An Exploratory Study of Itolizumab on the Preservation of Beta Cell Function in Adults with Recent-Onset Type 1 Diabetes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Study Design
2.2. Adverse Events
2.3. Physical Examination and Insulin Dose
2.4. Laboratory Measurements
2.5. Evaluations of the Outcome of the Trial
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2021. Diabetes Care 2021, 44, S15–S33. Available online: https://pubmed.ncbi.nlm.nih.gov/33298413/ (accessed on 22 November 2021). [CrossRef] [PubMed]
- Barnett, R. Type 1 diabetes. Lancet 2018, 391, 195. Available online: https://pubmed.ncbi.nlm.nih.gov/30277879/ (accessed on 22 November 2021). [CrossRef]
- Skyler, J.S. Hope vs. hype: Where are we in type 1 diabetes? Diabetologia 2018, 61, 509–516. Available online: https://pubmed.ncbi.nlm.nih.gov/29275427/ (accessed on 22 November 2021). [CrossRef] [Green Version]
- Steele, C.; Hagopian, W.A.; Gitelman, S.; Masharani, U.; Cavaghan, M.; Rother, K.I.; Donaldson, D.; Harlan, D.M.; Bluestone, J.; Herold, K.C. Insulin secretion in type 1 diabetes. Diabetes 2004, 53, 426–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherry, N.A.; Kushner, J.A.; Glandt, M.; Kitamura, T.; Brillantes, A.M.; Herold, K.C. Effects of autoimmunity and immune therapy on β-cell turnover in type 1 diabetes. Diabetes 2006, 55, 3238–3245. Available online: https://pubmed.ncbi.nlm.nih.gov/17130466/ (accessed on 22 November 2021). [CrossRef] [Green Version]
- Alonso, R.; Huerta, V.; de Leon, J.; Piedra, P.; Puchades, Y.; Guirola, O.; Chinea, G.; Montero, E. Towards the definition of a chimpanzee and human conserved CD6 domain 1 epitope recognized by T1 monoclonal antibody. Hybridoma 2008, 27, 291–301. Available online: https://pubmed.ncbi.nlm.nih.gov/18707547/ (accessed on 22 November 2021). [CrossRef]
- Hernández, P.; Moreno, E.; Aira, L.E.; Rodríguez, P.C. Therapeutic targeting of CD6 in autoinmune diseases: A review of Cuban clinical studies with the antibodies IOR-T1 and Itolizumab. Curr. Drug Targets 2016, 17, 666–677. Available online: https://pubmed.ncbi.nlm.nih.gov/26844560/ (accessed on 22 November 2021). [CrossRef]
- Nair, P.; Melarkode, R.; Rajkumar, D.; Montero, E. CD6 synergistic co-stimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin. Exp. Immunol. 2010, 162, 116–130. Available online: https://pubmed.ncbi.nlm.nih.gov/20726988/ (accessed on 20 July 2021). [CrossRef]
- Schneider, D.A.; Sarikonda, G.; Montero, E.; von Herrath, M.G. Combination therapy with anti-CD6 and oral insulin immunization reverses recent onset diabetes in non obese diabetic mice but fails to induce lasting tolerance. Clin. Immunol. 2013, 149, 440–441. Available online: https://pubmed.ncbi.nlm.nih.gov/24211845/ (accessed on 20 July 2021). [CrossRef]
- Physical Status: The Use of and Interpretation of Anthropometry, Report of a WHO Expert Committee; World Health Organization: Geneva, Switzerland, 1995; Available online: https://apps.who.int/iris/handle/10665/37003 (accessed on 20 July 2021).
- Cabrera-Rode, E.; Torres-López, Y.; Cubas-Dueñas, I.; Rodríguez-Acosta, J.; Vázquez-Izada, B.M.; Ruíz-Reinoso, M.; García-García, Y.; Prieto-Noa, C.; Echevarría-Valdés, R.; Álvarez-Álvarez, A.; et al. Utilidad de la prueba de tolerancia de comida mixta con Nutrial I para la evaluación de la función de las células beta en diabetes tipo 1. Rev. Cubana Endocrinol. 2020, 31, e205. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-29532020000100004&lng=es (accessed on 20 July 2021).
- Greenbaum, C.J.; Mandrup-Poulsen, T.; McGee, P.F.; Battelino, T.; Haastert, B.; Ludvigsson, J.; Pozzilli, P.; Lachin, J.M.; Kolb, H. Type 1 Diabetes Trial Net Research Group; European C-Peptide Trial Study Group. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 2008, 31, 1966–1971. Available online: https://pubmed.ncbi.nlm.nih.gov/18628574/ (accessed on 12 July 2021). [CrossRef] [PubMed] [Green Version]
- Besser, R.E.J.; Shields, B.M.; Casas, R.; Hatterley, A.T.; Ludvigsson, J. Lessons from the mixed-meal tolerance test: Use of 90-minute and fasting C-peptide in pediatric diabetes. Diabetes Care 2013, 36, 195–201. Available online: https://pubmed.ncbi.nlm.nih.gov/23111058/ (accessed on 20 July 2021). [CrossRef] [Green Version]
- Ruan, Y.; Willemsen, R.H.; Wilinska, M.E.; Tauschmann, M.; Dunger, D.B.; Hovorka, R. Mixed-meal tolerance test to assess residual beta-cell secretion: Beyond the area under-curve of plasma C-peptide concentration. Pediatr. Diabetes 2019, 20, 282–285. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6487945/ (accessed on 22 November 2021). [CrossRef] [PubMed] [Green Version]
- The Diabetes Control and Complications Trial Research Group. Influence of intensive diabetes treatment on body weight and composition of adults with type 1 diabetes in the diabetes control and complications Trial. Diabetes Care 2001, 24, 1711–1721. Available online: https://pubmed.ncbi.nlm.nih.gov/11574431/ (accessed on 12 July 2021). [CrossRef] [PubMed] [Green Version]
- Nansel, T.R.; Lipsky, L.M.; Iannotti, R.J. Cross-sectional and longitudinal relationships of body mass index with glycemic control in children and adolescents with type 1 diabetes mellitus. Diabetes Res. Clin. Pract. 2013, 100, 126–132. Available online: https://pubmed.ncbi.nlm.nih.gov/23339757/ (accessed on 12 July 2021). [CrossRef] [Green Version]
- Janež, A.; Guja, C.; Mitrakou, A.; Lalic, N.; Tankova, T.; Czupryniak, L.; Tabák, A.G.; Prazny, M.; Martinka, E.; Smircic-Duvnjak, L. Insulin therapy in adults with type 1 diabetes mellitus: A narrative review. Diabetes Ther. 2020, 11, 387–409. Available online: https://pubmed.ncbi.nlm.nih.gov/31902063/ (accessed on 22 November 2021). [CrossRef] [Green Version]
- Thomas, N.; Lynam, A.L.; Hill, A.V.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; McDonald, T.J.; Hattersley, A.T.; Jones, A.G. Type 1 diabetes defined by severe insulin deficiency occurs after 30 years of age and is commonly treated as type 2 diabetes. Diabetologia 2019, 62, 1167–1172. [Google Scholar] [CrossRef] [Green Version]
- Edqvist, J.; Rawshani, A.; Rawshani, A.; Adiels, M.; Franzén, S.; Bjorck, L.; Svensson, A.M.; Lind, M.; Sattar, N.; Rosengren, A. Trajectories in HbA1c and other risk factors among adults with type 1 diabetes by age at onset. BMJ. Open. Diab. Res. Care. 2021, 9, e002187. [Google Scholar] [CrossRef]
- Pai, G.; Pai, A.H. Itolizumab—A new biologic for management of Psoriasis and Psoriatic Arthritis. Case Rep. Dermatol. 2017, 9, 141–145. Available online: https://pubmed.ncbi.nlm.nih.gov/29033818/ (accessed on 22 November 2021). [CrossRef] [Green Version]
- Aira, L.E.; Hernández, P.; Prada, D.; Chico, A.; Gómez, J.A.; González, Z.; Fuentes, K.; Viada, C.; Mazorra, Z. Immunological evaluation of rheumatoid arthritis patients treated with itolizumab. mAbs 2016, 8, 187–195. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4966522/ (accessed on 22 November 2021). [CrossRef] [Green Version]
- Rodríguez, P.C.; Prada, D.M.; Moreno, E.; Aira, L.E.; Molinero, C.; López, A.M.; Gómez, J.A.; Hernández, I.M.; Martínez, J.P.; Reyes, Y.; et al. The anti-CD6 antibody itolizumab provides clinical benefit without lymphopenia in rheumatoid arthritis patients: Results from a 6-month, open-label Phase I clinical trial. Clin. Exp. Immunol. 2018, 191, 229–239. Available online: https://pubmed.ncbi.nlm.nih.gov/28963724/ (accessed on 22 November 2021). [CrossRef] [PubMed] [Green Version]
- Budamakuntla, L.; Madaiah, M.; Sarvajnamurthy, S.; Kapanigowda, S. Itolizumab provides sustained remission in plaque psoriasis: A 5-year follow-up experience. Clin. Exp. Dermatol. 2015, 40, 152–155. Available online: https://pubmed.ncbi.nlm.nih.gov/25495868/ (accessed on 20 July 2021). [CrossRef] [PubMed]
- Dogra, S.; Krupashankar, D.S.; Budamakuntla, L.; Srinivas, C.R.; Khopkar, U.; Gupta, S.; Shetty, N.; Pratap, D.V.S.; Gopal, M.G.; Rao, T.N.; et al. Long-term efficacy and safety of itolizumab in patients with moderate-to-severe chronic plaque psoriasis: A double-blind, randomized-withdrawal, placebo-controlled study. J. Am. Acad. Dermatol. 2015, 73, 331–333. Available online: https://pubmed.ncbi.nlm.nih.gov/26183983/ (accessed on 22 November 2021). [CrossRef] [PubMed]
- Aira, L.E.; López-Requena, A.; Fuentes, D.; Sánchez, L.; Pérez, T.; Urquiza, A.; Bautista, H.; Falcón, L.; Hernández, P.; Mazorra, Z. Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti-CD6 itolizumab. MAbs 2014, 6, 783–792. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4011922/ (accessed on 20 July 2021). [CrossRef] [PubMed] [Green Version]
- Navarrete-Cabrera, J.; Carvajal-Martínez, F.; Díaz-Díaz, O.; Domínguez-Alonso, E.; Cabrera-Benítez, E.; Villamil-Menéndez, Y. Caracterización clínica y epidemiológica de los pacientes menores de 15 años de edad con diabetes mellitus tipo 1. Rev. Cubana Endocrinol. 2012, 23, 30–43. Available online: http://scielo.sld.cu/scielo.php?script=sci_arttext&pid=S1561-29532012000100003&lng=es (accessed on 20 July 2021).
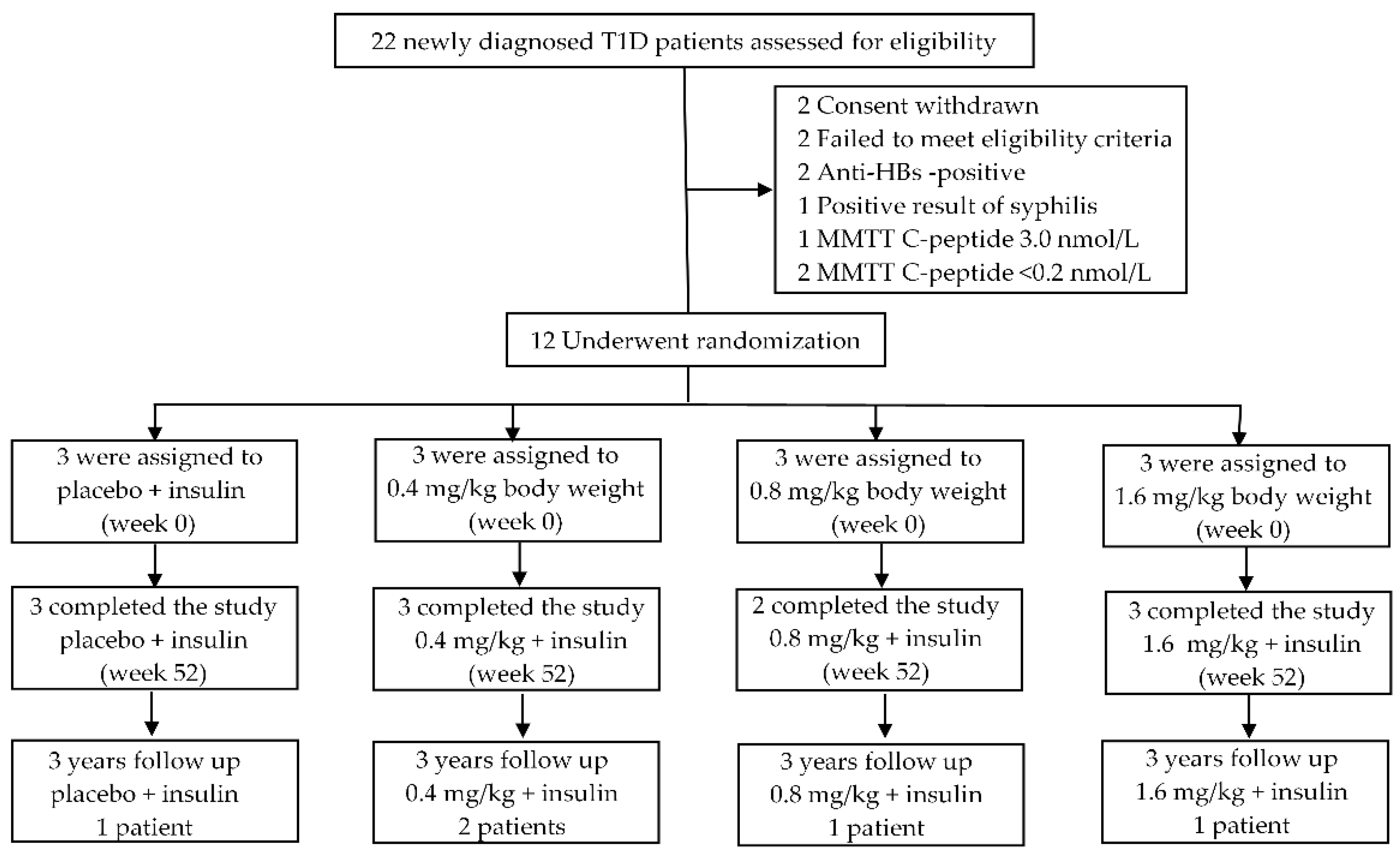
| Variable | Newly Diagnosed T1D Patients (n = 12) |
|---|---|
| Male (%) | 7 (58.3) |
| Autoantibodies (ICA and ZnT8A) (%) | 8 (66.7) |
| Age (years) | 24.08 ± 4.68 |
| Body mass index (kg/m2) | 18.40 (CI: 17.83–20.51) |
| Insulin dose (U/kg) | 0.55 (CI: 0.39–0.67) |
| HbA1c (%) | 7.85 (CI: 6.45–9.20) |
| Fasting blood glucose (mmol/L) | 7.05 (CI: 5.83–10.11) |
| Fasting blood C-peptide (nmol/L) | 0.36 (CI: 0.29–0.41) |
| AUC glucose (mmol × 120 min) | 1463.25 (CI: 1178.77–1797.23) |
| AUC C-peptide (nmol × 120 min) | 78.38 (CI: 53.21–110.56) |
| Total cholesterol (mmol/L) | 3.63 ± 0.60 |
| Triglycerides (mmol/L) | 0.97 ± 0.26 |
| HDL-c (mmol/L) | 1.21 ± 0.17 |
| Creatinine (µmol/L) | 64.25 ± 9.43 |
| Itolizumab mg/kg + Insulin | Sex | Autoantibodies (ICA and ZnT8A) | Insulin Dosage u/kg | HbA1c% | Fasting C-Peptide | AUC CP |
|---|---|---|---|---|---|---|
| 0 | F | − | 0.6 | 9.8 | 0.43 | 81.2 |
| M | + * | 0.7 | 9.7 | 0.25 | 47.9 | |
| M | + | 0.4 | 3.2 | 0.44 | 92.4 | |
| Mean ± SD | n = 2 | 0.6 ± 0.2 | 7.6 ± 3.8 | 0.37 ± 0.11 | 73.8 ± 23.2 | |
| 0.4 | F | + * | 0.80 | 10.7 | 0.48 | 60.0 |
| M | + * | 0.80 | 7.9 | 0.27 | 50.4 | |
| M | − | 0.30 | 7.8 | 0.3 | 193.5 | |
| Mean ± SD | n = 2 | 0.6 ± 0.3 | 8.8 ± 1.6 | 0.35 ± 0.11 | 101.3 ± 80.0 | |
| 0.8 | F | − | 0.70 | 8.6 | 0.42 | 89.6 |
| M | + | 0.70 | 9.9 | 0.24 | 33.3 | |
| F k | + * | 0.40 | 7.3 | 0.41 | 82.2 | |
| Mean ± SD | n = 2 | 0.6 ± 0.2 | 8.6 ± 1.3 | 0.36 ± 0.10 | 68.4 ± 30.6 | |
| 1.6 | M | + * | 0.50 | 7.0 | 0.43 | 136.7 |
| M | − | 0.10 | 6.9 | 0.30 | 75.6 | |
| F k | + | 0.40 | 5.1 | 0.24 | 40.05 | |
| Mean ± SD | n = 2 | 0.3 ± 0.2 | 6.3 ± 1.1 | 0.32 ± 0.10 | 84.1 ± 48.8 |
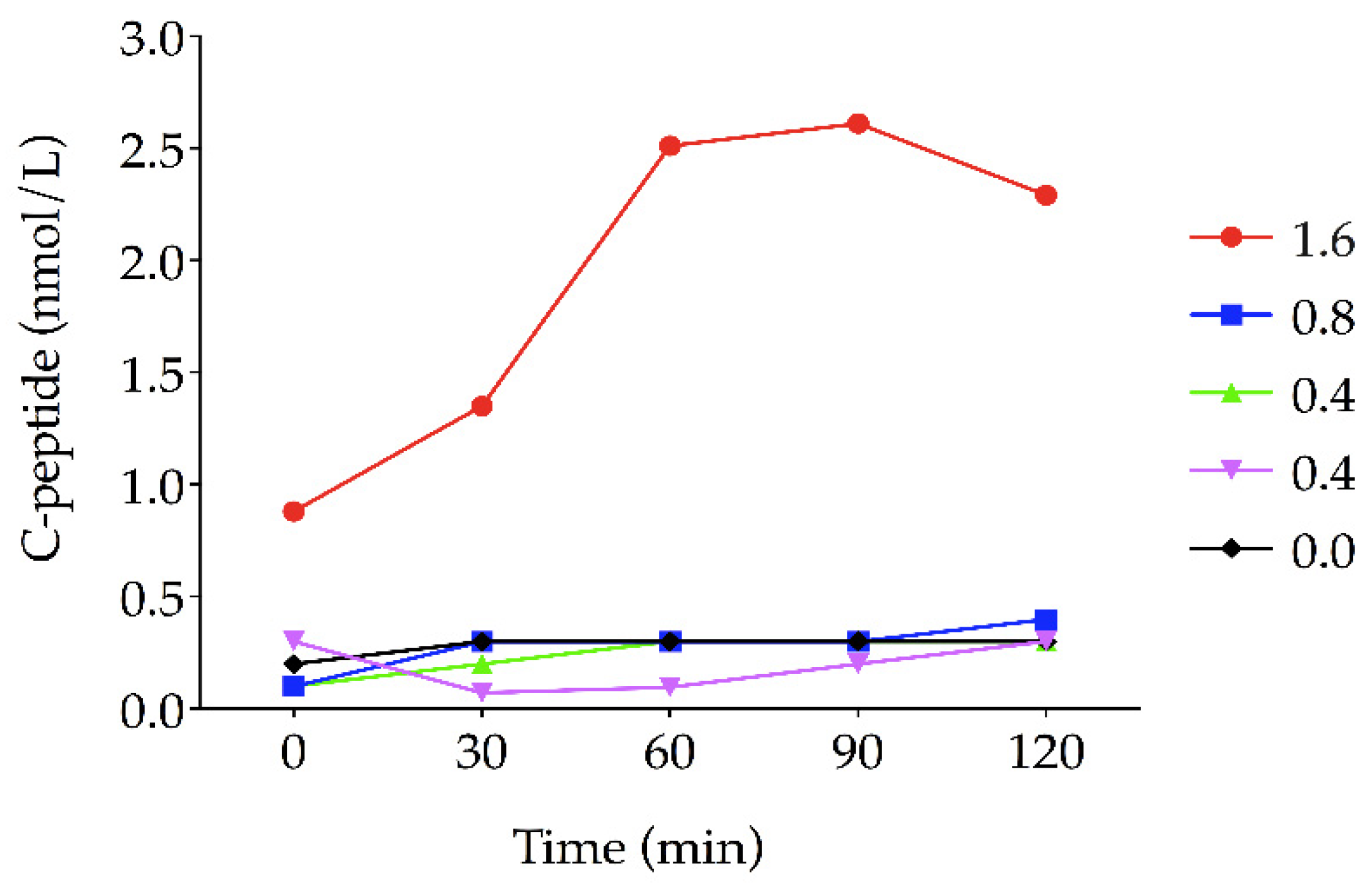
| Itolizumab Dose + Insulin | MMTT-Stimulated C-Peptide (nmol/L) | ||||
|---|---|---|---|---|---|
| 0 min | 30 min | 60 min | 90 min | 120 min | |
| 1.6 mg/kg | |||||
| Baseline | 0.43 | 1.08 | 1.4 | 1.44 | 0.84 |
| 6 months | 0.38 | 0.85 | 0.85 | 1.07 | 0.82 |
| 12 months | 0.24 | 1.08 | 0.91 | 0.6 | 0.72 |
| 36 months * | 0.88 | 1.35 | 2.51 | 2.61 | 2.29 |
| 0.8 mg/kg | |||||
| Baseline | 0.41 | 0.60 | 0.85 | 0.63 | 0.91 |
| 6 months | 0.38 | 0.38 | 0.71 | 0.93 | 0.85 |
| 12 months | 0.33 | 0.30 | 0.24 | 0.13 | 0.30 |
| 36 months * | 0.10 | 0.30 | 0.30 | 0.30 | 0.40 |
| 0.4 mg/kg | |||||
| Baseline | 0.48 | 0.31 | 0.57 | 0.59 | 0.58 |
| 6 months | 0.39 | 0.52 | 0.47 | 0.63 | 0.66 |
| 12 months | 0.38 | 0.41 | 0.41 | 0.74 | 0.74 |
| 36 months * | 0.10 | 0.20 | 0.30 | 0.30 | 0.30 |
| 0.4 mg/kg | |||||
| Baseline | 0.27 | 0.31 | 0.55 | 0.40 | 0.57 |
| 6 months | 0.22 | 0.33 | 0.41 | 0.60 | 0.60 |
| 12 months | 0.33 | 0.33 | 0.30 | 0.38 | 0.30 |
| 36 months * | 0.30 | 0.07 | 0.095 | 0.20 | 0.30 |
| 0.0 mg/kg | |||||
| Baseline | 0.25 | 0.36 | 0.44 | 0.45 | 0.44 |
| 6 months | 0.28 | 0.30 | 0.47 | 0.61 | 0.69 |
| 12 months | 0.08 | 0.11 | 0.25 | 0.33 | 0.30 |
| 36 months * | 0.20 | 0.30 | 0.30 | 0.30 | 0.30 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabrera-Rode, E.; Cubas-Dueñas, I.; Rodríguez-Acosta, J.; García-García, Y.; Torres-López, Y.; Prieto-Noa, C.; Vázquez-Izada, B.M.; Ruíz-Reinoso, M.; Echevarría-Valdés, R.; Álvarez-Álvarez, A.; et al. An Exploratory Study of Itolizumab on the Preservation of Beta Cell Function in Adults with Recent-Onset Type 1 Diabetes. J. Clin. Med. 2022, 11, 1789. https://doi.org/10.3390/jcm11071789
Cabrera-Rode E, Cubas-Dueñas I, Rodríguez-Acosta J, García-García Y, Torres-López Y, Prieto-Noa C, Vázquez-Izada BM, Ruíz-Reinoso M, Echevarría-Valdés R, Álvarez-Álvarez A, et al. An Exploratory Study of Itolizumab on the Preservation of Beta Cell Function in Adults with Recent-Onset Type 1 Diabetes. Journal of Clinical Medicine. 2022; 11(7):1789. https://doi.org/10.3390/jcm11071789
Chicago/Turabian StyleCabrera-Rode, Eduardo, Ileana Cubas-Dueñas, Janet Rodríguez-Acosta, Yudith García-García, Yelena Torres-López, Claudia Prieto-Noa, Bárbara M. Vázquez-Izada, Maité Ruíz-Reinoso, Ragmila Echevarría-Valdés, Aimee Álvarez-Álvarez, and et al. 2022. "An Exploratory Study of Itolizumab on the Preservation of Beta Cell Function in Adults with Recent-Onset Type 1 Diabetes" Journal of Clinical Medicine 11, no. 7: 1789. https://doi.org/10.3390/jcm11071789
APA StyleCabrera-Rode, E., Cubas-Dueñas, I., Rodríguez-Acosta, J., García-García, Y., Torres-López, Y., Prieto-Noa, C., Vázquez-Izada, B. M., Ruíz-Reinoso, M., Echevarría-Valdés, R., Álvarez-Álvarez, A., Domínguez-Alonso, E., Conesa-González, A. I., González-Calero, T., Robles-Torres, E., Turcios-Tristá, S. E., Senra-Estévez, E., Hernández-Casaña, P., & Sarmiento, L. (2022). An Exploratory Study of Itolizumab on the Preservation of Beta Cell Function in Adults with Recent-Onset Type 1 Diabetes. Journal of Clinical Medicine, 11(7), 1789. https://doi.org/10.3390/jcm11071789






