; Edwards, P.J.; Hardwicke, F.; Short, N.J.; Borthakur, G.; Maiti, A. Risk of Infection Associated with Ibrutinib in Patients with B-Cell Malignancies: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Clin. Lymphoma Myeloma Leuk. 2020, 20, 87–97.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Lu, H.; Yang, M.; Xu, C. Adverse drug events associated with ibrutinib for the treatment of elderly patients with chronic lymphocytic leukemia. A systematic review and meta-analysis of randomized trials. Medicine 2019, 98, e16915. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhao, A.; Zhou, H.; Zhu, J.; Niu, T. Risk of Bleeding Associated with Ibrutinib in Patients with B-Cell Malignancies: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Pharmacol. 2020, 11, 1731. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, D.; Alves, D.; Costa, J.; Ferreira, J.J.; Pinto, F.J. Ibrutinib increases the risk of hypertension and atrial fibrillation: Systematic review and meta-analysis. PLoS ONE 2019, 14, e0211228. [Google Scholar] [CrossRef]
- Pacheco-Paez, T.; Conte, C.; Rousseau, V.; Chebane, L.; Ysebaert, L.; Levy, V.; Montastruc, J.L.; Despas, F. Cardiovascular adverse drug reactions of ibrutinib, idelalisib, acalabrutinib, and venetoclax used in chronic lymphocytic leukemia: Systematic review-meta-analysis and Signal detection by disproportionality analysis from VigiBase®. Fund. Clin. Pharmacol. 2021, 35 (Suppl. 1), 38–39. [Google Scholar]
- Hilal, T.; Hillegass, W.B.; Gonzalez-Velez, M.; Leis, J.F.; Rosenthal, A.C. Adverse Events in Clinical Trials of Ibrutinib and Acalabrutinib for B-Cell Lymphoproliferative Disorders: A Systematic Review and Network Meta-Analysis. Blood 2020, 136, 23. [Google Scholar] [CrossRef]
- Coll Bastus, N.; Bavids, M.S.; Huntington, S.F.; Moreno, C.; Follows, G.; Cuneo, A.; Humpphrey, K.; Schary, W.; Sail, K.; Song, Y.; et al. Indirect treatment comparison analysis of venetoclax + obinutuzumab with standard front-line regimens for chronic lymphocytic leukaemia. Br. J. Haematol. 2020, 189 (Suppl. 1), 219–220. [Google Scholar]
- Khalid, Y.; Dasu, N.; Dasu, K.; Fradley, M.; Shah, A. Ventricular arrhythmias with ibrutinib use a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2021, 77, 3333. [Google Scholar] [CrossRef]
- A Haddad, P.; Ganey, N.; Gallagher, K.M. Comparative Efficacy of First-Line Chemotherapy-Free Combinations in Chronic Lymphocytic Leukemia (CLL): A Network Meta-Analysis. Blood 2020, 136, 25–26. [Google Scholar] [CrossRef]
- Sudhapalli, P.; Piena, M.; Palaka, A.; Mato, A.; van de Wetering, G.; Manzoor, B.; Sail, K. Systematic literature review and network meta-analysis comparing therapies for treatment naive patients with chronic lymphocytic leukemia. HemaSphere 2020, 4 (Suppl. 1), 320. [Google Scholar]
- Tang, X.; Zou, W.; Peng, P.; Bai, Y. Venetoclax alone or in combination with other regimens treatment achieve deep and sustained remission of relapsed/refractory chronic lymphocytic leukemia: A meta-analysis. Clin. Exp. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Habib RAiman, W.; Garg, I.; Niaz, R.; Butt, S.K.; Saeed, M.; Zubair, H.; Kashif, H.; Farrukh, M.; Naveed MTahir, N.; Fatima, A.; et al. Efficacy and safety of chimeric antigen receptor T cell therapy in chronic lymphocytic leukemia: A systematic review. Blood 2021, 138 (Suppl. 1), 4822. [Google Scholar]
- Cuneo, A.; Follows, G.; Rigolin, G.M.; Piciocchi, A.; Tedeschi, A.; Trentin, L.; Perez, A.M.; Coscia, M.; Laurenti, L.; Musuraca, G.; et al. Efficacy of bendamustine and rituximab as first salvage treatment in chronic lymphocytic leukemia and indirect comparison with ibrutinib: A GIMEMA, ERIC and UK CLL FORUM study. Haematologica 2018, 103, 1209–1217. [Google Scholar] [CrossRef]
- Marchetti, M.; Carobbio, A.; Capitoni, E.; Barbui, T. Lymphoproliferative disorders in patients with chronic myeloproliferative neoplasms. Am. J. Hematol. 2018, 93, 698–703. [Google Scholar] [CrossRef] [PubMed]
- Vitale, C.; Salvetti, C.; Griggio, V.; Porrazzo, M.; Schiattone, L.; Zamprogna, G.; Visentin, A.; Vassallo, F.; Cassin, R.; Rigolin, G.M.; et al. Preexisting and treatment-emergent autoimmune cytopenias in patients with CLL treated with targeted drugs. Blood 2021, 137, 3507–3517. [Google Scholar] [CrossRef] [PubMed]
- Brugiatelli, M.; Bandini, G.; Barosi, G.; Lauria, F.; Liso, V.; Marchetti, M.; Mauro, F.R.; Meloni, G.; Zinzani, P.L.; Tura, S. Management of chronic lymphocytic leukemia: Practice guidelines from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 2006, 91, 1662–1673. [Google Scholar]
- Zinzani, P.L.; Rambaldi, A.; Gaidano, G.; Girmenia, C.; Marchetti, M.; Pane, F.; Tura, S.; Barosi, G. Infection control in patients candidate to treatment with ibrutinib or idelalisib in chronic lymphocytic leukemia: Recommendations from Italian Society of Hematology. Leuk. Res. 2019, 81, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; Barosi, G.; Liberato, L.N. Fludarabine for chronic lymphocytic leukemia. N. Engl. J. Med. 2001, 344, 1166–1167. [Google Scholar]
- Marchetti, M.; Montillo, M.; Cuneo, A.; Mauro, F.R.; Martelli, E.; Pedone, M.P. Cost-effectiveness of idelalisib-rituximab for the treatment of relapsed-refractory chronic lymphocytic leukemia. Hematol. Int. J. 2017, 1, 000106. [Google Scholar] [CrossRef]
- Marchetti, M. Cost-effectiveness of kinase inhibitors for hematologic malignancies: A systematic and critical review. Expert Rev. Pharm. Outcomes Res. 2017, 17, 469–480. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).