Sensory Profiles in School-Aged Children with Autism Spectrum Disorder: A Descriptive Study Using the Sensory Processing Measure-2 (SPM-2)
Abstract
1. Introduction
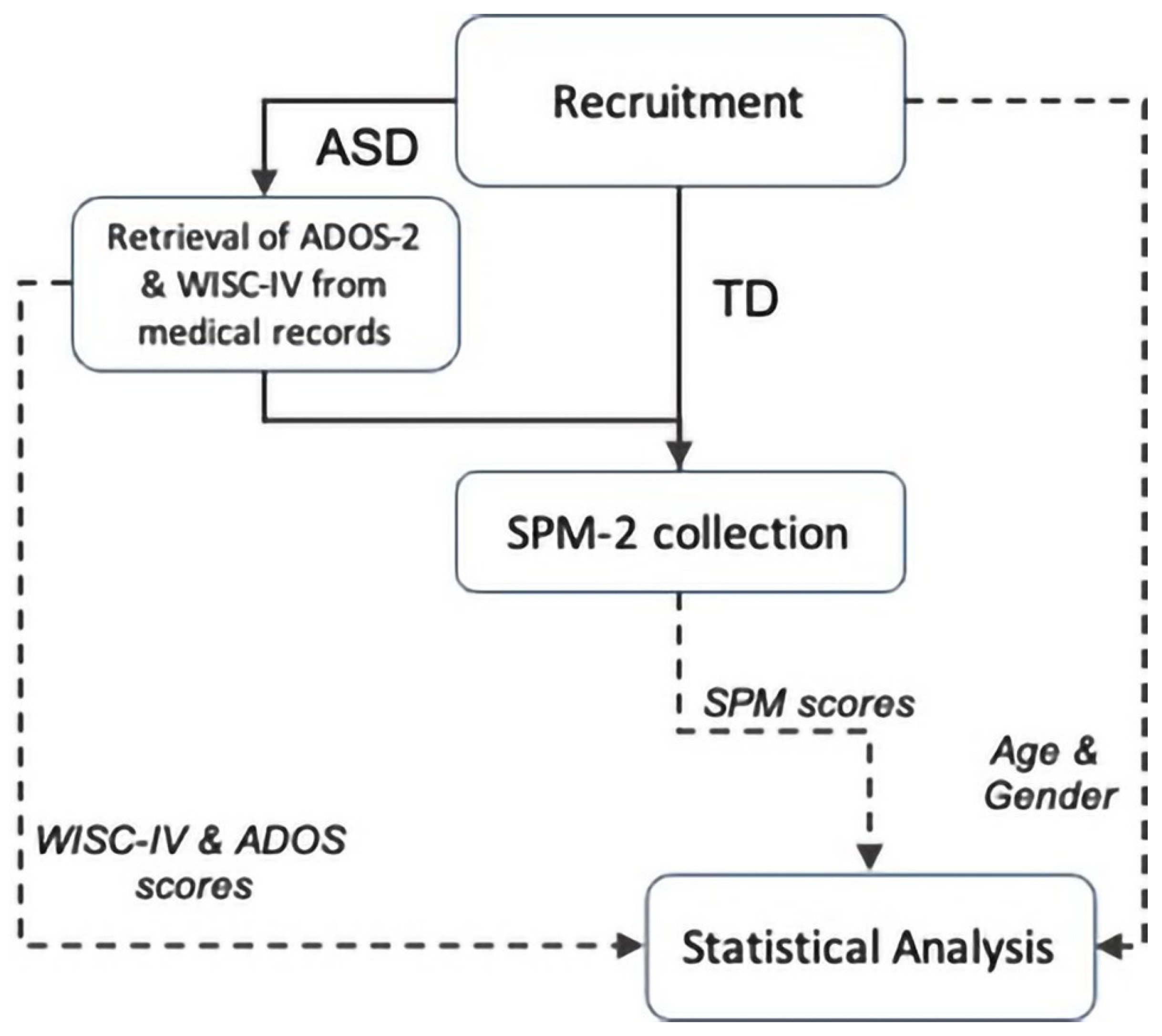
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Data Analysis
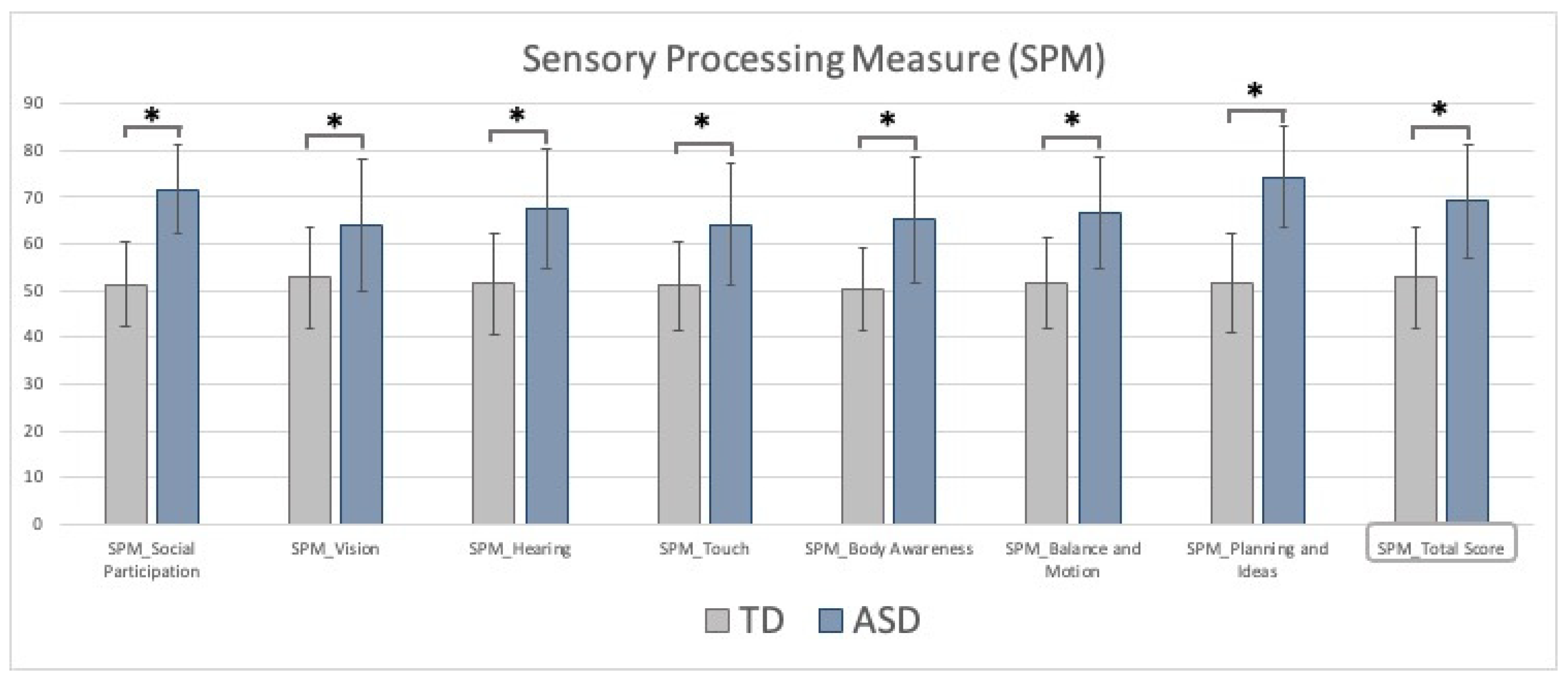
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kanner, L. Autistic disturbances of affective contact. Nervous Child 1943, 2, 217–250. [Google Scholar]
- Robertson, C.E.; Baron-Cohen, S. Sensory perception in autism. Nat. Rev. Neurosci. 2017, 18, 671–684. [Google Scholar] [CrossRef] [PubMed]
- DuBois, D.; Lymer, E.; Gibson, B.E.; Desarkar, P.; Nalder, E. Assessing Sensory Processing Dysfunction in Adults and Adolescents with Autism Spectrum Disorder: A Scoping Review. Brain Sci. 2017, 7, 108. [Google Scholar] [CrossRef] [PubMed]
- Tomchek, S.D.; Dunn, W. Sensory processing in children with and without autism: A comparative study using the short sensory profile. Am. J. Occup. Ther. 2007, 61, 190–200. [Google Scholar] [CrossRef]
- Tavassoli, T.; Miller, L.J.; Schoen, S.A.; Nielsen, D.M.; Baron-Cohen, S. Sensory over-responsivity in adults with autism spectrum conditions. Autism 2014, 18, 428–432. [Google Scholar] [CrossRef]
- Ahn, R.R.; Miller, L.J.; Milberger, S.; McIntosh, D.N. Prevalence of parents’ perceptions of sensory processing disorders among kindergarten children. Am. J. Occup. Ther. 2004, 58, 287–293. [Google Scholar] [CrossRef]
- Ben-Sasson, A.; Hen, L.; Fluss, R.; Cermak, S.A.; Engel-Yeger, B.; Gal, E. A meta-analysis of sensory modulation symptoms in individuals with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Miller, L.J.; Anzalone, M.E.; Lane, S.; Cermak, S.A.; Osten, E. Concept evolution in sensory integration: A proposed nosology for diagnosis. Am. J. Occup. Ther. 2007, 61, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Cesaroni, L.; Garber, M. Exploring the experience of autism through firsthand accounts. J. Autism Dev. Disord. 1991, 21, 303–313. [Google Scholar] [CrossRef]
- Ermer, J.; Dunn, W. The sensory profile: A discriminant analysis of children with and without disabilities. Am. J. Occup. Ther. 1998, 52, 283–290. [Google Scholar] [CrossRef][Green Version]
- Grandin, T. Thinking in Pictures: My Life with Autism; Doubleday: New York, NY, USA, 1995. [Google Scholar]
- Chamak, B.; Bonniau, B.; Jaunay, E.; Cohen, D. What can we learn about autism from autistic persons? Psychother. Psychosom. 2008, 77, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T. Visual abilities and sensory differences in a person with autism. Biol. Psychiatry 2009, 65, 15–16. [Google Scholar] [CrossRef] [PubMed]
- Dahlgren, S.O.; Gillberg, C. Symptoms in the first two years of life. A preliminary population study of infantile autism. Eur. Arch. Psychiatry Neurol. Sci. 1989, 238, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, Y.; Kumashiro, H.; Yashima, Y.; Tachibana, R.; Watanabe, M.; Furukawa, H. Early symptoms of autistic children and its diagnostic significance. Folia Psychiatr Neurol. J. 1982, 36, 367–374. [Google Scholar] [CrossRef]
- Glod, M.; Riby, D.M.; Honey, E.; Rodgers, J. Psychological Correlates of Sensory Processing Patterns in Individuals with Autism Spectrum Disorder: A Systematic Review. Rev. J. Autism Dev. Disord. 2015, 2, 199–221. [Google Scholar] [CrossRef]
- Schauder, K.B.; Bennetto, L. Toward an Interdisciplinary Understanding of Sensory Dysfunction in Autism Spectrum Disorder: An Integration of the Neural and Symptom Literatures. Front. Neurosci. 2016, 17, 268. [Google Scholar] [CrossRef] [PubMed]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5, 5th ed.; American Psychiatric Publishing Inc.: Arlington, VA, USA, 2013. [Google Scholar]
- Ben-Sasson, A.; Gal, E.; Fluss, R.; Katz-Zetler, N.; Cermak, S.A. Update of a Meta-analysis of Sensory Symptoms in ASD: A New Decade of Research. J. Autism Dev. Disord. 2019, 49, 4974–4996. [Google Scholar] [CrossRef] [PubMed]
- Gillberg, C.; Coleman, M. Autism and medical disorders: A review of the literature. Dev. Med. Child Neurol. 1996, 38, 191–202. [Google Scholar] [CrossRef]
- Rimland, B.; Edelson, S.M. Brief report: A pilot study of auditory integration training in autism. J. Autism Dev. Disord. 1995, 25, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Osterling, J.; Dawson, G. Early recognition of children with autism: A study of first birthday home videotapes. J. Autism Dev. Disord. 1994, 24, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T. Autism during infancy: A retrospective video analysis of sensorymotor and social behaviors at 9-12 months of age. J. Autism Dev. Disord. 1999, 29, 213–224. [Google Scholar] [CrossRef]
- Wiggins, L.D.; Robins, D.L.; Bakeman, R.; Adamson, L.B. Brief report: Sensory abnormalities as distinguishing symptoms of Autism Spectrum Disorders in young children. J. Autism Dev. Disord. 2009, 39, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- Wing, L. Autistic spectrum disorders. BMJ 1996, 312, 327–328. [Google Scholar] [CrossRef]
- Kientz, M.A.; Dunn, W. A comparison of the performance of children with and without autism on the Sensory Profile. Am. J. Occup. Ther. 1997, 51, 530–537. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; Foster, L.G.; Berkson, G. Tactile defensiveness and mtyped behaviors. Am. J. Occup. Ther. 1997, 51, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Afif, I.Y.; Farkhan, M.; Kurdi, O.; Maula, M.I.; Ammarullah, M.I.; Setiyana, B.; Jamari, J.; Winarni, T.I. Effect of Short-Term Deep-Pressure Portable Seat on Behavioral and Biological Stress in Children with Autism Spectrum Disorders: A Pilot Study. Bioengineering 2022, 9, 48. [Google Scholar] [CrossRef]
- Adamson, A.; O’Hare, A.; Graham, C. Impairments in sensory modulation in children with autistic spectrum disorder. Br. J. Occup. Ther. 2006, 69, 357–364. [Google Scholar] [CrossRef]
- Dawson, G.; Finley, C.; Phillips, S.; Lewy, A. A comparison of hemispheric asymmetries in speech-related brain potentials of autistic and dysphasic children. Brain Lang. 1989, 37, 26–41. [Google Scholar] [CrossRef]
- Gillberg, C.; Ehlers, S.; Schaumann, H.; Jakobsson, G.; Dahlgren, S.O.; Lindblom, R.; Bågenholm, A.; Tjuus, T.; Blidner, E. Autism under age 3 years: A clinical study of 28 cases referred for autistic symptoms in infancy. J. Child Psychol. Psychiatry 1990, 31, 921–934. [Google Scholar] [CrossRef]
- Levante, A.; Petrocchi, S.; Lecciso, F. The Criterion Validity of the First Year Inventory and the Quantitative-CHecklist for Autism in Toddlers: A Longitudinal Study. Brain Sci. 2020, 10, 729. [Google Scholar] [CrossRef] [PubMed]
- Kern, J.K.; Trivedi, M.H.; Grannemann, B.D.; Garver, C.R.; Johnson, D.G.; Andrews, A.; Savla, J.S.; Mehta, J.A.; Schroeder, J.L. Sensory correlations in autism. Autism 2007, 11, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Baranek, G.T.; David, F.J.; Poe, M.D.; Stone, W.L.; Watson, L.R. Sensory Experiences Questionnaire: Discriminating sensory features in young children with autism, developmental delays, and typical development. J. Child Psychol. Psychiatry 2006, 47, 591–601. [Google Scholar] [CrossRef]
- Rogers, S.J.; Hepburn, S.; Wehner, E. Parent reports of sensory symptoms in toddlers with autism and those with other developmental disorders. J. Autism Dev. Disord. 2003, 33, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Lord, C.; Rutter, M.; DiLavore, P.C.; Risi, S.; Gotham, K.; Bishop, S. Autism Diagnostic Observation Schedule 2; Western Psychological Services: Torrance, CA, USA, 2012. [Google Scholar]
- Wechsler, D. Wechsler Intelligence Scale for Children, 4th ed.; PsychCorp: San Antonio, TX, USA, 2003. [Google Scholar]
- Parham, D.L.; Ecker, C.L.; Kuhaneck, H.; Henry, D.A.; Glennon, T.J. Sensory Processing Measure (SPM-2); WPS: Torrance, CA, USA, 2013. [Google Scholar]
- Edgar, J.C.; Khan, S.Y.; Blaskey, L.; Chow, V.Y.; Rey, M.; Gaetz, W.; Cannon, K.M.; Monroe, J.F.; Cornew, L.; Qasmieh, S.; et al. Neuromagnetic oscillations predict evoked-response latency delays and core language deficits in Autism Spectrum Disorders. J. Autism Dev. Disord. 2015, 45, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Roberts, T.P.; Khan, S.Y.; Rey, M.; Monroe, J.F.; Cannon, K.; Blaskey, L.; Woldoff, S.; Qasmieh, S.; Gandal, M.; Schmidt, G.L.; et al. MEG detection of delayed auditory evoked responses in Autism Spectrum Disorders: Towards an imaging biomarker for autism. Autism Res. 2010, 3, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Brandwein, A.B.; Foxe, J.J.; Butler, J.S.; Frey, H.-P.; Bates, J.C.; Shulman, L.H.; Molholm, S. Neurophysiological indices of atypical auditory processing and multisensory integration are associated with symptom severity in autism. J. Autism Dev. Disord. 2015, 45, 230–244. [Google Scholar] [CrossRef]
- Oram Cardy, J.E.; Flagg, E.J.; Roberts, W.; Roberts, T.P. Auditory evoked fields predict language ability and impairment in children. Int. J. Psychophysiol. 2008, 68, 170–175. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, R.A.; Segers, M.; Ferber, S.; Barense, M.D.; Wallace, M.T. The impact of multisensory integration deficits on speech perception in children with Autism Spectrum Disorders. Front. Psychol. 2014, 21, 379. [Google Scholar] [CrossRef] [PubMed]
- Mansour, Y.; Burchell, A.; Kulesza, R.J. Central Auditory and Vestibular Dysfunction Are Key Features of Autism Spectrum Disorder. Front. Integr. Neurosci. 2021, 15, 743561. [Google Scholar] [CrossRef] [PubMed]
- Remington, A.; Fairnie, J. A Sound advantage: Increased auditory capacity in autism. Cognition 2017, 166, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Estes, A.; Zwaigenbaum, L.; Gu, H.; St John, T.; Paterson, S.; Elison, J.T.; Hazlett, H.; Botteron, K.; Dager, S.R.; Schultz, R.T.; et al. Behavioral, cognitive, and adaptive development in infants with autism spectrum disorder in the first 2 years of life. J. Neurodev. Disord. 2015, 7, 24. [Google Scholar] [CrossRef]
- Pellicano, E.; Burr, D. When the world becomes ’too real’: A Bayesian explanation of autistic perception. Trends Cogn. Sci. 2012, 16, 504–510. [Google Scholar] [CrossRef]
- Cermak, S.A.; Daunhauer, L.A. Sensory processing in the postinstitutionalized child. Am. J. Occup. Ther. 1997, 51, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Lombardo, M.V.; Chakrabarti, B.; Ruigrok, A.N.; Bullmore, E.T.; Suckling, J.; Auyeung, B.; Happé, F.; Szatmari, P.; Baron-Cohen, S.; et al. Neural self-representation in autistic women and association with ‘compensatory camouflaging’. Autism 2019, 23, 1210–1223. [Google Scholar] [CrossRef] [PubMed]
- Hull, L.; Petrides, K.V.; Allison, C.; Smith, P.; Baron-Cohen, S.; Lai, M.C.; Mandy, W. “Putting on My Best Normal”: Social Camouflaging in Adults with Autism Spectrum Conditions. J. Autism Dev. Disord. 2017, 47, 2519–2534. [Google Scholar] [CrossRef] [PubMed]
- Fabbri-Destro, M.; Gizzonio, V.; Avanzini, P. Autism, motor dysfunctions and mirror mechanism. Clin. Neuropsychiatry 2013, 10, 177–187. [Google Scholar]
- Ritvo, E.R.; Ornitz, E.M.; Eviatar, A.; Markham, C.H.; Brown, M.B.; Mason, A. Decreased postrotatory nystagmus in early infantile autism. Neurology 1969, 19, 653–658. [Google Scholar] [CrossRef]
- Ornitz, E.M. Vestibular dysfunction in schizophrenia and childhood autism. Compr. Psychiatry 1970, 11, 159–173. [Google Scholar] [CrossRef]
- Molloy, C.A.; Dietrich, K.N.; Bhattacharya, A. Postural stability in children with autism spectrum disorder. J. Autism Dev. Disord. 2003, 33, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Baum, S.H.; Stevenson, R.A.; Wallace, M.T. Behavioral, perceptual, and neural alterations in sensory and multisensory function in autism spectrum disorder. Prog. Neurobiol. 2015, 134, 140–160. [Google Scholar] [CrossRef]
- Parham, L.D.; Cohn, E.S.; Spitzer, S.; Koomar, J.A.; Miller, L.J.; Burke, J.P.; Brett-Green, B.; Mailloux, Z.; May-Benson, T.A.; Roley, S.S.; et al. Fidelity in sensory integration intervention research. Am. J. Occup. Ther. 2007, 61, 216–227. [Google Scholar] [CrossRef]
- Smoot Reinert, S.; Jackson, K.; Bigelow, K. Using posturography to examine the immediate effects of vestibular therapy for children with Autism Spectrum Disorders: A feasibility study. Phys. Occup. Ther. Pediatr. 2015, 35, 365–380. [Google Scholar] [CrossRef] [PubMed]
- Slavik, B.; Kitsuwa-Lowe, J.; Danner, P.; Green, J.; Ayres, A. Vestibular stimulation and eye contact in autistic children. Neuropediatrics 1984, 15, 33–36. [Google Scholar] [CrossRef]
- Maurer, R.G.; Damasio, A.R. Vestibular dysfunction in autistic children. Dev. Med. Child Neurol. 1979, 21, 656–659. [Google Scholar] [CrossRef] [PubMed]
| N | Age | Gender | ADOS-2 | IQ | ||
|---|---|---|---|---|---|---|
| TD | 70 | 8.8 ± 2.0 | 33 f; 37 m | |||
| ASD | 105 | 9.3 ± 2.0 | 17 f; 88 m | 5.8 ± 1.7 | 88.4 ± 25.5 | |
| HF | 66 | 9.4 ± 2.1 | 7 f; 59 m | 5.4 ± 1.5 | 105.1 ± 14.7 | |
| LF | 39 | 8.9 ± 1.7 | 10 f; 29 m | 6.6 ± 1.9 | 60.4 ± 10.4 | |
| LF | HF | Symptoms | Functioning | |
|---|---|---|---|---|
| Social Participation | 74.1 ± 8.5 | 71.3 ± 9.9 | p = 0.002 * | p = 0.05 * |
| Vision | 63.6 ± 13.3 | 67 ± 14.7 | p < 0.001 * | p = 0.75 |
| Hearing | 68.7 ± 12.1 | 67.4 ± 13.6 | p < 0.001 * | p = 0.02 * |
| Touch | 60.1 ± 12.2 | 66.6 ± 13 | p < 0.001 * | p = 0.10 |
| Body Awareness | 62 ± 12.9 | 69.1 ± 13.3 | p < 0.001 * | p = 0.14 |
| Balance and Motion | 63.3 ± 12.2 | 69.6 ± 11.6 | p < 0.001 * | p = 0.01 * |
| Planning and Ideas | 76.2 ± 8.4 | 73.7 ± 11.8 | p = 0.002 * | p = 0.16 |
| Total | 67 ± 11.8 | 73 ± 12.2 | p < 0.001 * | p = 0.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Narzisi, A.; Fabbri-Destro, M.; Crifaci, G.; Scatigna, S.; Maugeri, F.; Berloffa, S.; Fantozzi, P.; Prato, A.; Muccio, R.; Valente, E.; et al. Sensory Profiles in School-Aged Children with Autism Spectrum Disorder: A Descriptive Study Using the Sensory Processing Measure-2 (SPM-2). J. Clin. Med. 2022, 11, 1668. https://doi.org/10.3390/jcm11061668
Narzisi A, Fabbri-Destro M, Crifaci G, Scatigna S, Maugeri F, Berloffa S, Fantozzi P, Prato A, Muccio R, Valente E, et al. Sensory Profiles in School-Aged Children with Autism Spectrum Disorder: A Descriptive Study Using the Sensory Processing Measure-2 (SPM-2). Journal of Clinical Medicine. 2022; 11(6):1668. https://doi.org/10.3390/jcm11061668
Chicago/Turabian StyleNarzisi, Antonio, Maddalena Fabbri-Destro, Giulia Crifaci, Stefano Scatigna, Federica Maugeri, Stefano Berloffa, Pamela Fantozzi, Adriana Prato, Rosy Muccio, Elena Valente, and et al. 2022. "Sensory Profiles in School-Aged Children with Autism Spectrum Disorder: A Descriptive Study Using the Sensory Processing Measure-2 (SPM-2)" Journal of Clinical Medicine 11, no. 6: 1668. https://doi.org/10.3390/jcm11061668
APA StyleNarzisi, A., Fabbri-Destro, M., Crifaci, G., Scatigna, S., Maugeri, F., Berloffa, S., Fantozzi, P., Prato, A., Muccio, R., Valente, E., Viglione, V., Pecchini, E., Pelagatti, S., Rizzo, R., Milone, A., Barone, R., & Masi, G. (2022). Sensory Profiles in School-Aged Children with Autism Spectrum Disorder: A Descriptive Study Using the Sensory Processing Measure-2 (SPM-2). Journal of Clinical Medicine, 11(6), 1668. https://doi.org/10.3390/jcm11061668







