Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review
Abstract
1. Introduction
1.1. Conventional Treatments and Management after SCI
1.2. Functional Electrical Stimulation (FES) Therapy for SCI Rehabilitation
1.3. Spinal Cord Stimulation (SCS) Therapy for SCI Rehabilitation
2. Trans-Spinal Direct Current Stimulation (tsDCS)
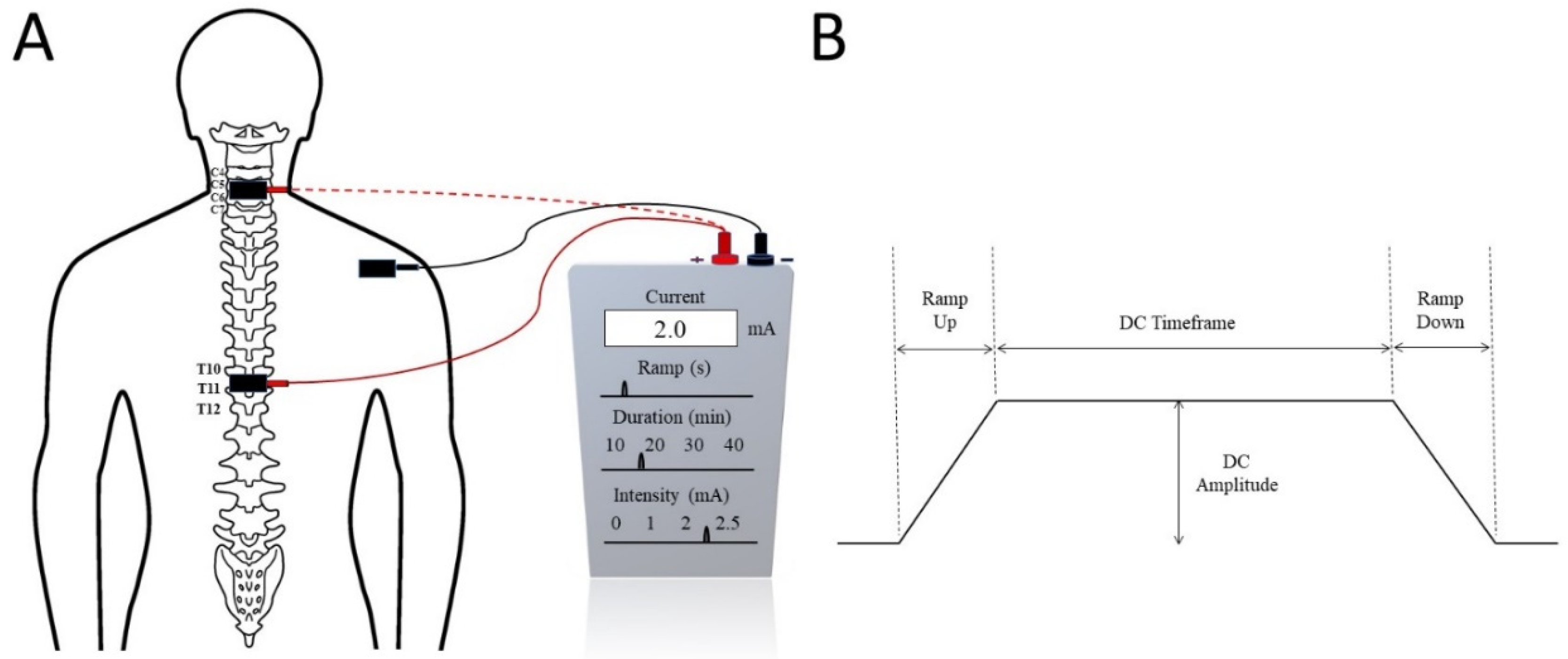
2.1. Mechanism of tsDCS
2.2. tsDCS in SCI Rehabilitation
3. Trans-Spinal Pulsed Current Stimulation (tsPCS)
3.1. Mechanism of tsPCS
3.2. tsPCS in SCI Rehabilitation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.S.; Harvey, L.A.; Islam, S.; Rahman, A.; Muldoon, S.; Biering-Sorensen, F.; Jan, S.; Liu, H.; Li, Q.; Cameron, I.D.; et al. A community-based intervention to prevent serious complications and death 2 years after discharge in people with spinal cord injury in Bangladesh (CIVIC): A randomised trial. Spinal Cord 2020, 59, 649–658. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Ahmed, S.; Sultana, R.; Taoheed, F.; Andalib, A.; Arafat, S.Y. Epidemiology of Spinal Cord Injury in Bangladesh: A Five Year Observation from a Rehabilitation Center. J. Spine 2017, 6, 100367. [Google Scholar] [CrossRef]
- Hoffmann, D.D.; Sundby, J.; Biering-Sørensen, F.; Kasch, H. Implementing volunteer peer mentoring as a supplement to professional efforts in primary rehabilitation of persons with spinal cord injury. Spinal Cord 2019, 57, 881–889. [Google Scholar] [CrossRef]
- Ginis, K.A.M.; Latimer, A.; Hicks, A.L.; Craven, B. Development and Evaluation of an Activity Measure for People with Spinal Cord Injury. Med. Sci. Sports Exerc. 2005, 37, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- Chafetz, R.S.; Vogel, L.C.; Betz, R.R.; Gaughan, J.P.; Mulcahey, M.J. International Standards for Neurological Classification of Spinal Cord Injury: Training Effect on Accurate Classification. J. Spinal Cord Med. 2008, 31, 538–542. [Google Scholar] [CrossRef]
- Roberts, T.T.; Leonard, G.R.; Cepela, D.J. Classifications in brief: American spinal injury association (ASIA) impairment scale. Clin. Orthop. Relat. Res. 2017, 475, 1499–1504. [Google Scholar] [CrossRef]
- Kirshblum, S.C.; Burns, S.P.; Biering-Sørensen, F.; Donovan, W.; Graves, D.E.; Jha, A.; Johansen, M.; Jones, L.; Krassioukov, A.; Mulcahey, M.J.; et al. International standards for neurological classification of spinal cord injury (Revised 2011). J. Spinal Cord Med. 2011, 34, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Van den Berg, M.E.; Castellote, J.M.; de Pedro-Cuesta, J.; Mahillo-Fernandez, I. Survival after spinal cord injury: A systematic review. J. Neurotrauma 2010, 27, 1517–1528. [Google Scholar] [CrossRef] [PubMed]
- Hitzig, S.L.; Tonack, M.; Campbell, K.A.; McGillivray, C.F.; Boschen, K.A.; Richards, K.; Craven, B.C. Secondary Health Complications in an Aging Canadian Spinal Cord Injury Sample. Am. J. Phys. Med. Rehabil. 2008, 87, 545–555. [Google Scholar] [CrossRef]
- Sezer, N.; Akkuş, S.; Uğurlu, F.G. Chronic complications of spinal cord injury. World J. Orthop. 2015, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Whiteneck, G.; Cassaway, J.; Dijkers, M.; Jha, A. New Approach to Study the Contents and Outcomes of Spinal Cord Injury Rehabilitation: The SCIRehab Project. J. Spinal Cord Med. 2009, 32, 251–259. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beekhuizen, K.S.; Field-Fote, E.C. Sensory stimulation augments the effects of massed practice training in persons with tetraplegia. Arch. Phys. Med. Rehabil. 2008, 89, 602–608. [Google Scholar] [CrossRef] [PubMed]
- McGee, M.J.; Amundsen, C.L.; Grill, W.M. Electrical stimulation for the treatment of lower urinary tract dysfunction after spinal cord injury. J. Spinal Cord Med. 2013, 38, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Xu, H.; Zuo, Y.; Liu, X.; All, A.H. A Review of Functional Electrical Stimulation Treatment in Spinal Cord Injury. Neuromol. Med. 2020, 22, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Grecco, L.; Li, S.; Michel, S.; Castillo-Saavedra, L.; Mourdoukoutas, A.; Bikson, M. Transcutaneous spinal stimulation as a therapeutic strategy for spinal cord injury: State of the art. J. Neurorestoratology 2015, 73, 77813. [Google Scholar] [CrossRef]
- Mekhail, N.A.; Mathews, M.; Nageeb, F.; Guirguis, M.; Mekhail, M.N.; Cheng, J. Retrospective Review of 707 Cases of Spinal Cord Stimulation: Indications and Complications. Pain Pract. 2010, 11, 148–153. [Google Scholar] [CrossRef] [PubMed]
- Al’Joboori, Y.; Massey, S.J.; Knight, S.L.; Donaldson, N.D.N.; Duffell, L.D. The Effects of Adding Transcutaneous Spinal Cord Stimulation (tSCS) to Sit-To-Stand Training in People with Spinal Cord Injury: A Pilot Study. J. Clin. Med. 2020, 9, 2765. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Vette, A.H.; Ramasubramaniam, S.; Li, K.; Burdick, J.; Edgerton, V.R.; Gerasimenko, Y.P.; Sayenko, D.G. Trunk Stability Enabled by Noninvasive Spinal Electrical Stimulation after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2540–2553. [Google Scholar] [CrossRef] [PubMed]
- Eng, J.J.; Teasell, R.; Miller, W.C.; Wolfe, D.L.; Townson, A.F.; Aubut, J.-A.; Abramson, C.; Hsieh, J.T.; Connolly, S.; Konnyu, K. Spinal Cord Injury Rehabilitation Evidence: Method of the SCIRE Systematic Review. Top. Spinal Cord Inj. Rehabil. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Shields, R.K. Muscular, skeletal, and neural adaptations following spinal cord injury. J. Orthop. Sports Phys. Ther. 2002, 32, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Galea, M.P.; Dunlop, S.A.; Davis, G.M.; Nunn, A.; Geraghty, T.; Hsueh, Y.-S.; Churilov, L. Intensive exercise program after spinal cord injury (“Full-On”): Study protocol for a randomized controlled trial. Trials 2013, 14, 291. [Google Scholar] [CrossRef] [PubMed]
- Cameron, A.P.; Wallner, L.P.; Tate, D.G.; Sarma, A.V.; Rodriguez, G.M.; Clemens, J.Q. Bladder Management After Spinal Cord Injury in the United States 1972 to 2005. J. Urol. 2010, 184, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Elbasiouny, S.M.; Moroz, D.; Bakr, M.M.; Mushahwar, V.K. Management of Spasticity After Spinal Cord Injury: Current Techniques and Future Directions. Neurorehabilit. Neural Repair 2009, 24, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Budh, C.N.; Lundeberg, T. Non-pharmacological pain-relieving therapies in individuals with spinal cord injury: A patient perspective. Complementary Ther. Med. 2004, 12, 189–197. [Google Scholar] [CrossRef]
- Weaver, L.C.; Marsh, D.R.; Gris, D.; Brown, A.; Dekaban, G.A. Autonomic dysreflexia after spinal cord injury: Central mechanisms and strategies for prevention. Prog. Brain Res. 2006, 152, 245–263. [Google Scholar] [CrossRef]
- Eldahan, K.C.; Rabchevsky, A.G. Autonomic dysreflexia after spinal cord injury: Systemic pathophysiology and methods of management. Auton. Neurosci. 2017, 209, 59–70. [Google Scholar] [CrossRef]
- Karlsson, A.K. Autonomic dysreflexia. Spinal Cord 1999, 37, 383–391. [Google Scholar] [CrossRef]
- Craig, A.; Tran, Y.; Wijesuriya, N.; Middleton, J. Fatigue and tiredness in people with spinal cord injury. J. Psychosom. Res. 2012, 73, 205–210. [Google Scholar] [CrossRef]
- Wijesuriya, N.; Tran, Y.; Middleton, J.; Craig, A. Impact of Fatigue on the Health-Related Quality of Life in Persons with Spinal Cord Injury. Arch. Phys. Med. Rehabil. 2012, 93, 319–324. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic spinal cord injury. Nat. Rev. Dis. Primers 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Popovic, M.R.; Zivanovic, V.; Valiante, T.A. Restoration of Upper Limb Function in an Individual with Cervical Spondylotic Myelopathy using Functional Electrical Stimulation Therapy: A Case Study. Front. Neurol. 2016, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Shen, D.; Wang, Y.; Wang, N.; Liang, Y. A Review of Different Stimulation Methods for Functional Reconstruction and Comparison of Respiratory Function after Cervical Spinal Cord Injury. Appl. Bionics Biomech. 2020, 2020, 8882430. [Google Scholar] [CrossRef]
- Creasey, G.H.; Ho, C.H.; Triolo, R.J.; Gater, D.R.; DiMarco, A.F.; Bogie, K.M.; Keith, M.W. Clinical applications of electrical stimulation after spinal cord injury. J. Spinal Cord Med. 2004, 27, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Arora, M.; Harvey, L.A.; Glinsky, J.V.; Nier, L.; Lavrencic, L.; Kifley, A.; Cameron, I.D. Electrical stimulation for treating pressure ulcers. Cochrane Database Syst. Rev. 2020, CD012196. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, N.; Moineau, B.; Popovic, M.R. Functional Electrical Stimulation Therapy for Retraining Reaching and Grasping after Spinal Cord Injury and Stroke. Front. Neurosci. 2020, 14, 718. [Google Scholar] [CrossRef] [PubMed]
- Melzack, R.J.F.N. Neuromodulation. Spinal Cord Stimul. 2018, 4, 43. [Google Scholar]
- Durbhakula, S.; Malik, K. Spinal Cord Stimulation. In Essentials of Pain Medicine; Elsevier: Amsterdam, The Netherlands, 2018; pp. 663–676.e662. [Google Scholar]
- Cuellar, C.A.; Mendez, A.A.; Islam, R.; Calvert, J.; Grahn, P.J.; Knudsen, B.E.; Pham, T.; Lee, K.H.; Lavrov, I.A. The Role of Functional Neuroanatomy of the Lumbar Spinal Cord in Effect of Epidural Stimulation. Front. Neuroanat. 2017, 11, e00082. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Minassian, K.; Mushahwar, V.K. Spinal cord stimulation: Therapeutic benefits and movement generation after spinal cord injury. Handb. Clin. Neurol. 2012, 109, 283–296. [Google Scholar] [PubMed]
- Nardone, R.; Höller, Y.; Leis, S.; Höller, P.; Thon, N.; Thomschewski, A.; Golaszewski, S.; Brigo, F.; Trinka, E. Invasive and non-invasive brain stimulation for treatment of neuropathic pain in patients with spinal cord injury: A review. J. Spinal Cord Med. 2013, 37, 19–31. [Google Scholar] [CrossRef] [PubMed]
- Bendersky, D.; Yampolsky, C. Is Spinal Cord Stimulation Safe? A Review of Its Complications. World Neurosurg. 2014, 82, 1359–1368. [Google Scholar] [CrossRef] [PubMed]
- Eldabe, S.; Buchser, E.; Duarte, R.V. Complications of Spinal Cord Stimulation and Peripheral Nerve Stimulation Techniques: A Review of the Literature. Pain Med. 2015, 17, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Shkorbatova, P.; Lyakhovetskii, V.; Pavlova, N.; Popov, A.; Bazhenova, E.; Kalinina, D.; Gorskii, O.; Musienko, P. Mapping of the Spinal Sensorimotor Network by Transvertebral and Transcutaneous Spinal Cord Stimulation. Front. Syst. Neurosci. 2020, 14, 555593. [Google Scholar] [CrossRef]
- Saito, A.; Masugi, Y.; Nakagawa, K.; Obata, H.; Nakazawa, K. Repeatability of spinal reflexes of lower limb muscles evoked by transcutaneous spinal cord stimulation. PLoS ONE 2019, 14, e0214818. [Google Scholar] [CrossRef] [PubMed]
- Megía-García, Á.; Serrano-Muñoz, D.; Taylor, J.; Avendaño-Coy, J.; Comino-Suárez, N.; Gómez-Soriano, J. Transcutaneous Spinal Cord Stimulation Enhances Quadriceps Motor Evoked Potential in Healthy Participants: A Double-Blind Randomized Controlled Study. J. Clin. Med. 2020, 9, 3275. [Google Scholar] [CrossRef]
- García, A.M.; Serrano-Muñoz, D.; Taylor, J.; Avendaño-Coy, J.; Gómez-Soriano, J. Transcutaneous Spinal Cord Stimulation and Motor Rehabilitation in Spinal Cord Injury: A Systematic Review. Neurorehabilit. Neural Repair 2019, 34, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Alam, M.; Li, S.; Ahmed, R.U.; Yam, Y.M.; Thakur, S.; Wang, X.-Y.; Tang, D.; Ng, S.; Zheng, Y.-P. Development of a battery-free ultrasonically powered functional electrical stimulator for movement restoration after paralyzing spinal cord injury. J. Neuroeng. Rehabil. 2019, 16, 36. [Google Scholar] [CrossRef]
- James, N.D.; McMahon, S.; Field-Fote, E.; Bradbury, E.J. Neuromodulation in the restoration of function after spinal cord injury. Lancet Neurol. 2018, 17, 905–917. [Google Scholar] [CrossRef]
- Meyer, C.; Hofstoetter, U.S.; Hubli, M.; Hassani, R.H.; Rinaldo, C.; Curt, A.; Bolliger, M. Immediate Effects of Transcutaneous Spinal Cord Stimulation on Motor Function in Chronic, Sensorimotor Incomplete Spinal Cord Injury. J. Clin. Med. 2020, 9, 3541. [Google Scholar] [CrossRef] [PubMed]
- McHugh, L.V.; Miller, A.A.; Leech, K.A.; Salorio, C.; Martin, R.H. Feasibility and utility of transcutaneous spinal cord stimulation combined with walking-based therapy for people with motor incomplete spinal cord injury. Spinal Cord Ser. Cases 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.L.; Campêlo, M.; Mendonça, T.; Fontes, L.A.M.; Brito, R.D.M.; Monte-Silva, K. Effects of repetitive transcranial magnetic stimulation and trans-spinal direct current stimulation associated with treadmill exercise in spinal cord and cortical excitability of healthy subjects: A triple-blind, randomized and sham-controlled study. PLoS ONE 2018, 13, e0195276. [Google Scholar] [CrossRef]
- Awosika, O.O.; Sandrini, M.; Volochayev, R.; Thompson, R.M.; Fishman, N.; Wu, T.; Floeter, M.K.; Hallett, M.; Cohen, L.G. Transcutaneous spinal direct current stimulation improves locomotor learning in healthy humans. Brain Stimul. 2019, 12, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Bocci, T.; Marceglia, S.; Vergari, M.; Cognetto, V.; Cogiamanian, F.; Sartucci, F.; Priori, A. Transcutaneous spinal direct current stimulation modulates human corticospinal system excitability. J. Neurophysiol. 2015, 114, 440–446. [Google Scholar] [CrossRef]
- Bocci, T.; Vannini, B.; Torzini, A.; Mazzatenta, A.; Vergari, M.; Cogiamanian, F.; Priori, A.; Sartucci, F. Cathodal transcutaneous spinal direct current stimulation (tsDCS) improves motor unit recruitment in healthy subjects. Neurosci. Lett. 2014, 578, 75–79. [Google Scholar] [CrossRef]
- Ahmed, Z. Trans-spinal direct current stimulation modulates motor cortex-induced muscle contraction in mice. J. Appl. Physiol. 2011, 110, 1414–1424. [Google Scholar] [CrossRef] [PubMed]
- Ardolino, G.; Bocci, T.; Nigro, M.; Vergari, M.; Di Fonzo, A.; Bonato, S.; Cogiamanian, F.; Cortese, F.; Cova, I.; Barbieri, S.; et al. Spinal direct current stimulation (tsDCS) in hereditary spastic paraplegias (HSP): A sham-controlled crossover study. J. Spinal Cord Med. 2018, 44, 46–53. [Google Scholar] [CrossRef]
- Powell, E.S.; Carrico, C.; Salyers, E.; Westgate, P.M.; Sawaki, L. The effect of transcutaneous spinal direct current stimulation on corticospinal excitability in chronic incomplete spinal cord injury. Neurorehabilitation 2018, 43, 125–134. [Google Scholar] [CrossRef]
- Gómez-Soriano, J.; Megía-García, A.; Serrano-Muñoz, D.; Osuagwu, B.; Taylor, J. Non-invasive spinal direct current simulation for spasticity therapy following spinal cord injury: Mechanistic insights contributing to long-term treatment effects. J. Physiol. 2019, 597, 2121. [Google Scholar] [CrossRef]
- Gogeascoechea, A.; Kuck, A.; Van Asseldonk, E.; Negro, F.; Buitenweg, J.R.; Yavuz, U.S.; Sartori, M. Interfacing with Alpha Motor Neurons in Spinal Cord Injury Patients Receiving Trans-spinal Electrical Stimulation. Front. Neurol. 2020, 11, e00493. [Google Scholar] [CrossRef] [PubMed]
- Woods, A.J.; Antal, A.; Bikson, M.; Boggio, P.S.; Brunoni, A.R.; Celnik, P.; Cohen, L.G.; Fregni, F.; Herrmann, C.S.; Kappenman, E.S.; et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin. Neurophysiol. 2016, 127, 1031–1048. [Google Scholar] [CrossRef]
- Niérat, M.-C.; Similowski, T.; Lamy, J.-C. Does Trans-Spinal Direct Current Stimulation Alter Phrenic Motoneurons and Respiratory Neuromechanical Outputs in Humans? A Double-Blind, Sham-Controlled, Randomized, Crossover Study. J. Neurosci. 2014, 34, 14420–14429. [Google Scholar] [CrossRef] [PubMed]
- Berra, E.; Bergamaschi, R.; De Icco, R.; Dagna, C.; Perrotta, A.; Rovaris, M.; Grasso, M.G.; Anastasio, M.G.; Pinardi, G.; Martello, F.; et al. The Effects of Transcutaneous Spinal Direct Current Stimulation on Neuropathic Pain in Multiple Sclerosis: Clinical and Neurophysiological Assessment. Front. Hum. Neurosci. 2019, 13, 31. [Google Scholar] [CrossRef] [PubMed]
- Cogiamanian, F.; Vergari, M.; Schiaffi, E.; Marceglia, S.; Ardolino, G.; Barbieri, S.; Priori, A. Transcutaneous spinal cord direct current stimulation inhibits the lower limb nociceptive flexion reflex in human beings. Pain 2011, 152, 370–375. [Google Scholar] [CrossRef]
- Kuck, A.; Stegeman, D.F.; Van Asseldonk, E.H.F. Modeling Trans-Spinal Direct Current Stimulation in the Presence of Spinal Implants. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 790–797. [Google Scholar] [CrossRef] [PubMed]
- ElBasiouny, S.M.; Mushahwar, V.K. Suppressing the excitability of spinal motoneurons by extracellularly applied electrical fields: Insights from computer simulations. J. Appl. Physiol. 2007, 103, 1824–1836. [Google Scholar] [CrossRef]
- Radman, T.; Su, Y.; An, J.H.; Parra, L.C.; Bikson, M. Spike Timing Amplifies the Effect of Electric Fields on Neurons: Implications for Endogenous Field Effects. J. Neurosci. 2007, 27, 3030–3036. [Google Scholar] [CrossRef] [PubMed]
- Cogiamanian, F.; Vergari, M.; Pulecchi, F.; Marceglia, S.; Priori, A. Effect of spinal transcutaneous direct current stimulation on somatosensory evoked potentials in humans. Clin. Neurophysiol. 2008, 119, 2636–2640. [Google Scholar] [CrossRef] [PubMed]
- Parazzini, M.; Fiocchi, S.; Liorni, I.; Rossi, E.; Cogiamanian, F.; Vergari, M.; Priori, A.; Ravazzani, P. Modeling the current density generated by transcutaneous spinal direct current stimulation (tsDCS). Clin. Neurophysiol. 2014, 125, 2260–2270. [Google Scholar] [CrossRef] [PubMed]
- De Groat, W.C.; Yoshimura, N. Mechanisms underlying the recovery of lower urinary tract function following spinal cord injury. Prog. Brain Res. 2006, 152, 59–84. [Google Scholar] [PubMed]
- Leoni, M.G.; De Ruz, A.E. Management of urinary tract infection in patients with spinal cord injuries. Clin. Microbiol. Infect. 2003, 9, 780–785. [Google Scholar] [CrossRef] [PubMed]
- Winkler, T.; Hering, P.; Straube, A. Spinal DC stimulation in humans modulates post-activation depression of the H-reflex depending on current polarity. Clin. Neurophysiol. 2010, 121, 957–961. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z. Effects of cathodal trans-spinal direct current stimulation on lower urinary tract function in normal and spinal cord injury mice with overactive bladder. J. Neural Eng. 2017, 14, 056002. [Google Scholar] [CrossRef] [PubMed]
- Sjölund, B.H. Pain and rehabilitation after spinal cord injury: The case of sensory spasticity? Brain Res. Rev. 2002, 40, 250–256. [Google Scholar] [CrossRef]
- Smania, N.; Picelli, A.; Munari, D.; Geroin, C.; Ianes, P.; Waldner, A.; Gandolfi, M. Rehabilitation procedures in the management of spasticity. Eur. J. Phys. Rehabil. Med. 2010, 46, 423–438. [Google Scholar] [PubMed]
- Hubli, M.; Dietz, V.; Schrafl-Altermatt, M.; Bolliger, M. Modulation of spinal neuronal excitability by spinal direct currents and locomotion after spinal cord injury. Clin. Neurophysiol. 2013, 124, 1187–1195. [Google Scholar] [CrossRef]
- Schweizer, L.; Meyer-Frießem, C.H.; Zahn, P.K.; Tegenthoff, M.; Schmidt-Wilcke, T. Transcutaneous Spinal Direct Current Stimulation Alters Resting-State Functional Connectivity. Brain Connect. 2017, 7, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Truini, A.; Vergari, M.; Biasiotta, A.; La Cesa, S.; Gabriele, M.; Di Stefano, G.; Cambieri, C.; Cruccu, G.; Inghilleri, M.; Priori, A. Transcutaneous spinal direct current stimulation inhibits nociceptive spinal pathway conduction and increases pain tolerance in humans. Eur. J. Pain 2011, 15, 1023–1027. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, A.; Bolla, M.; Anastasio, M.; Serrao, M.; Sandrini, G.; Pierelli, F. Modulation of temporal summation threshold of the nociceptive withdrawal reflex by transcutaneous spinal direct current stimulation in humans. Clin. Neurophysiol. 2016, 127, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Meyer-Frießem, C.; Haag, L.; Schmidt-Wilcke, T.; Magerl, W.; Pogatzki-Zahn, E.; Tegenthoff, M.; Zahn, P. Transcutaneous spinal DC stimulation reduces pain sensitivity in humans. Neurosci. Lett. 2015, 589, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Murray, L.M.; Tahayori, B.; Knikou, M. Transspinal Direct Current Stimulation Produces Persistent Plasticity in Human Motor Pathways. Sci. Rep. 2018, 8, 717. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.L.; Mendonça, T.; Campêlo, M.; Shirahige, L.; Monte-Silva, K. Does trans-spinal direct current stimulation modulate the Hoffmann reflexes of healthy individuals? A systematic review and meta-analysisc. Spinal Cord 2018, 56, 1022–1031. [Google Scholar] [CrossRef]
- Lu, D.C.; Edgerton, V.R.; Modaber, M.; AuYong, N.; Morikawa, E.; Zdunowski, S.; Sarino, M.E.; Sarrafzadeh, M.; Nuwer, M.R.; Roy, R.R.; et al. Engaging Cervical Spinal Cord Networks to Reenable Volitional Control of Hand Function in Tetraplegic Patients. Neurorehabilit. Neural Repair 2016, 30, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Inanici, F.; Samejima, S.; Gad, P.; Edgerton, V.R.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Electrical Spinal Stimulation Promotes Long-Term Recovery of Upper Extremity Function in Chronic Tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z. Trans-spinal direct current stimulation modifies spinal cord excitability through synaptic and axonal mechanisms. Physiol. Rep. 2014, 2, e12157. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Atkinson, D.A.; Dy, C.J.; Gurley, K.M.; Smith, V.L.; Angeli, C.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Spinal segment-specific transcutaneous stimulation differentially shapes activation pattern among motor pools in humans. J. Appl. Physiol. 2015, 118, 1364–1374. [Google Scholar] [CrossRef] [PubMed]
- Laufer, Y.; Elboim-Gabyzon, M. Does Sensory Transcutaneous Electrical Stimulation Enhance Motor Recovery following a Stroke? A Systematic Review. Neurorehabilit. Neural Repair 2011, 25, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Keller, T.; Micera, S.; Morari, M. Array electrode design for transcutaneous electrical stimulation: A simulation study. Med. Eng. Phys. 2009, 31, 945–951. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, A.; Keller, T.; Lawrence, M.; Morari, M. The Influence of Electrode Size on Selectivity and Comfort in Transcutaneous Electrical Stimulation of the Forearm. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 255–262. [Google Scholar] [CrossRef]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef] [PubMed]
- Lobov, G.I.; Gerasimenko, Y.P.; Moshonkina, T.R. Mechanisms of Vasodilation in Skin during Lumbar Transcutaneous Spinal Cord Stimulation. Hum. Physiol. 2019, 45, 389–396. [Google Scholar] [CrossRef]
- Benavides, F.D.; Jo, H.J.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and Subcortical Effects of Transcutaneous Spinal Cord Stimulation in Humans with Tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef]
- Hughes, G.B.; Bottomy, M.B.; Dickins, J.R.; Jackson, C.G.; Sismanis, A.; Glasscock, M.E., III. A comparative study of neuropathologic changes following pulsed and direct current stimulation of the mouse sciatic nerve. Am. J. Otolaryngol. 1980, 1, 378–384. [Google Scholar] [CrossRef]
- Wu, Y.-K.; Levine, J.M.; Wecht, J.R.; Maher, M.T.; Limonta, J.M.; Saeed, S.; Santiago, T.M.; Bailey, E.; Kastuar, S.; Guber, K.S.; et al. Posteroanterior cervical transcutaneous spinal stimulation targets ventral and dorsal nerve roots. Clin. Neurophysiol. 2019, 131, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-Invasive Activation of Cervical Spinal Networks after Severe Paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cord stimulation in humans. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Donaldson, N.D. A comparison of FES and SCS for neuroplastic recovery after SCI: Historical perspectives and future directions. Front. Neurol. 2020, 11, 607. [Google Scholar] [CrossRef]
- Alam, M.; Ling, Y.T.; Wong, A.Y.; Zhong, H.; Edgerton, V.R.; Zheng, Y. Reversing 21 years of chronic paralysis via non-invasive spinal cord neuromodulation: A case study. Ann. Clin. Transl. Neurol. 2020, 7, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Ke, A.; Zhang, P.; Gao, Z.; Zou, X.; He, J. Bioheat transfer model of transcutaneous spinal cord stimulation-induced temperature changes. PeerJ 2018, 6, e4921. [Google Scholar] [CrossRef]
- Manson, G.A.; Calvert, J.; Ling, J.; Tychhon, B.; Ali, A.; Sayenko, D.G. The relationship between maximum tolerance and motor activation during transcutaneous spinal stimulation is unaffected by the carrier frequency or vibration. Physiol. Rep. 2020, 8, e14397. [Google Scholar] [CrossRef]
- Ievins, A.; Moritz, C.T. Therapeutic Stimulation for Restoration of Function After Spinal Cord Injury. Physiology 2017, 32, 391–398. [Google Scholar] [CrossRef]
- Mayr, W.; Krenn, M.; Dimitrijevic, M.R. Epidural and transcutaneous spinal electrical stimulation for restoration of movement after incomplete and complete spinal cord injury. Curr. Opin. Neurol. 2016, 29, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.A.; Squair, J.; Sayenko, D.G.; Edgerton, V.R.; Gerasimenko, Y.; Krassioukov, A.V. An Autonomic Neuroprosthesis: Noninvasive Electrical Spinal Cord Stimulation Restores Autonomic Cardiovascular Function in Individuals with Spinal Cord Injury. J. Neurotrauma 2018, 35, 446–451. [Google Scholar] [CrossRef]
- Keller, A.; Singh, G.; Sommerfeld, J.H.; King, M.; Parikh, P.; Ugiliweneza, B.; D’Amico, J.; Gerasimenko, Y.; Behrman, A.L. Noninvasive spinal stimulation safely enables upright posture in children with spinal cord injury. Nat. Commun. 2021, 12, 5850. [Google Scholar] [CrossRef]
- Száva, Z.; Danner, S.M.; Minassian, K. Transcutaneous Electrical Spinal Cord Stimulation: Biophysics of a New Rehabilitation Method after Spinal Cord Injury; VDM Publishing: Saarbrücken, Germany, 2011. [Google Scholar]
- Murray, L.M.; Knikou, M. Repeated cathodal transspinal pulse and direct current stimulation modulate cortical and corticospinal excitability differently in healthy humans. Exp. Brain Res. 2019, 237, 1841–1852. [Google Scholar] [CrossRef]
- Minassian, K.; Hofstoetter, U.S.; Danner, S.M.; Mayr, W.; Bruce, J.A.; McKay, W.B.; Tansey, K.E. Spinal Rhythm Generation by Step-Induced Feedback and Transcutaneous Posterior Root Stimulation in Complete Spinal Cord–Injured Individuals. Neurorehabilit. Neural Repair 2015, 30, 233–243. [Google Scholar] [CrossRef]
- Sivanesan, E.; Maher, D.P.; Raja, S.N.; Linderoth, B.; Guan, Y. Supraspinal mechanisms of spinal cord stimulation for modulation of pain: Five decades of research and prospects for the future. Anesthesiology 2019, 130, 651–665. [Google Scholar] [CrossRef]
- Nardone, R.; Höller, Y.; Taylor, A.; Thomschewski, A.; Orioli, A.; Frey, V.; Trinka, E.; Brigo, F. Noninvasive Spinal Cord Stimulation: Technical Aspects and Therapeutic Applications. Neuromodul. Technol. Neural Interface 2015, 18, 580–591. [Google Scholar] [CrossRef]
- Salchow-Hömmen, C.; Dikau, C.; Müller, P.; Hofstötter, U.; Kühn, A.; Schauer, T.; Wenger, N. Characterization of Optimal Electrode Configurations for Transcutaneous Spinal Cord Stimulation. In Proceedings of the IFESS Conference Proc. 23, Toronto, ON, Canada, 25 September 2019. [Google Scholar]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight Bearing Over-ground Stepping in an Exoskeleton with Non-invasive Spinal Cord Neuromodulation after Motor Complete Paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.R.; Pereira, M.; Salvador, R.; Miranda, P.; De Carvalho, M. Cervical trans-spinal direct current stimulation: A modelling-experimental approach. J. Neuroeng. Rehabil. 2019, 16, 123. [Google Scholar] [CrossRef]
- Fregni, F.; Boggio, P.; Lima, M.; Ferreira, M.J.; Wagner, T.; Rigonatti, S.P.; Castro, A.W.; Souza, D.R.; Riberto, M.; Freedman, S.D.; et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006, 122, 197–209. [Google Scholar] [CrossRef]
- Barss, T.S.; Parhizi, B.; Mushahwar, V.K. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J. Neurophysiol. 2020, 123, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Krenn, M.; Toth, A.; Danner, S.M.; Hofstoetter, U.S.; Minassian, K.; Mayr, W. Selectivity of transcutaneous stimulation of lumbar posterior roots at different spinal levels in humans. Biomed. Eng. Biomed. Tech. 2013, 58. [Google Scholar] [CrossRef] [PubMed]
- Pérez Gámez, E.; Hernández Rodríguez, O. Efectos de diferentes técnicas de fisioterapia en el tratamiento de la espasticidad. Revisión Bibliográfica 2020. Available online: http://riull.ull.es/xmlui/handle/915/19841 (accessed on 8 March 2022).
- Calvert, J.; Manson, G.A.; Grahn, P.J.; Sayenko, D.G. Preferential activation of spinal sensorimotor networks via lateralized transcutaneous spinal stimulation in neurologically intact humans. J. Neurophysiol. 2019, 122, 2111–2118. [Google Scholar] [CrossRef] [PubMed]
- Danner, S.M.; Krenn, M.; Hofstoetter, U.S.; Toth, A.; Mayr, W.; Minassian, K. Body Position Influences Which Neural Structures Are Recruited by Lumbar Transcutaneous Spinal Cord Stimulation. PLoS ONE 2016, 11, e0147479. [Google Scholar] [CrossRef] [PubMed]
- Moshonkina, T.R.; Stolbkov, Y.K.; Kozlovskaya, I.B.; Gerasimenko, Y.P. Mechanisms of spinal cord electrical stimulation action on autonomic functions. Hum. Physiol. 2016, 42, 694–704. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Atkinson, D.A.; Floyd, T.C.; Gorodnichev, R.M.; Moshonkina, T.R.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci. Lett. 2015, 609, 229–234. [Google Scholar] [CrossRef][Green Version]
- Gorodnichev, R.M.; Pivovarova, E.A.; Puhov, A.; Moiseev, S.A.; Savochin, A.; Moshonkina, T.R.; Chsherbakova, N.A.; Kilimnik, V.A.; Selionov, V.; Kozlovskaya, I.B.; et al. Transcutaneous electrical stimulation of the spinal cord: A noninvasive tool for the activation of stepping pattern generators in humans. Hum. Physiol. 2012, 38, 158–167. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Sayenko, D.; Gad, P.; Kozesnik, J.; Moshonkina, T.; Grishin, A.; Pukhov, A.; Moiseev, S.; Gorodnichev, R.; Selionov, V.; et al. Electrical Spinal Stimulation, and Imagining of Lower Limb Movements to Modulate Brain-Spinal Connectomes That Control Locomotor-Like Behavior. Front. Physiol. 2018, 9, e01196. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Rath, M.; Ferguson, A.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-Assisted Standing Enabled by Non-Invasive Spinal Stimulation after Spinal Cord Injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hu, X. Elicited upper limb motions through transcutaneous cervical spinal cord stimulation. J. Neural Eng. 2020, 17, 036001. [Google Scholar] [CrossRef]
- Moshonkina, T.; Grishin, A.; Bogacheva, I.; Gorodnichev, R.; Ovechkin, A.; Siu, R.; Edgerton, V.R.; Gerasimenko, Y. Novel Non-invasive Strategy for Spinal Neuromodulation to Control Human Locomotion. Front. Hum. Neurosci. 2021, 14, 622533. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.P.; McKinney, Z.; Sayenko, D.G.; Gad, P.; Gorodnichev, R.M.; Grundfest, W.; Edgerton, V.R.; Kozlovskaya, I.B. Spinal and sensory neuromodulation of spinal neuronal networks in humans. Hum. Physiol. 2017, 43, 492–500. [Google Scholar] [CrossRef]
- Zhang, F.; Momeni, K.; Ramanujam, A.; Ravi, M.; Carnahan, J.; Kirshblum, S.; Forrest, G.F. Cervical Spinal Cord Transcutaneous Stimulation Improves Upper Extremity and Hand Function in People with Complete Tetraplegia: A Case Study. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 3167–3174. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W.; Binder, H.; Minassian, K. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 37, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Hofer, C.; Kern, H.; Danner, S.M.; Mayr, W.; Dimitrijevic, M.R.; Minassian, K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Eng. Biomed. Tech. 2013, 58. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Krenn, M.; Danner, S.M.; Hofer, C.; Kern, H.; McKay, W.B.; Mayr, W.; Minassian, K. Augmentation of Voluntary Locomotor Activity by Transcutaneous Spinal Cord Stimulation in Motor-Incomplete Spinal Cord-Injured Individuals. Artif. Organs 2015, 39, E176–E186. [Google Scholar] [CrossRef]
- Doherty, S.; Vanhoestenberghe, A.; Duffell, L.; Hamid, R.; Knight, S. A Urodynamic Comparison of Neural Targets for Transcutaneous Electrical Stimulation to Acutely Suppress Detrusor Contractions Following Spinal Cord Injury. Front. Neurosci. 2019, 13, 1360. [Google Scholar] [CrossRef] [PubMed]
- Kreydin, E.; Zhong, H.; Latack, K.; Ye, S.; Edgerton, V.R.; Gad, P. Transcutaneous Electrical Spinal Cord Neuromodulator (TESCoN) Improves Symptoms of Overactive Bladder. Front. Syst. Neurosci. 2020, 14. [Google Scholar] [CrossRef] [PubMed]
- DiMarco, A.; Geertman, R.; Tabbaa, K.; Nemunaitis, G.; Kowalski, K. Case report: Effects of lower thoracic spinal cord stimulation on bowel management in a person with spinal cord injury. J. Neuro Neurobiol. 2019, 5. [Google Scholar] [CrossRef]
- Havton, L.A.; Christe, K.L.; Edgerton, V.R.; Gad, P.N. Noninvasive spinal neuromodulation to map and augment lower urinary tract function in rhesus macaques. Exp. Neurol. 2019, 322, 113033. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.N.; Kreydin, E.; Zhong, H.; Edgerton, V.R. Enabling respiratory control after severe chronic tetraplegia: An exploratory case study. J. Neurophysiol. 2020, 124, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Lobov, G.I.; Gerasimenko, Y.P.; Moshonkina, T.R. Mechanisms of Blood Flow Regulation in the Skin during Stimulation of the Spinal Cord in Humans. Dokl. Biol. Sci. 2019, 485, 27–29. [Google Scholar] [CrossRef] [PubMed]
- Lobov, G.I.; Shcherbakova, N.A.; Gorodnichev, R.M.; Grishin, A.A.; Gerasimenko, Y.P.; Moshonkina, T.R. Effect of transcutaneous electrical spinal cord stimulation on the blood flow in the skin of lower limbs. Hum. Physiol. 2017, 43, 518–523. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2013, 37, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Jutzeler, C.R.; Streijger, F.; Aguilar, J.; Shortt, K.; Manouchehri, N.; Okon, E.; Hupp, M.; Curt, A.; Kwon, B.K.; Kramer, J.L.K. Sensorimotor plasticity after spinal cord injury: A longitudinal and translational study. Ann. Clin. Transl. Neurol. 2018, 6, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Jurkiewicz, M.T.; Mikulis, D.J.; McIlroy, W.E.; Fehlings, M.G.; Verrier, M.C. Sensorimotor Cortical Plasticity During Recovery Following Spinal Cord Injury: A Longitudinal fMRI Study. Neurorehabilit. Neural Repair 2007, 21, 527–538. [Google Scholar] [CrossRef]
- Jurkiewicz, M.T.; Mikulis, D.; Fehlings, M.; Verrier, M.C. Sensorimotor Cortical Activation in Patients withCervical Spinal Cord Injury withPersisting Paralysis. Neurorehabilit. Neural Repair 2009, 24, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Wrigley, P.J.; Siddall, P.J.; Gustin, S. New evidence for preserved somatosensory pathways in complete spinal cord injury: A fMRI study. Hum. Brain Mapp. 2017, 39, 588–598. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.A.; Tharu, N.S.; Gustin, S.M.; Zheng, Y.-P.; Alam, M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. J. Clin. Med. 2022, 11, 1550. https://doi.org/10.3390/jcm11061550
Rahman MA, Tharu NS, Gustin SM, Zheng Y-P, Alam M. Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. Journal of Clinical Medicine. 2022; 11(6):1550. https://doi.org/10.3390/jcm11061550
Chicago/Turabian StyleRahman, Md. Akhlasur, Niraj Singh Tharu, Sylvia M. Gustin, Yong-Ping Zheng, and Monzurul Alam. 2022. "Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review" Journal of Clinical Medicine 11, no. 6: 1550. https://doi.org/10.3390/jcm11061550
APA StyleRahman, M. A., Tharu, N. S., Gustin, S. M., Zheng, Y.-P., & Alam, M. (2022). Trans-Spinal Electrical Stimulation Therapy for Functional Rehabilitation after Spinal Cord Injury: Review. Journal of Clinical Medicine, 11(6), 1550. https://doi.org/10.3390/jcm11061550







